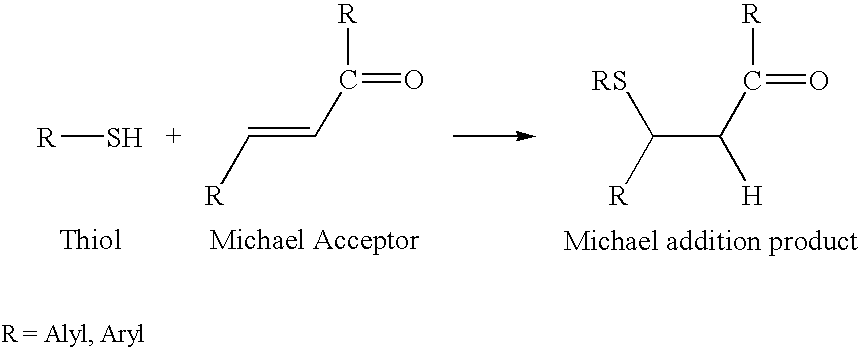
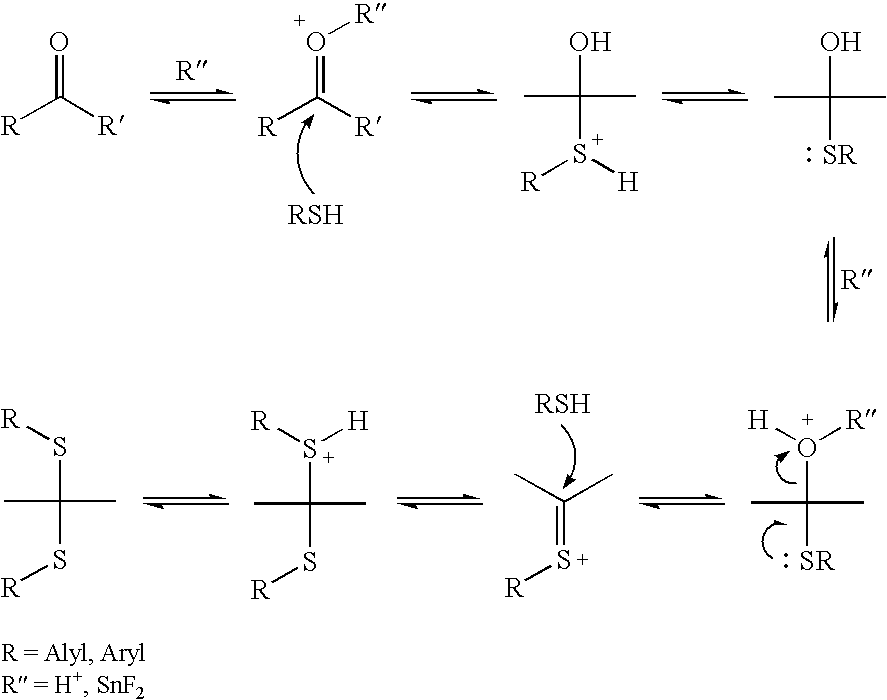
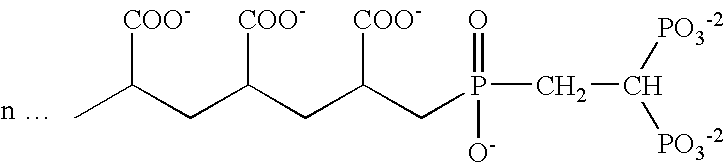
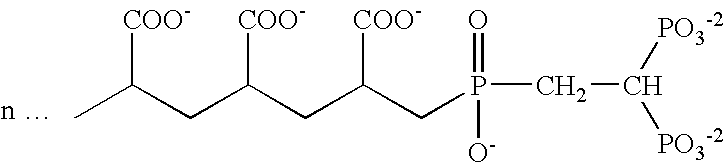
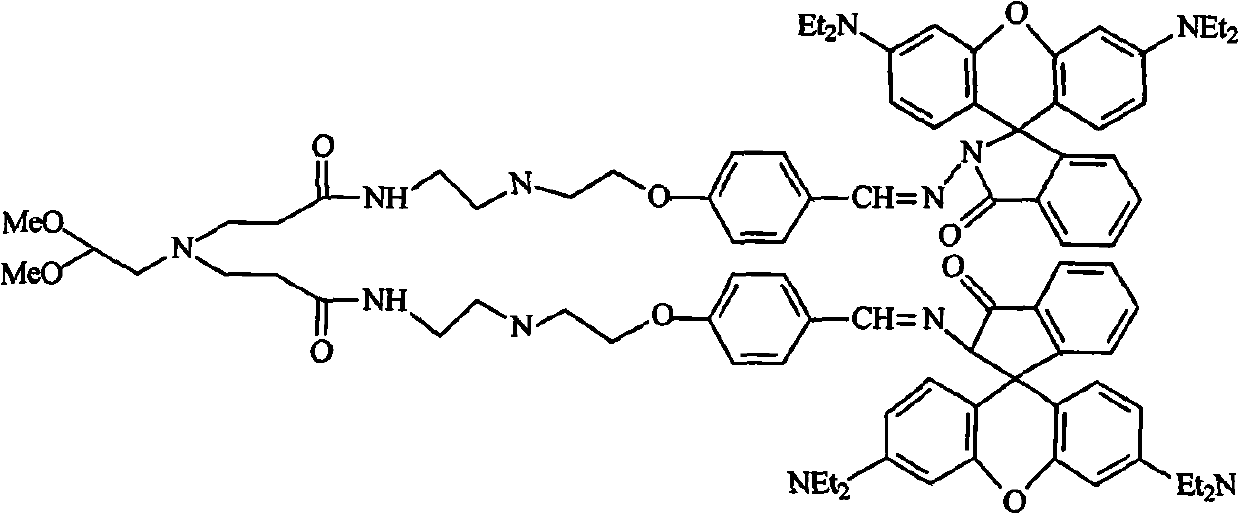
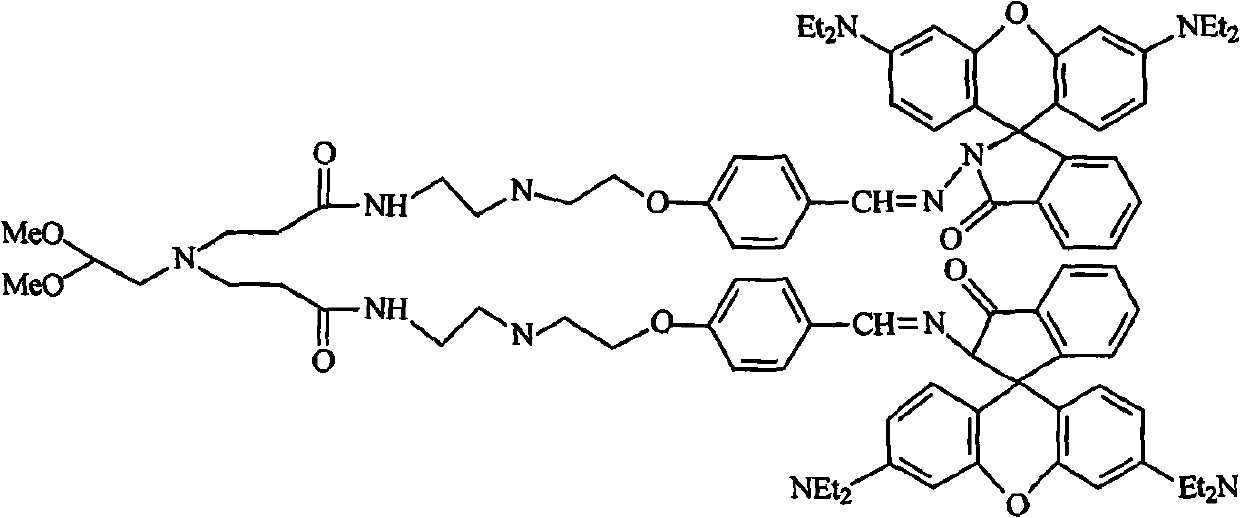
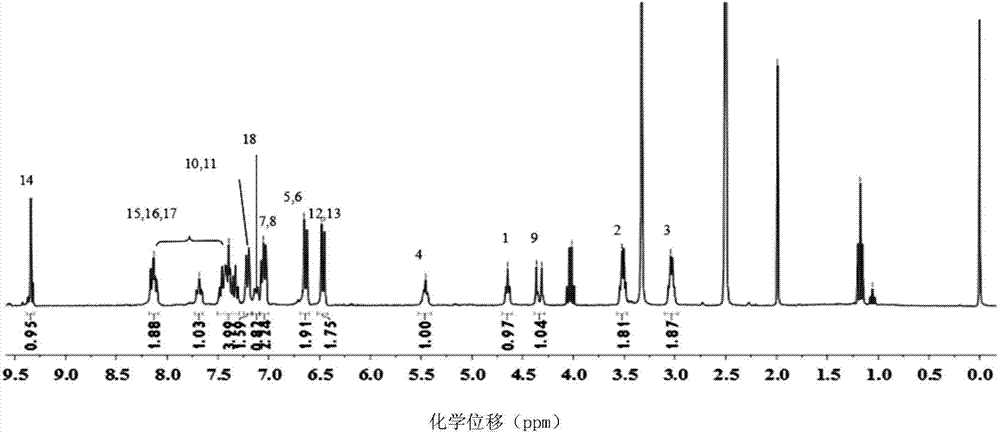
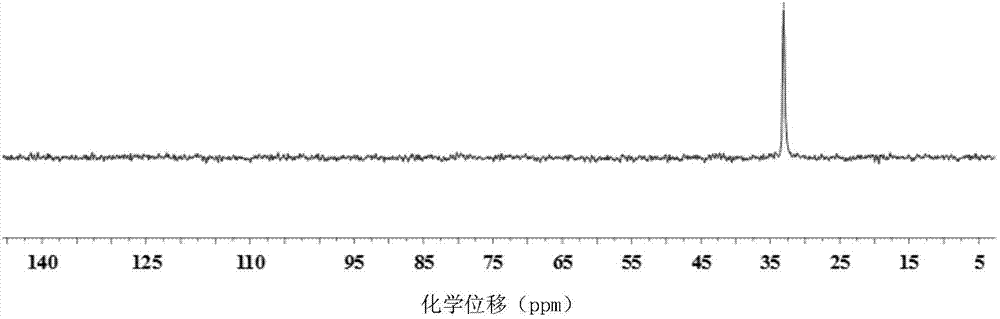
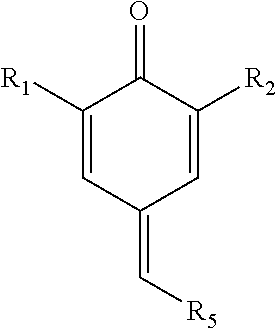
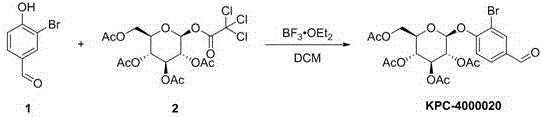
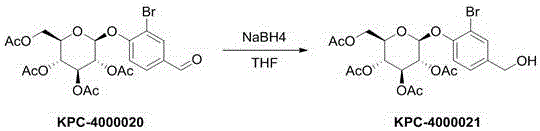
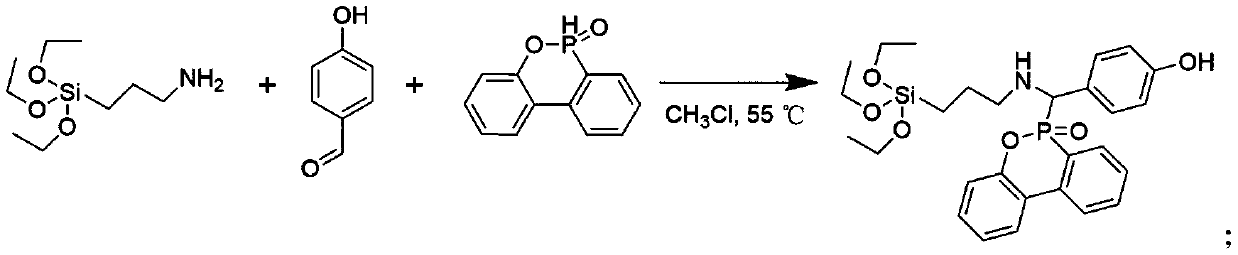
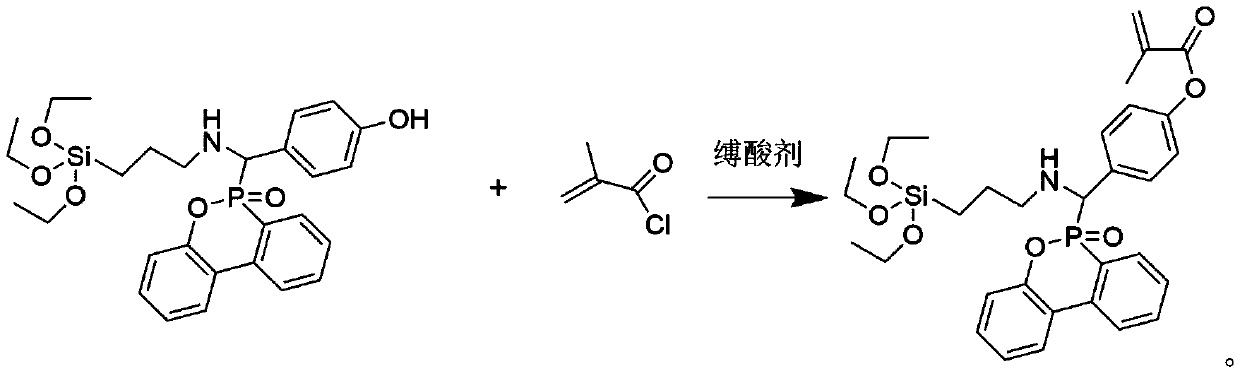
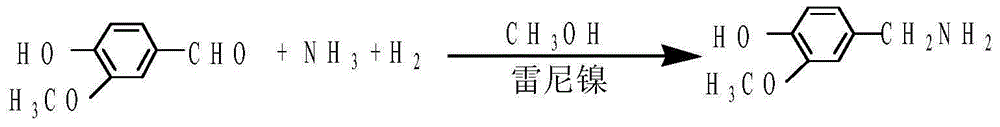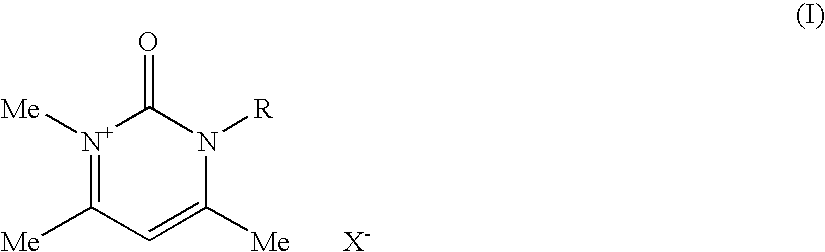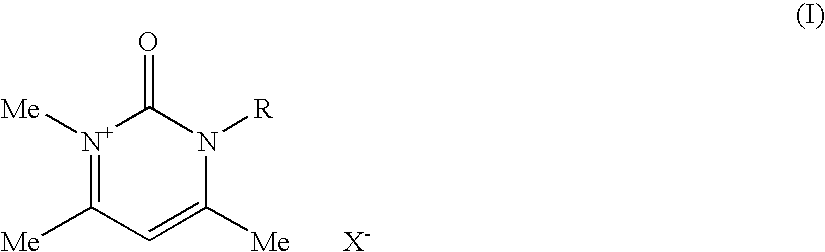Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
90 results about "4-Hydroxybenzaldehyde" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
4-Hydroxybenzaldehyde is one of the three isomers of hydroxybenzaldehyde. It can be found in the orchids Gastrodia elata and Galeola faberi. It is also found in vanilla.
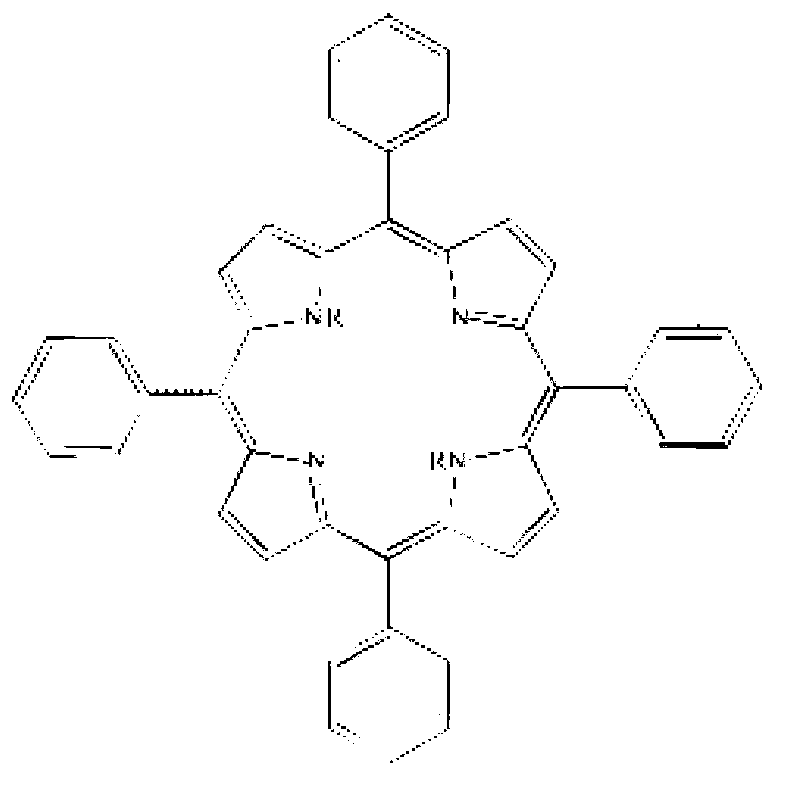
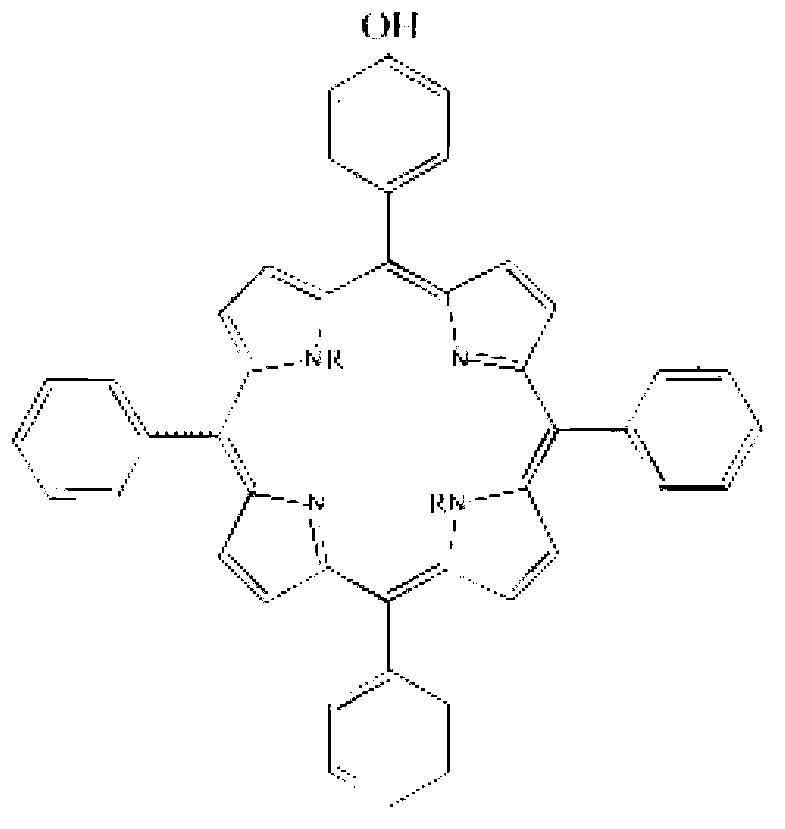
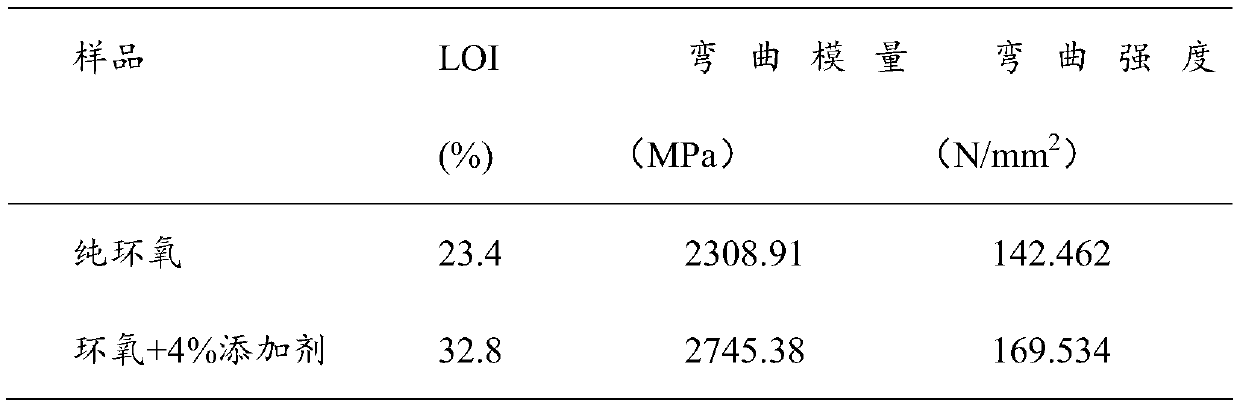
Corrosion-inhibition drag reducer for alkyl porphyrin compound natural gas pipelines and preparation method of corrosion-inhibition drag reducer
ActiveCN102838606AImprove anti-corrosion performanceOrganic chemistryPipeline systemsBenzaldehydePorphyrin
The invention relates to a corrosion-inhibition drag reducer for alkyl porphyrin compound natural gas pipelines of and a preparation method of the corrosion-inhibition drag reducer. The preparation method comprises the steps as follows: firstly, benzaldehyde, 4-hydroxyl benzaldehyde and pyrrole are used as raw materials to be reacted so as to prepare the mixture of six kinds of porphyrin compounds (H); then, the mixture H of the porphyrin compounds is reacted with acrylic acid long-chain alkyl esters to prepare six kinds of alkyl orphyrin compounds, wherein each kind of the compounds is 15 to 20wt%; and the prepared compounds are dissolved in 30 to 50wt% of solvent prepared with p-dioxane so as to form the corrosion-inhibition type drag reducer for the alkyl porphyrin compound natural gas pipelines. The proportion is that the mole ratio of methanal to the 4-hydroxyl benzaldehyde is (0.8:1) to (1.2:1); the mole ratio of the benzaldehyde to the pyrrole is (0.8:2) to (1.2:2); and the mole ratio of the acrylic acid long-chain alkyl esters to the pyrrole synthetizing H is (1:1) to (2:1). The drag reducer provided by the invention has the advantages of good drag reduction and anticorrosion property.
Owner:PIPECHINA SOUTH CHINA CO
Flavors for oral compositions
The present invention relates to oral care compositions comprising stannous ions, a mint-type flavor oil, a protectant component that prevents generation of off odor and off taste in the composition, and orally acceptable carriers. The mint-type oils include peppermint, spearmint and corn mint. Suitable protectant components include copper salts and carbonyl compounds such as ascorbic acid; cis-jasmone; 2,5-dimethyl-4-hydroxy-3(2H)-furanone; 5-ethyl-3-hydroxy-4-methyl-2(5H)-furanone; vanillin; ethyl vanillin; anisaldehyde; 3,4-methylenedioxybenzaldehyde; 3,4-dimethoxybenzaldehyde; 4-hydroxybenzaldehyde; 2-methyoxybenzaldehyde; 4-methoxybenzaldehyde; benzaldehyde; cinnamaldehyde (3-phenyl-2-propenal); hexyl cinnamaldehyde; α-methyl cinnamaldehyde; ortho-methoxy cinnamaldehyde; α-amyl cinnamaldehyde; and combinations thereof. The oral care composition may be a dentifrice.
Owner:PROCTER & GAMBLE CO
Flavors for oral compositions
ActiveUS20080008665A1Avoid generatingPrevents of off tasteBiocideCosmetic preparationsBenzaldehydePeppermints
The present invention relates to oral care compositions comprising stannous ions, a mint-type flavor oil, a protectant component that prevents generation of off odor and off taste in the composition, and orally acceptable carriers. The mint-type oils include peppermint, spearmint and corn mint. Suitable protectant components include copper salts and carbonyl compounds such as ascorbic acid; cis-jasmone; 2,5-dimethyl-4-hydroxy-3(2H)-furanone; 5-ethyl-3-hydroxy-4-methyl-2(5H)-furanone; vanillin; ethyl vanillin; anisaldehyde; 3,4-methylenedioxybenzaldehyde; 3,4-dimethoxybenzaldehyde; 4-hydroxybenzaldehyde; 2-methyoxybenzaldehyde; 4-methoxybenzaldehyde; benzaldehyde; cinnamaldehyde (3-phenyl-2-propenal); hexyl cinnamaldehyde; α-methyl cinnamaldehyde; ortho-methoxy cinnamaldehyde; α-amyl cinnamaldehyde; and combinations thereof. The oral care composition may be a dentifrice.
Owner:THE PROCTER & GAMBLE COMPANY
Self-inflaming-retarding epoxy resin curing agent containing phosphorus-nitrogen and preparation method thereof
InactiveCN104693421AGood compatibilityEasy to prepareGroup 5/15 element organic compoundsEpoxyBenzaldehyde
The invention discloses a self-inflaming-retarding epoxy resin curing agent containing phosphorus-nitrogen and a preparation method thereof and relates to epoxy resin curing agents. The method comprises the steps that N-phenyl-1, 4-phenylenediamine and 4-hydroxy benzaldehyde are added into a container provided with a condensation pipe and a stirrer for reacting with first solvent, the reaction system is filtered, and solids obtained after the filtering are washed and dried, so that an intermediate body is obtained; the intermediate body, DOPO and second solvent are added into a container provided with a condensation pipe and a stirrer for reacting, the reaction system is filtered, and solids obtained after the filtering are washed and dried, so that the self-inflaming-retarding epoxy resin curing agent containing the phosphorus-nitrogen is obtained; or alternatively, the N-phenyl-1, the 4-phenylenediamine, the 4-hydroxy benzaldehyde and the DOPO are added into a container provided with a condensation pipe and a stirrer sequentially for reacting with solvent, the reaction system is filtered, and articles obtained after the filtering are washed and dried, so that the self-inflaming-retarding epoxy resin curing agent containing the phosphorus-nitrogen is obtained. The curing agent contains two active imino groups, one active hydroxyl group and P and N synergistic flame retardant elements simultaneously and can be used for epoxy resin curing and inflaming retardation.
Owner:XIAMEN UNIV
Deodorization composition and application thereof
ActiveCN106620780AChemically stableMeet the requirements of green environmental protectionCosmetic preparationsToilet preparationsAntibacterial agentParaformaldehyde
The invention discloses a deodorization composition and application thereof. The deodorization composition contains an amino acid complex of (9Z,12R)-12-hydroxy-octadecanoic acid zinc salt and / or copper salt, paraformaldehyde and / or 3-methoxy-4-hydroxybenzaldehyde, a stabilizer, a penetrant, an antibacterial agent and an antistatic agent, wherein the weight ratio of the components is 1:(0.1-4.0):(0.05-0.5):(0.05-0.5):(0.001-0.2):(0.001-0.2). A method for removing foul odor, provided by the invention, comprises contacting an aqueous solution containing the deodorization composition with the foul odor. The deodorization composition disclosed by the invention can meet green and environment-friendly requirements, and can be applicable to elimination of off-odors of ammonium, hydrogen sulfide, methyl mercaptan, dimethyl sulfide and the like. The deodorization composition disclosed by the invention can effectively relieve the problem of pollution of the off-odors to the environment, has an excellent deodorization performance, is small in dosage, is simple in treatment process and obvious in effect, and is easy to promote and apply.
Owner:深圳市爱康泉水处理服务有限公司
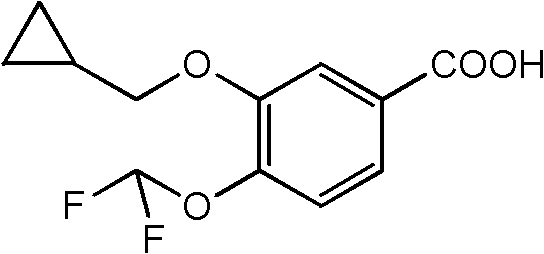
Preparation method of 3-cyclopropylmethoxy-4-difluoromethoxy-benzoic acid
InactiveCN102690194AAvoid pollutionHigh yieldOrganic compound preparationCarboxylic preparation by oxidationBenzoic acidBenzaldehyde
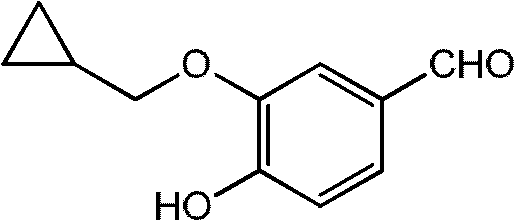
The invention provides a preparation method of 3-cyclopropylmethoxy-4-difluoromethoxy-benzoic acid shown in the formula I. The preparation method comprises the following step that 3-halogeno-4-hydroxybenzaldehyde shown in the formula II and cyclopropylmethanol undergo a reaction in the presence of one or more alkalis to produce a compound shown in the formula III; the compound shown in the formula III and chlorodifluoroacetic acid or its derivative shown in the formula IV undergo a reaction in the presence of one or more alkalis to produce 3-cyclopropylmethoxy-4-difluoromethoxy-benzaldehyde; and the 3-cyclopropylmethoxy-4-difluoromethoxy-benzaldehyde further undergoes an oxidation reaction to produce 3-cyclopropylmethoxy-4-difluoromethoxy-benzoic acid shown in the formula I. The preparation method has good reaction selectivity and a high reaction yield, adopts reagents having low costs, allows mild reaction conditions, and has safe and controllable processes. In the whole reaction process, highly toxic reagents and solvents are not used and thus the preparation method satisfies environmental protection requirements and is very suitable for industrial production.
Owner:SHANGHAI TONGYUAN BIOTECH
Method for manufacturing novel dendritic fluorescent chemosensor and application
InactiveCN102020985ASensing shortcutSensitiveOrganic chemistryChemiluminescene/bioluminescenceEthylenediamineFluorescence
The invention discloses a method for manufacturing a dendritic fluorescent chemosensor and the method for detecting Fe<3+> in a sample. The method for manufacturing the dendritic fluorescent chemosensor is mainly technically characterized by comprising the following steps of: performing reaction on aminoacetaldehyde dimethyl acetal, methyl acrylate and ethylenediamine in a methanol medium under the protection of argon at the temperature of 55 to 65 DEG C for 24 to 30 h to obtain a dendritic compound; and reacting in darkness the dendritic compound with rhodamine B hydrazide in the methanol medium under the protection of the argon at room temperature for 48 to 58 h by taking 4-hydroxybenzaldehyde as a crosslinker, and performing filtration and washing to obtain the dendritic fluorescent chemosensor. In the detection method, the mixed solution of ethanol and water serves as the medium, and the Fe<3+> content of various samples is determined by utilizing a fluorophotometer in Tris-HCl buffer solution with the pH value of 6.8 to 7.5. The dendritic fluorescent chemosensor has the linear range of 0 to 10.0 mu g.mL<-1>, the detection limit of 0.026 mu g.mL<-1>, and sensitivity and selectivity which are valuable in analytical chemistry.
Owner:UNIV OF JINAN
Halogen-free active fire retardant and preparation method thereof
ActiveCN104710651AImprove flame retardant performanceEasy to prepareGroup 5/15 element organic compoundsNitrogenMagnetic stirrer
The invention relates to fire retardants, and in particular relates to a halogen-free active fire retardant and a preparation method thereof. The method comprises the following steps: under the protection of nitrogen, sequentially adding reactants, namely N-hydroxyethylaniline, 4-hydroxybenzaldehyde and a solvent, into a container equipped with a condensate return pipe and a magnetic stirrer; after a heating reaction, adding DOPO to continue the reaction to obtain a light blue solid deposit; and cooling the deposit, keeping the deposit standing, pouring out the upper-layer solvent, washing the deposit with a solvent, and drying the deposit to obtain the halogen-free active fire retardant. Or the method comprises the following steps: under the protection of nitrogen, sequentially adding the reactants, namely N-hydroxyethylaniline, 4-hydroxybenzaldehyde and a solvent, into a container equipped with a condensate return pipe and a magnetic stirrer; after a heating reaction, adding DOPO to continue the reaction to obtain a light blue solution; and concentrating the solution, and precipitating the solution with a precipitator to obtain the halogen-free active fire retardant. The preparation method is simple, and the halogen-free active fire retardant contains a plurality of kinds of active groups, is well compatible with a matrix and has a good halogen-free flame-retardant property.
Owner:XIAMEN UNIV +1
Method for preparing antioxidant
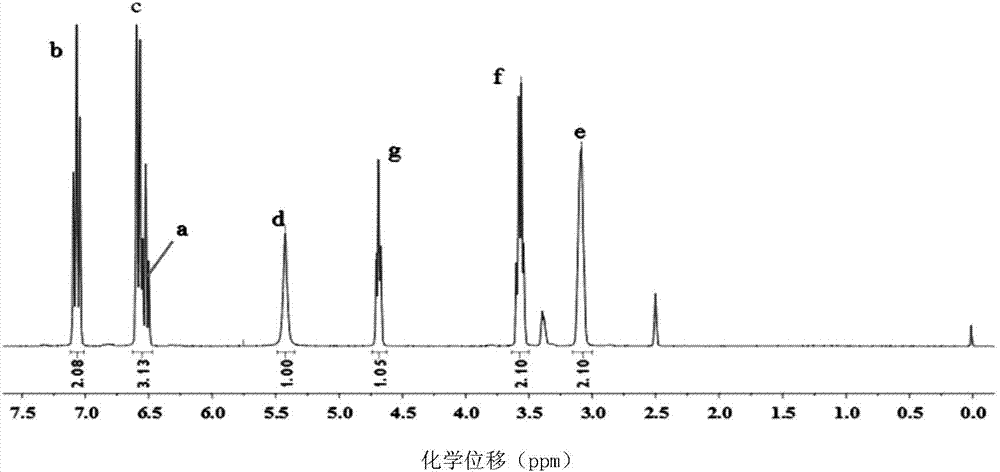
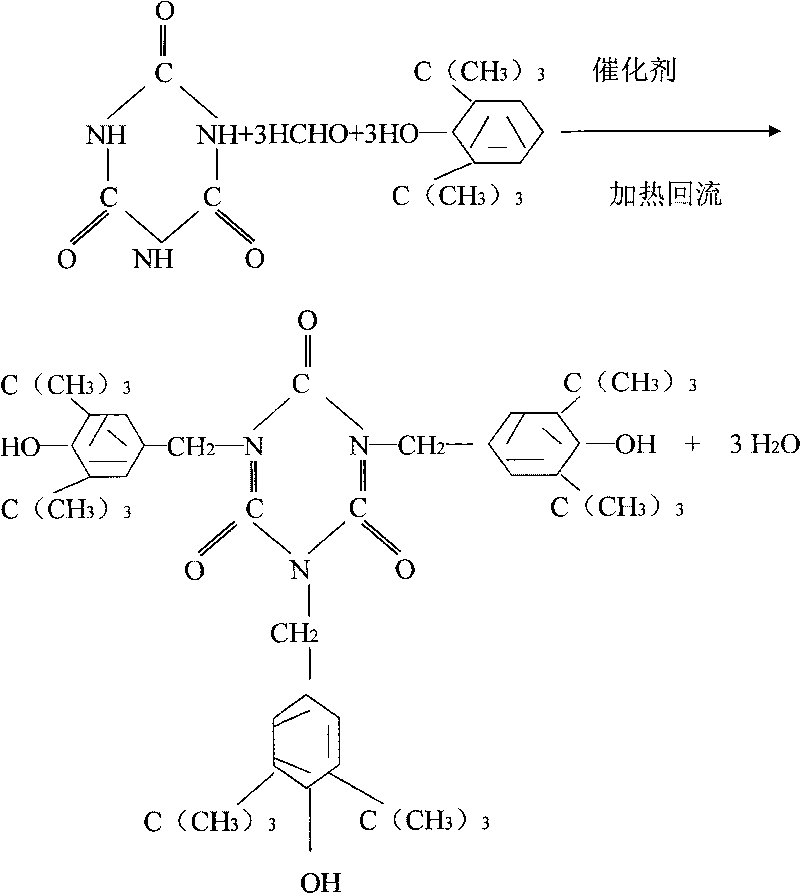
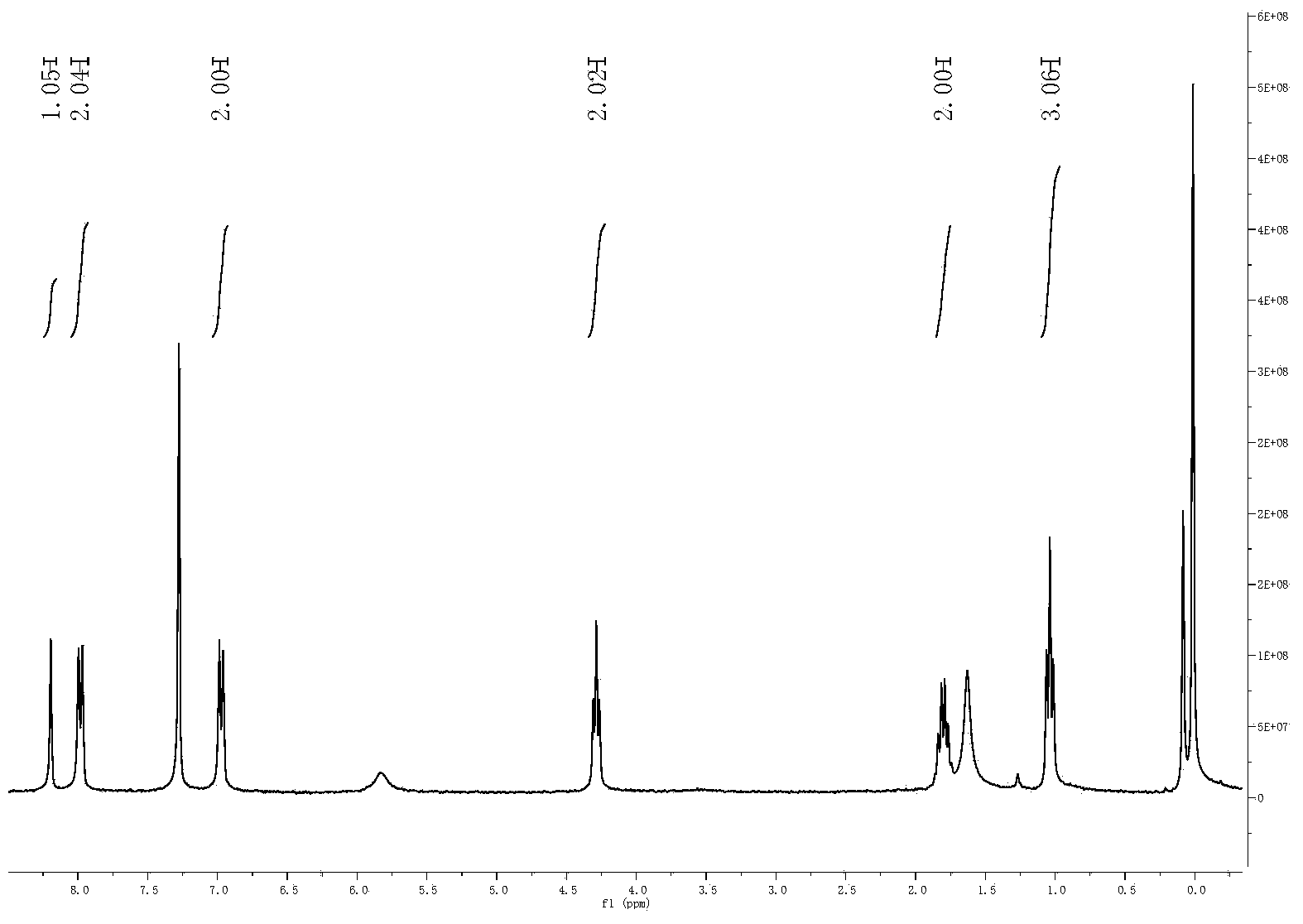
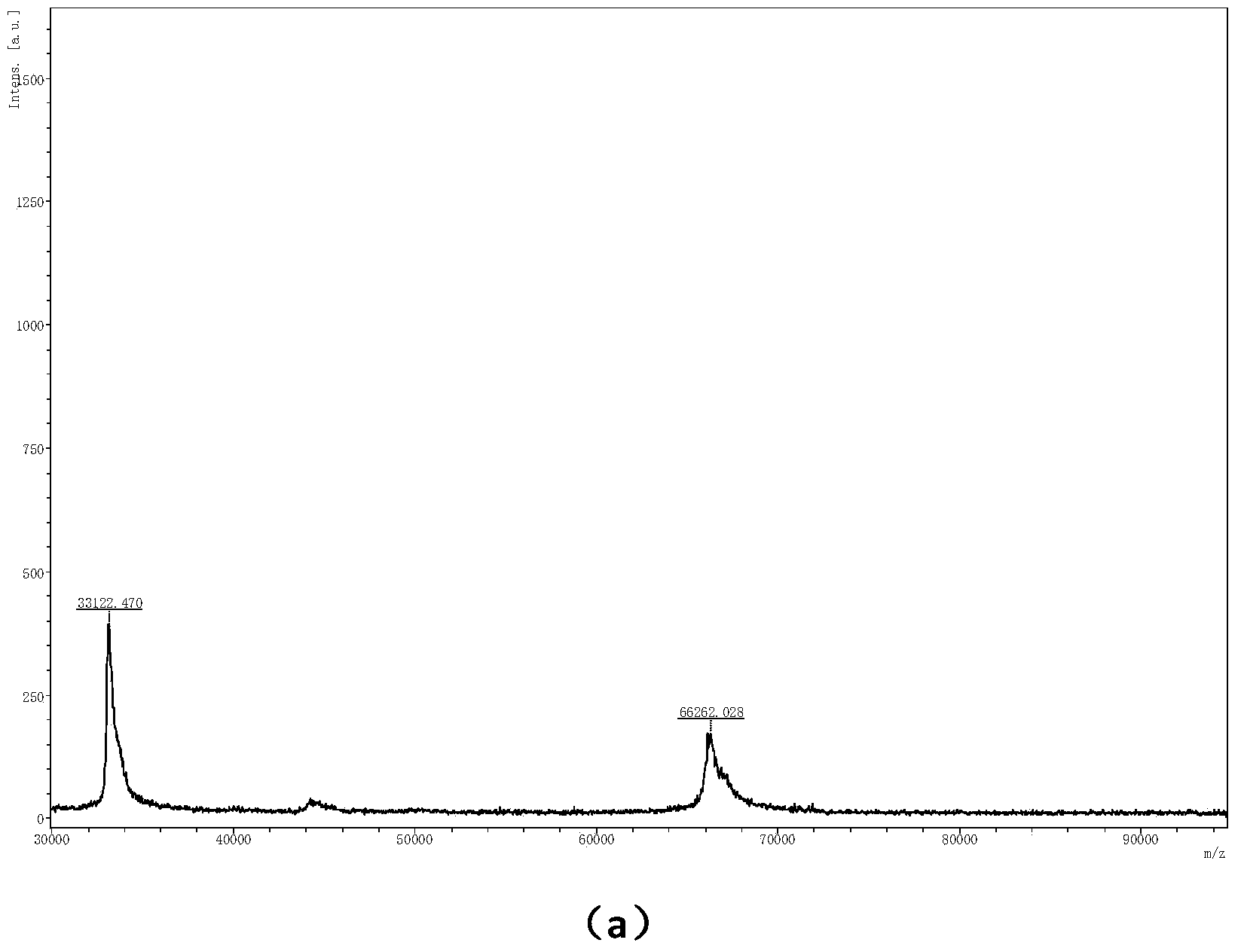
A method for preparing 1.3.5-tri(3.5-di tert butyl-4-hydroxybenzaldehyde)1.3.5-s-triazine.2.4.6-(1H, 2H, 3H) triketone is characterized by adding isopropanol and distilled water into a reactor, adding cyanuric acid and paraformaldehyde under stirring, adding catalysts including triethylamine and dibutylamine after uniform dispersion, after uniform stirring, raising the temperature to 60-80 DEG C under the protection of nitrogen, after adding 2.6-di tert butyl phenol, continuing raising the temperature until the reflux state, naturally cooling after reacting for 10-12h, carrying out pumping filtration after cooling to 10 DEG C, drying to obtain the crude product and obtaining the product through recrystallization and refining. The method has the following advantages and benefits: 1. the production technology process is more reasonable and convenient and the production efficiency is improved; 2. the pollution to the environment is lightened and the harm to the production workers is reduced; and 3. the yield of the product is improved and the total yield is over 90%.
Owner:天津市北方金恒新材料有限责任公司
Alpha-cyano-group-4-hydroxycinnamic acid normal propyl ester, preparation method and application
ActiveCN103483223AThe test effect is goodReduce processing requirementsCarboxylic acid nitrile preparationOrganic compound preparationLaser desorption ionization mass spectrometryBenzaldehyde
The invention discloses an alpha-cyano-group-4-hydroxycinnamic acid normal propyl ester, a preparation method and an application, and belongs to the technical field of MALDI mass spectra. The preparation method comprises the steps of adding piperidine into a reaction solvent by stirring at the room temperature, then adding cyano propyl acetate and 4-hydroxy benzaldehyde, after the drugs are dissolved, adding methylbenzene, raising the temperature, stopping the reaction after the reflux and water removal, after cooling to the room temperature, conducting rotary evaporation to obtain a concentrated solution containing the alpha-cyano-group-4-hydroxycinnamic acid normal propyl ester, purifying the concentrated solution, pouring the obtained concentrated solution into a large amount of deionized water, immediately separating out solid, conducting suction filtration to obtain a filter cake, and then conducting recrystallization on the filter cake 1-5 times by means of ethyl alcohol to obtain a yellowish product, namely the alpha-cyano-group-4-hydroxycinnamic acid normal propyl ester. By the adoption of a mass spectrometry to detect various samples, and compared with a traditional base material (alpha-cyano-group-4-hydroxycinnamic acid), the alpha-cyano-group-4-hydroxycinnamic acid normal propyl ester has good sensitivity, good detection limit and a better test effect.
Owner:JILIN UNIV
Synthesis of 7-acetyleno quinone methide derivatives and their application as vinylic polymerization retarders
ActiveUS20120313036A1Organic compounds purification/separation/stabilisationOrganic compound preparationReaction temperatureTert butyl
The invention provides a method for synthesizing 7-Acetyleno quinone methide compounds that is safe and inexpensive. The method avoids the need for extremely cold reaction temperatures and unlike the prior art does not require any highly explosive materials. The method comprises the steps ofa) performing a condensation reaction between 3,5-di-tert-butyl-4-hydroxybenzaldehyde and a secondary amine thereby forming a secondary amine quinone methide intermediate;b) removing water from the secondary amine quinone methide intermediate by azeotropic distillation;c) adding the dehydrated secondary amine quinone methide intermediate to an organic medium containing a metal acetylide to form a Mannich base intermediate; andd) adding a release agent to the Mannich base intermediate to yield a 7-Acetyleno quinone methide.
Owner:NALCO CO
Method for preparing hexa(4-carboxylphenoxy)cyclotriphosphazene by hydrogen peroxide oxidation
InactiveCN103467525AImprove oxidation capacityMild reaction conditionsGroup 5/15 element organic compoundsDistillationReaction temperature
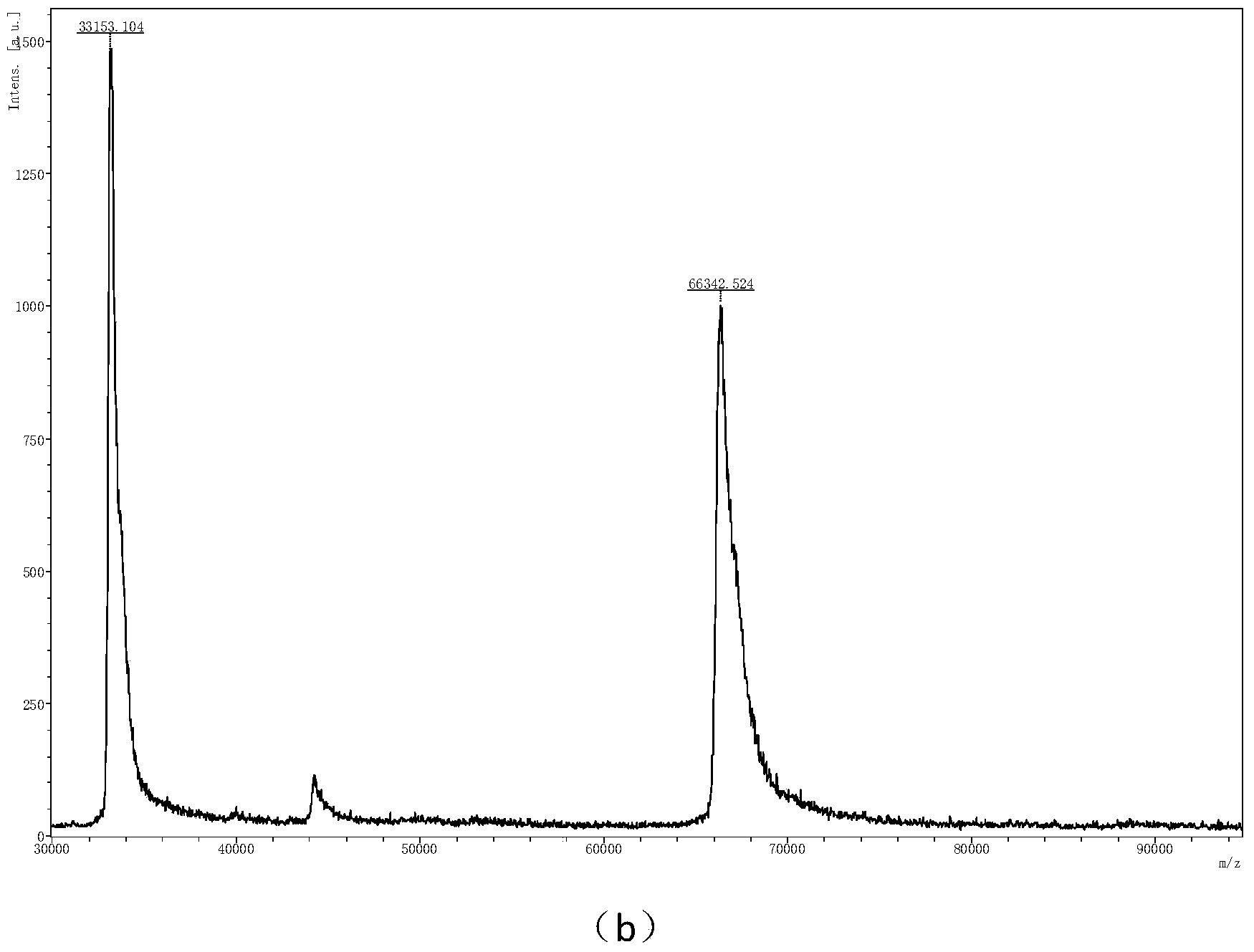
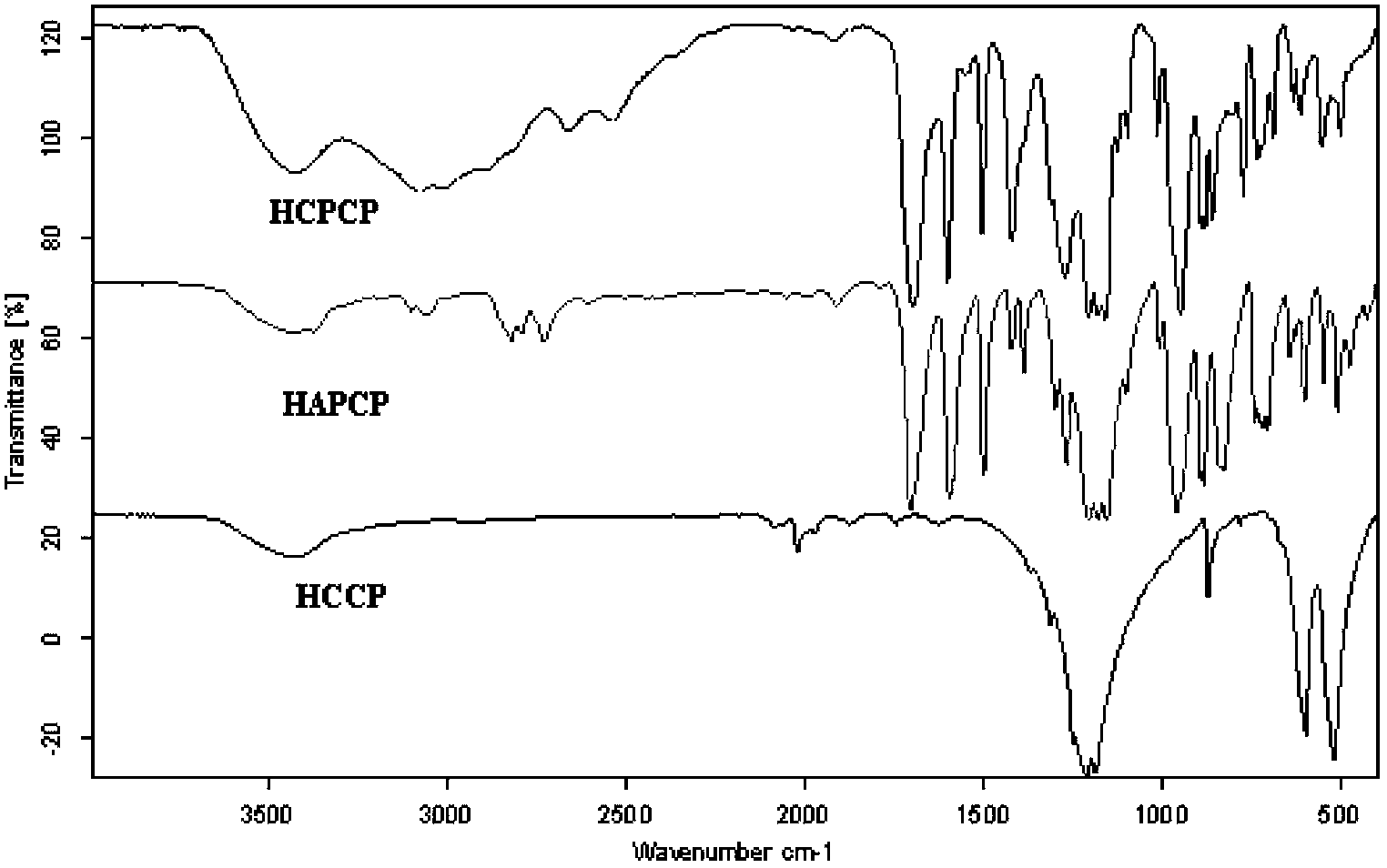
The invention relates to a method for preparing hexa(4-carboxylphenoxy)cyclotriphosphazene by hydrogen peroxide oxidation. The method comprises: taking hexachlorocyclotriphosphazene (HCCP) and 4-hydroxybenzaldehyde as raw materials and taking anhydrous tetrahydrofuran as a solvent, adding an acid binding agent, performing a nucleophilic substitution reaction to prepare hexa(4-formacylphenoxy) cyclotriphosphazene (HAPCP); and performing filtering, underpressure distillation and recrystallization purification, then under an alkaline condition, oxidizing hexa(4-formacylphenoxy) cyclotriphosphazene with hydrogen peroxide to obtain hexa(4-carboxylphenoxy)cyclotriphosphazene (HCPCP). The molar ratio of 4-hydroxybenzaldehyde, the acid binding agent and HCCP is (5-10):(5-8):1, the temperature of the nucleophilic substitution reaction is 50-80 DEG C and the reaction time is 10-30 h. Compared with the prior art, the method of the invention has the advantages of mild reaction condition, more thorough reaction, reduced reaction temperature, accelerated reaction speed and improved product yield and purity.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY +1
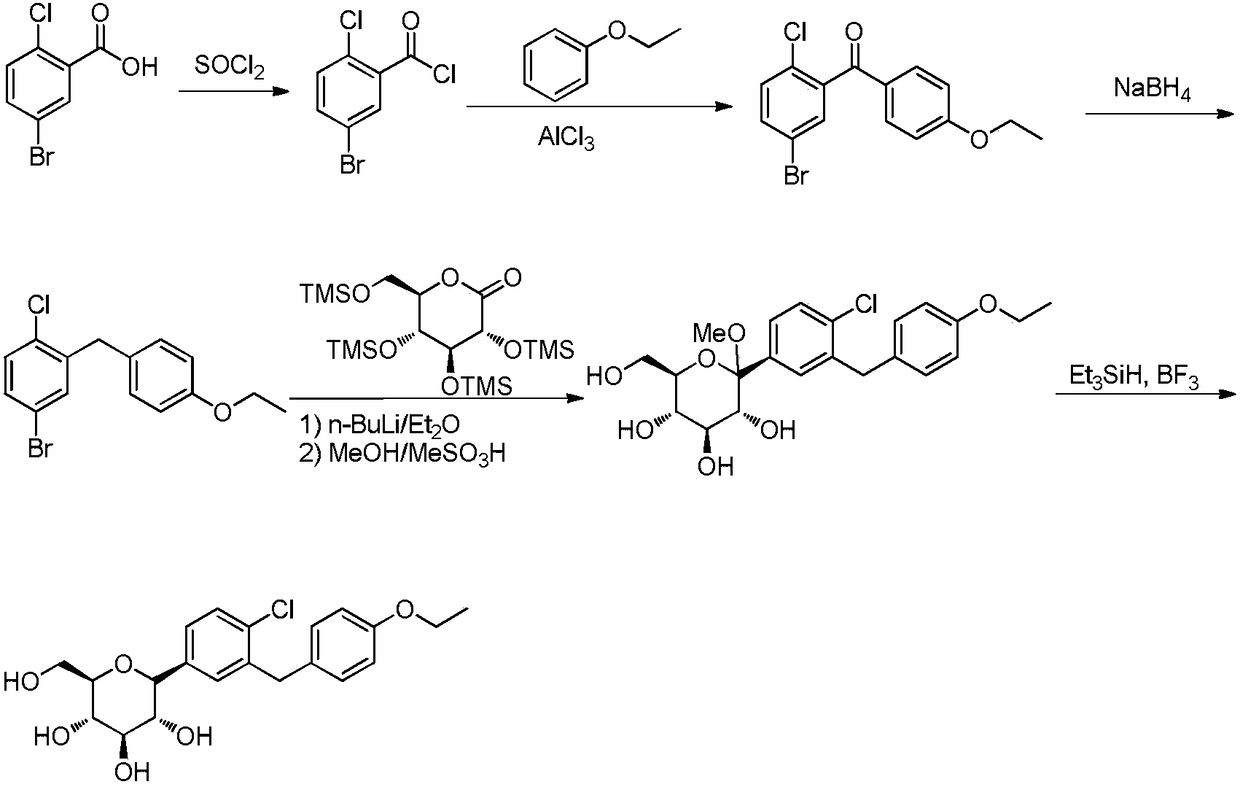
Preparation method for hypoglycemic drugdapagliflozin
ActiveCN108084130ACheap and easy to getHigh purityOrganic chemistryMetabolism disorderDiphenylmethaneAlkyl transfer
The invention discloses a preparation method for hypoglycemic drugdapagliflozin. The method comprises the steps that 4-hydroxybenzaldehyde is adopted as a starting raw material, alkylation, carbonyl reduction, chlorination and alkylation reaction with asepsin, diazotization and chlorination are performed to obtain a dapagliflozinmidbody 5-bromo-2-chloro-4'-ethyoxyl diphenylmethane, then, the midbody and 2,3,4,6-tetra-O-trimethyl silicone-D-glucolactone are subjected to condensation, etherification and methoxyl removal to obtain the hypoglycemic drugdapagliflozin. The raw materials adopted by the technological path are low in price and easy to obtain, the technology can achieve industrialization easily, and the final product is high in purity; the technological path is novel, the syntheticroute is short, no risky or complex technology exists in the reactions, equipment is simple, operation is easy and convenient, and the method is suitable for industrial production.
Owner:SOUTHEAST UNIV
Method for synthesizing mitochondria targeted spinning scavenger MitoPBNs (spinning probe)
InactiveCN101906118AHigh purityRealize early warningGroup 5/15 element organic compoundsBiological testingScavengerStructural formula
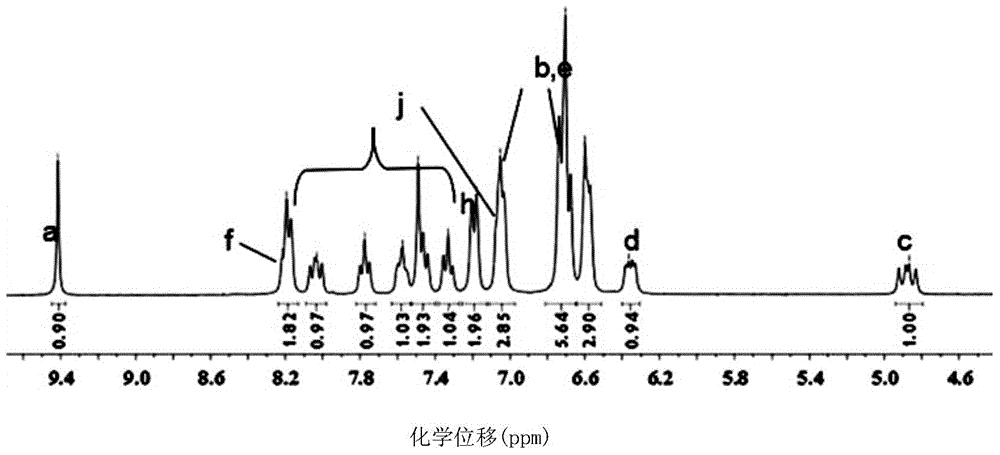
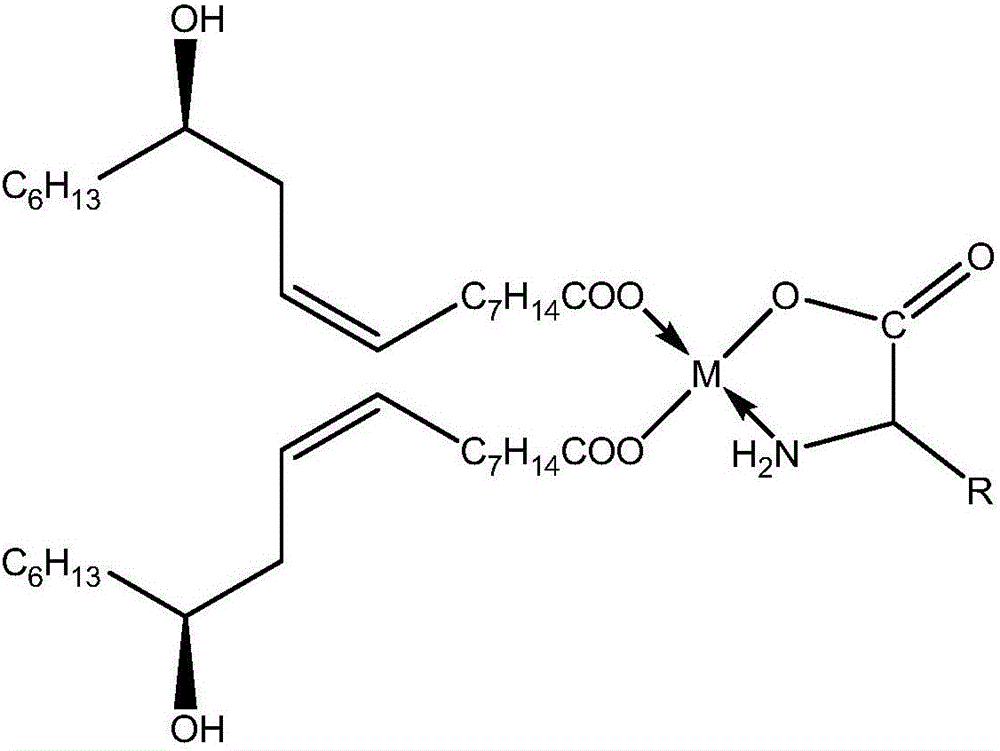
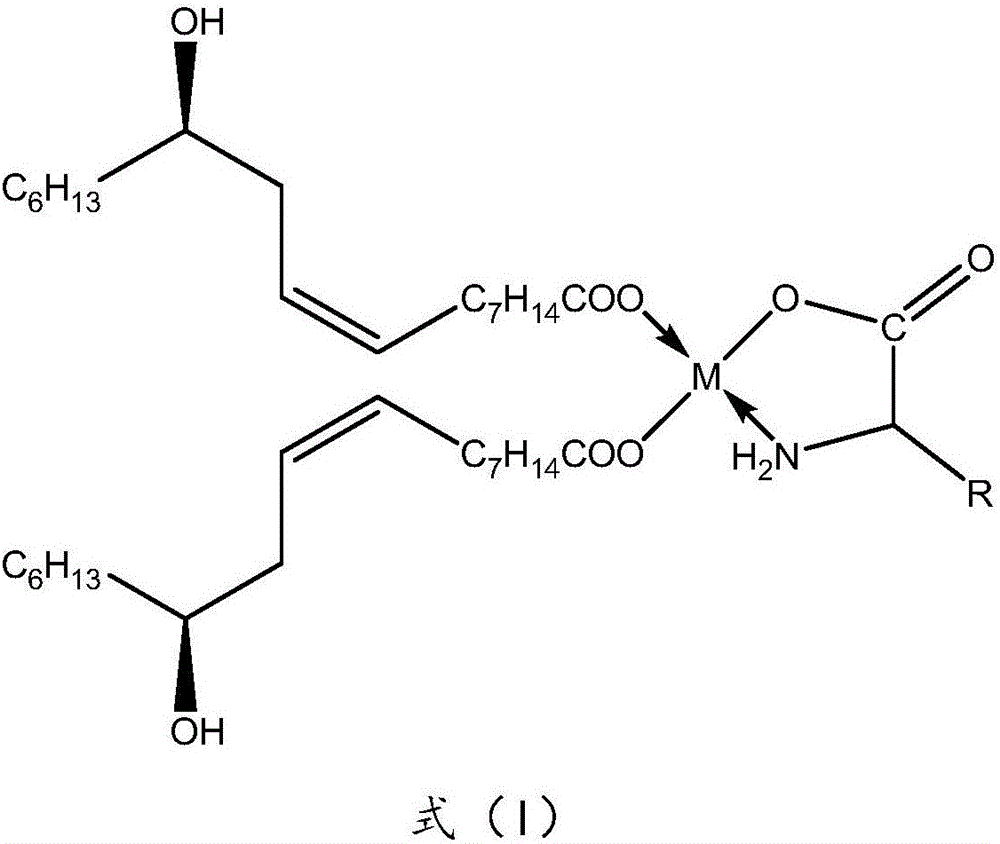
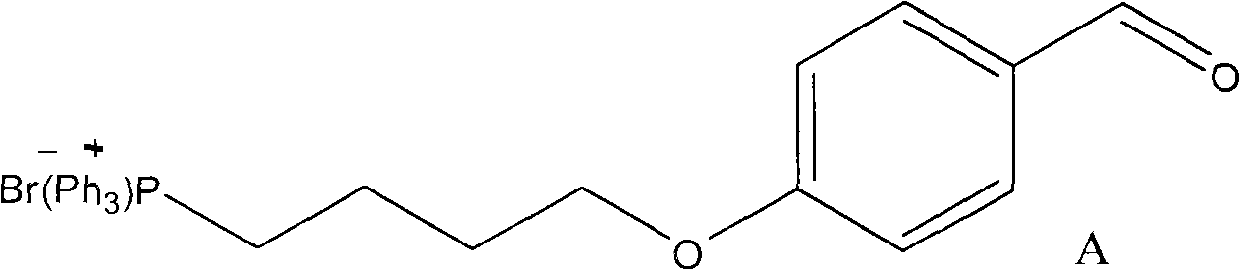
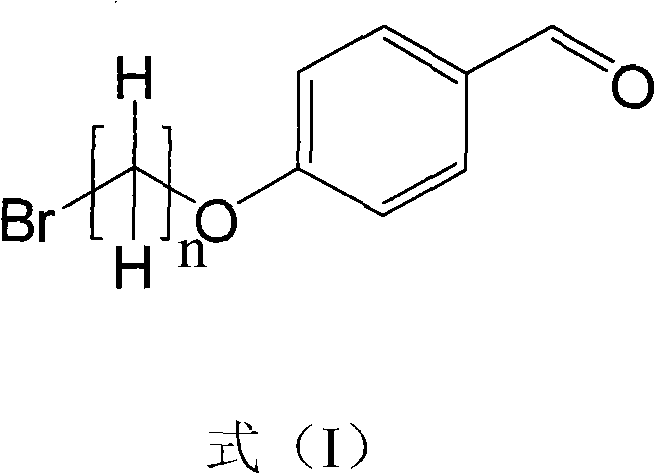
The invention discloses a method for preparing a mitochondria targeted spinning scavenger MitoPBNs (spinning probe). The structural formula of the MitoPBN is shown in a formula (I). A synthesis flow is shown in the formula (II), and comprises the following steps of: performing elimination reaction on 4-hydroxybenzaldehyde and dibromo-straight-chain paraffin under alkali condition to prepare a compound 3; reacting the compound 3 with triphenylphosphine to prepare the compound 4; in ethanol, mixing the compound 4, 2-nitro-2-methylpropane, a 4A molecular sieve and zinc powder in a solvent, adding dropwise a glacial acetic acid, stirring and reacting the mixed solution at room temperature, placing the mixed solution in a refrigerator for refrigeration, and performing separation and purification to prepare the spinning probe MitoPBN; and performing liposome packing treatment. The method has the characteristics of relatively fewer synthesis steps, low raw material cost, readily available raw materials and relatively higher product purity. The probe for scavenging free radical signals can enter cell mitochondria.
Owner:刘珊林
Synthesis of 7-acetyleno quinone methide derivatives and their application as vinylic polymerization retarders
ActiveUS8884038B2Organic compounds purification/separation/stabilisationOrganic compound preparationReaction temperatureTert butyl
The invention provides a method for synthesizing 7-Acetyleno quinone methide compounds that is safe and inexpensive. The method avoids the need for extremely cold reaction temperatures and unlike the prior art does not require any highly explosive materials. The method comprises the steps of:a) performing a condensation reaction between 3,5-di-tert-butyl-4-hydroxybenzaldehyde and a secondary amine thereby forming a secondary amine quinone methide intermediate;b) removing water from the secondary amine quinone methide intermediate by azeotropic distillation;c) adding the dehydrated secondary amine quinone methide intermediate to an organic medium containing a metal acetylide to form a Mannich base intermediate; andd) adding a release agent to the Mannich base intermediate to yield a 7-Acetyleno quinone methide.
Owner:NALCO CO
Synthetic method for methoxy-cephalosporin intermediate 7-MAC
InactiveCN102850379ASolve the difficult problem of storing diphenyldiazomethaneEasy to operateOrganic chemistryTetrazoleTert butyl
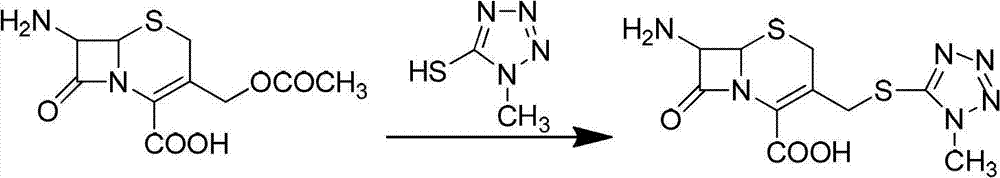
The invention provides a synthetic method for methoxy-cephalosporin intermediate 7-MAC. According to the invention, 7-ACA is used as a raw material to react with 1-methyl-5-mercapto-1, 2, 3, 4-tetrazole to obtain intermediate I; the intermediate I reacts with newly-prepared diphenyldiazomethane to produce intermediate II; the intermediate II reacts with 3, 5-di-tert-butyl-4-hydroxylbenzaldehyde to produce intermediate III; and the intermediate III reacts with a compound oxidizer and methanol to produce intermediate IV, and the intermediate IV reacts with a Girard-T reagent to obtain 7-MAC, wherein overall yield is 61% to 65%, and liquid phase purity reaches 99.20%. The synthetic method provided by the invention has the advantages of easily available raw materials, advanced technology and easy industrial production and is an improved synthetic method for 7-MAC.
Owner:湖北伟德合创新材料科技有限公司
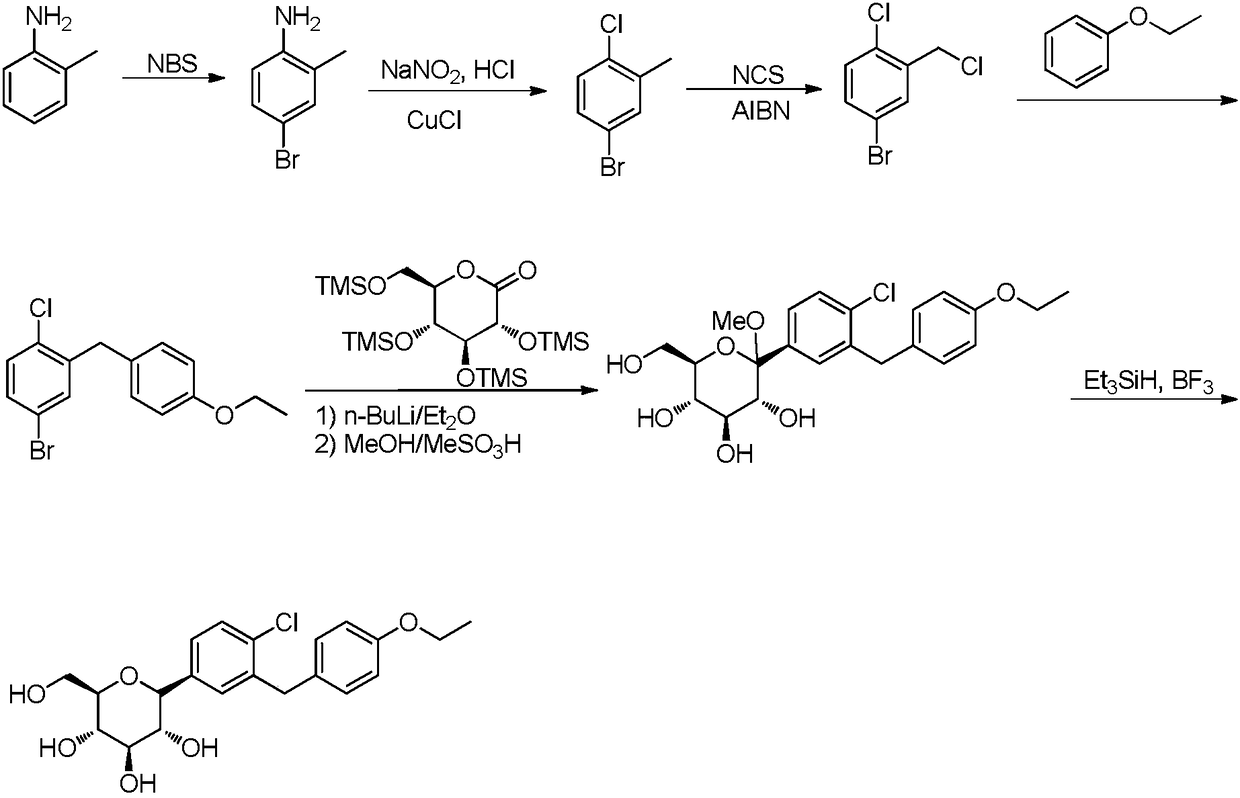
Preparation method of 5-bromine-2-chlorine-4'-ethyoxyl diphenylmethane
ActiveCN107311847AShort synthetic routeHigh purityOrganic compound preparationPreparation by hydrocarbon ammoxidationDiphenylmethaneBenzaldehyde
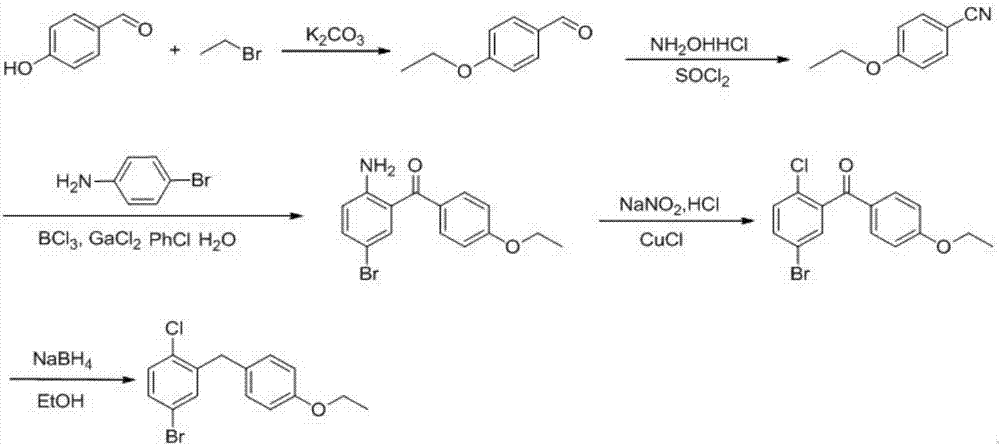
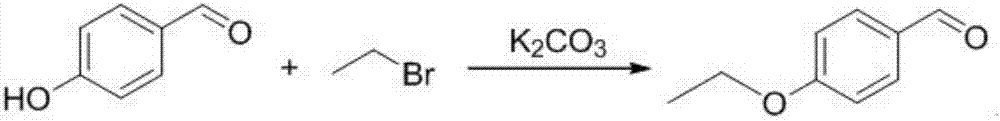
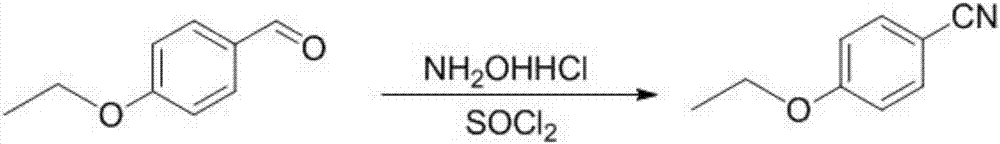
The invention provides a preparation method of 5-bromine-2-chlorine-4'-ethyoxyl diphenylmethane. The preparation method comprises the following steps: (1) using 4-hydroxybenzaldehyde and bromoethane as raw materials, a polar solvent as a reaction solvent and inorganic base as a catalyst to react so as to prepare 4-ethoxy-benzaldehyde; (2) using hydroxylamine hydrochloride and 4-hydroxybenzaldehyde as raw materials and a hydroxyl-containing solvent as a reaction solvent to react so as to obtain oxime, and then reacting under the effect of a dehydrating agent so as to generate 4-ethoxyl benzonitrile; (3) using 4-bromaniline and 4-ethoxyl benzonitrile as raw materials, using lewis acid as a catalyst, and performing a Hoesch reaction to generate 5-bromine-2-amino-4'-ethyoxyl benzophenone; (4) performing a diazo-reaction on 5-bromine-2-amino-4'-ethyoxyl benzophenone, and then reacting with cuprous chloride to synthesize 5-bromine-2-chlorine-4'-ethyoxyl benzophenone; and (5) performing a reduction reaction on 5-bromine-2-chlorine-4'-ethyoxyl benzophenone to obtain 5-bromine-2-chlorine-4'-ethyoxyl diphenylmethane. The method is low in cost, low in environment stress and short in synthetic route.
Owner:安徽省诚联医药科技有限公司
Synthesis method of isoliquiritigenin
InactiveCN108440264AHigh yieldReduce generation riskGroup 4/14 element organic compoundsOrganic compound preparationOrganic synthesisSynthesis methods
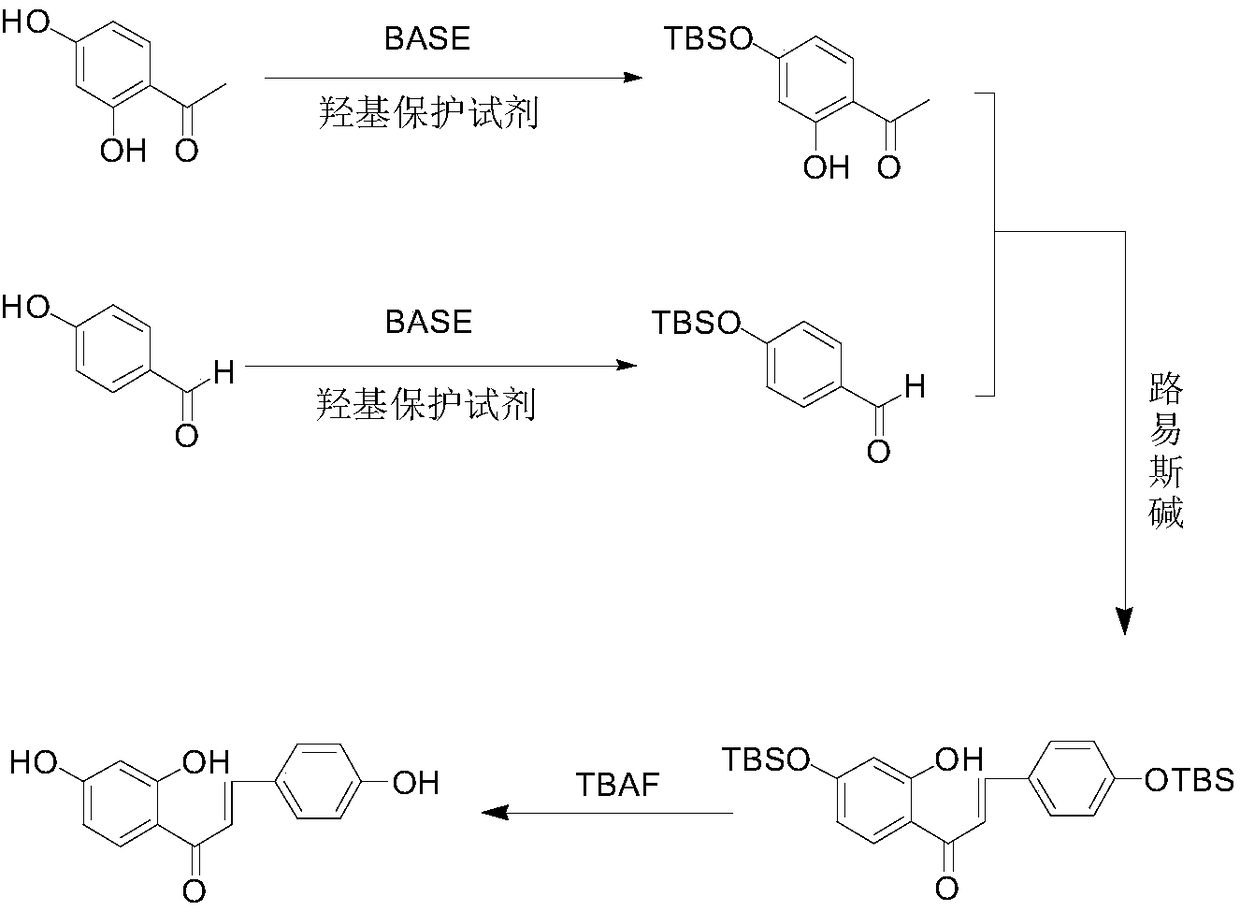
The invention relates to the technical field of organic synthesis, and particularly discloses a synthesis method of isoliquiritigenin. According to the synthesis method of isoliquiritigenin, 2,4-hydroxyacetophenone and 4-hydroxybenzaldehyde are used as raw materials, and the product namely isoliquiritigenin can be obtained through hydroxyl protection, aldol condensation and hydroxyl deprotection reaction. The synthesis method of isoliquiritigenin, provided by the invention, is simple and easy to implement, mild in reaction conditions, safe, reliable, high in raw material availability, low in price and low in cost, adopts at least one of dimethyl tert-butyl chlorosilane and triisopropyl chlorosilane to protect 4-hydroxyl groups of 4-hydroxybenzaldehyde and 2,4-dihydroxyacetophenone, reducesthe risk of byproduct generation, improves the yield of isoliquiritigenin, and is suitable for industrial production.
Owner:湖北凌晟药业股份有限公司
Compositions
ActiveUS20100284942A1Reduction of spore countExtended shelf lifeCosmetic preparationsBiocide3-HydroxybenzaldehydeBenzaldehyde
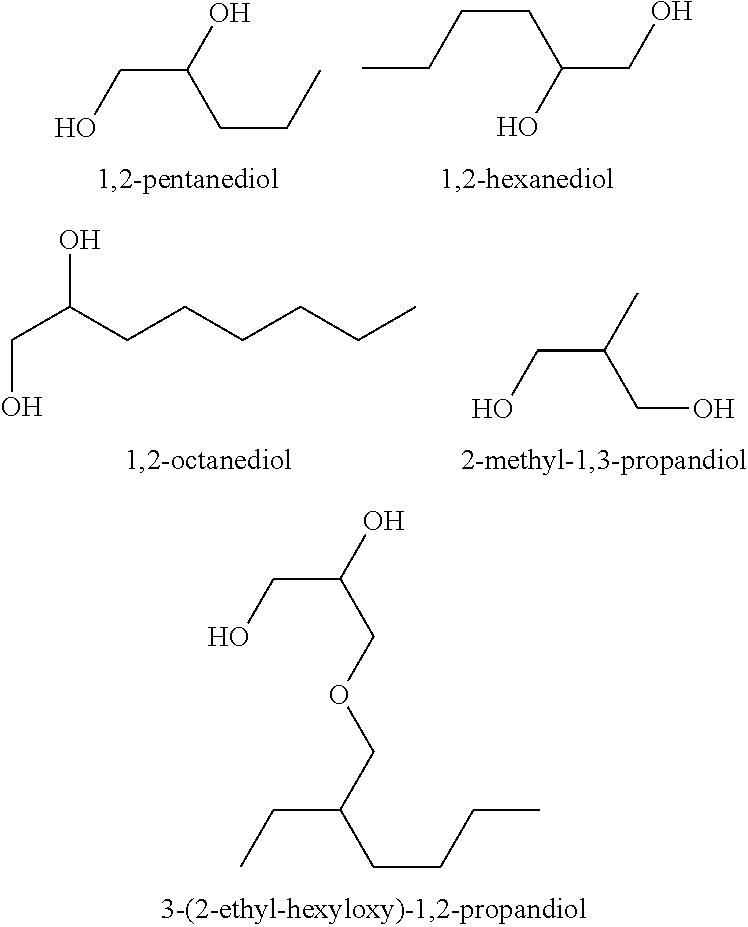
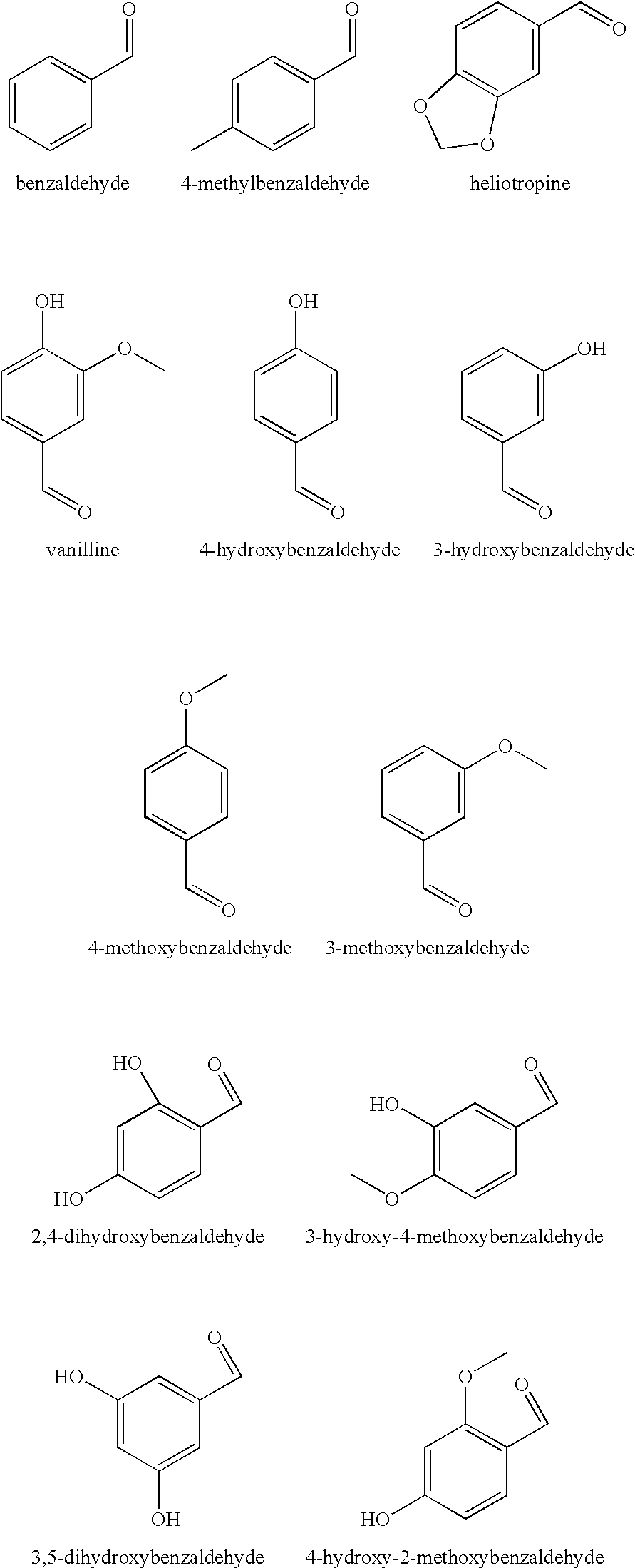
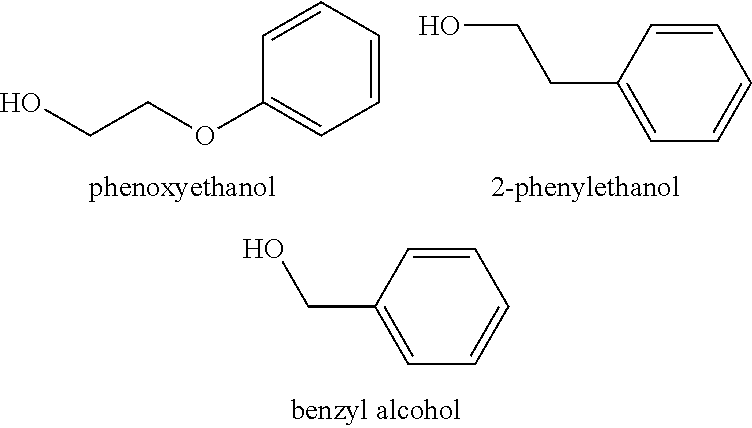
There are provided personal care products and compositions that comprise at least one diol compound selected from the group consisting of 1,2-pentanediol, 1,2-hexanediol, 1,2-octanediol, 2-methyl-1,3-propandiol, and 3-(2-ethyl-hexyloxy)-1,2-propandiol in a total concentration of 0.1% to 0.5% (w / w); and at least one preservative enhancer compound selected from the group consisting of benzaldehyde, 4-methylbenzaldehyde, heliotropine, vanilline, 4-hydroxybenzaldehyde, 3-hydroxybenzaldehyde, 4-methoxybenzaldehyde and 3-methoxybenzaldehyde, 2,4-dihydroxybenzaldehyde, 3-hydroxy-4-methoxybenzaldehyde, 3,5-dihydroxybenzaldehyde, and 4-hydroxy-2-methoxybenzaldehyde, in a total concentration of 0.05 to 0.5% (w / w), in a cosmetically acceptable base. The composition optionally contains at least one compound selected from the group consisting of phenoxyethanol, 2-phenylethanol, and benzylalcohol, in a total concentration of 0.05 to 0.3% (w / w), but does not contain other classic bactericidal, fungicidal, sporicidal or preservative compounds. The invention is further directed to methods of forming such compositions and products and the use of preservatives and preservative enhancers in such compositions and products.
Owner:GIVAUDAN SA
Application of Bacillus in degrading aromatic compounds
InactiveCN106698678ARich diversityImprove degradation efficiencyWater contaminantsWaste water treatment from textile industryBenzoic acidMicroorganism
The invention provides application of Bacillus in degrading aromatic compounds, belonging to the technical field of microbes. The experiment proves that the Bacillus L1 strain has the capacity for degrading 3,4-dihydroxybenzoic acid, 3-methoxy-4-hydroxybenzaldehyde, catechol and other environmental pollutants; the degradation rates for para-hydroxybenzoic acid, 3,4-dihydroxybenzoic acid, 2,5-dihydroxybenzoic acid, 3-methoxy-4-hydroxybenzoic acid, 3-methoxy-4-hydroxybenzaldehyde, catechol and benzoic acid with the concentration of 500 mg / L can respectively reach 90% or above; and thus, the Bacillus L1 strain has wide application prospects in the aspect of environmental treatment.
Owner:JIANGSU UNIV
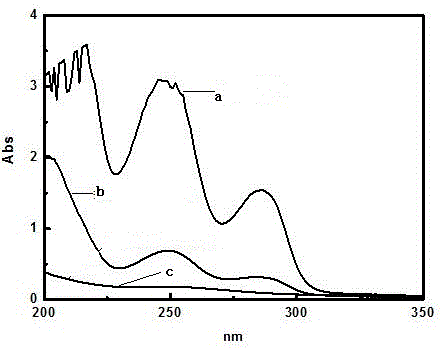
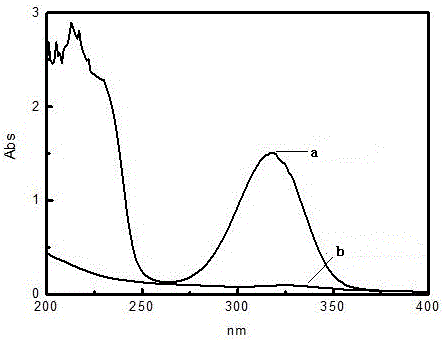
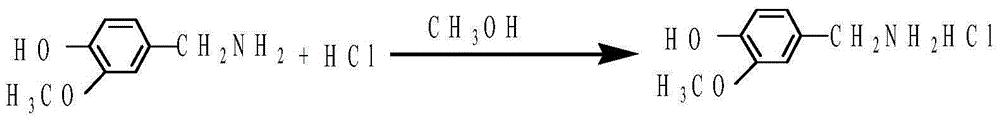
Preparation method of 3-methoxy-4-hydroxybenzylamine hydrochloride
InactiveCN105061231AHigh yieldLow costOrganic compound preparationAmino-hyroxy compound preparation4-hydroxybenzylamineReaction temperature
The invention provides a preparation method of 3-methoxy-4-hydroxybenzylamine hydrochloride. According to the method, 3-methoxy-4-hydroxybenzaldehyde reacts with liquid ammonia and hydrogen at the reaction temperature of 45-50 DEG C with a methanol solvent and a raney nickel catalyst, a product 3-methoxy-4-hydroxybenzylamine is obtained and dissolved in a methanol solvent, the mixture reacts with hydrogen chloride at the room temperature, and 3-methoxy-4-hydroxybenzylamine hydrochloride is obtained. Hydroxylamine hydrochloride in the traditional synthetic process of 3-methoxy-4-hydroxybenzylamine hydrochloride is replaced with the liquid ammonia, the product yield is increased, the adopted raw materials are easy to obtain, the methanol solvent can be recycled, and the raw material cost is reduced.
Owner:黄石市利福达医药化工有限公司
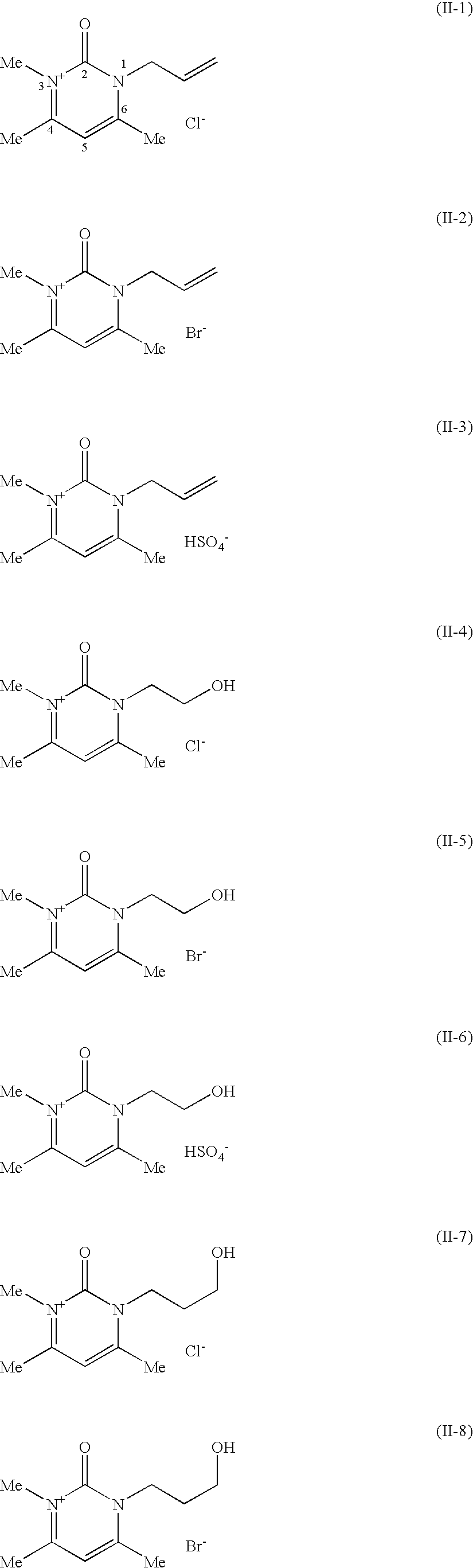
Compositions for treating keratinic fibers, methods of treating such fibers therewith and compounds contained therein
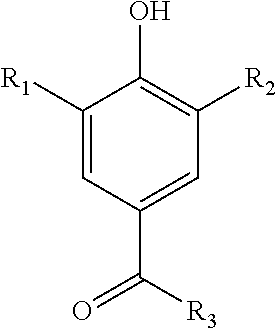
Compositions suitable for treating keratinic fibers, in particular human hair, are disclosed, which compositions comprise: (i) at least one component selected from the group consisting of salt compounds of general formula I, enamine counterparts of salt compounds of general formula I, and mixtures thereof:Where each Me represents a methyl group, R represents a substituent selected from the group consisting of an allyl group, a hydroxy-(C2-6)-alkyl group, a benzyl group, and substituted benzyl groups, and X− represents a physiologically compatible anion; and (ii) at least one aldehyde selected from the group consisting of 4-hydroxy-3-methoxybenzaldehyde, 3,5-dimethoxy-4-hydroxybenzaldehyde, 4-hydroxy-1-naphthaldehyde, 4-hydroxy-2-methoxybenzaldehyde, 3,4-dihydroxy-5-methoxybenzaldehyde, 3,4,5-trihydroxybenzaldehyde, 3,4-dibromo-4-hydroxybenzaldehyde, 4-hydroxy-3-nitrobenzaldehyde, 3-bromo-4-hydroxybenzaldehyde, 4-hydroxy-3-methylbenzaldehyde, 3,5-dimethyl-4-hydroxy-benzaldehyde, 5-bromo-4-hydroxy-3-methoxybenzaldehyde, 4-diethylamino-2-hydroxybenzaldehyde, 4-dimethylamino-2-methoxybenzaldehyde and mixtures thereof.
Owner:HENKEL KGAA
Preparation method for 4-hydroxybenzyl cyanide
The invention belongs to the field of organic chemistry synthesis, and specifically relates to the field of preparation of 4-hydroxybenzyl cyanide. A preparation method for 4-hydroxybenzyl cyanide comprises preparation of benzylalcohol and preparation of 4-hydroxybenzyl cyanide by employing 4-hydroxybenzaldehyde as a raw material and potassium borohydride as a reducing agent. The preparation method for 4-hydroxybenzyl cyanide is performed at normal temperature and normal pressure; the operation process is fast, simple and easy to control; the reaction time in the preparation process is shorter; and the yield of the finished product of 4-hydroxybenzyl cyanide is 95% or more.
Owner:TIANJIN KERMEL CHEM REAGENT
Compound as well as preparation method, preparation and application thereof
ActiveCN106831904ASignificant anticoagulant effectSimple manufacturing methodOrganic active ingredientsSugar derivativesDiseaseSchmidt reaction
The invention discloses a compound as well as a preparation method, a preparation and application thereof. The compound has the following structure shown in the specification, wherein X is hydrogen; R is other compound residues. The preparation method comprises the following steps: taking 2-chloro-4-hydroxybenzaldehyde or 2-bromo-4-hydroxybenzaldehyde as raw materials, performing Schmidt reaction with tetraacetylglucose trichloroacetimidate and then reacting with NaBH4 or DAST to obtain a target compound. The preparation is a tablet, a capsule, an injection or a freeze-dried powder injection prepared by adding pharmaceutically-acceptable excipients in the compound. The application is application of the compound to the preparation of an anticoagulant drug. The compound disclosed by the invention has an obvious anticoagulant effect and can be taken as a potential lead compound to be applied to the preparation of the drug for treating deep vein thrombus, pulmonary thromboembolism, cardiovascular and cerebrovascular diseases, stroke and other diseases related to blood coagulation; the preparation method is simple and easy to implement, good in repeatability and less in environmental pollution, and can be used for the preparation of a large amount of compounds.
Owner:KPC PHARM INC
Method for synthesizing phosphorus-containing silicon-containing organic inorganic internal-hybridized activated monomer and application
ActiveCN110498814AThe synthesis method is simplePrecise Control of CompositionGroup 5/15 element organic compoundsElemental compositionDouble bond
The invention discloses a method for synthesizing a phosphorus-containing silicon-containing organic or inorganic internal-hybridized activated monomer and an application. The method comprises the steps of preparing a phosphorus-containing silicon-containing phenol intermediate from 3-aminopropyl triethoxysilane, 4-hydroxybenzaldehyde and DOPO, which serve as raw materials, by utilizing a Kabachnik-Fields reaction, then, subjecting the phenol intermediate and methacryloyl chloride to a reaction in the presence of a solvent and an acid binding agent, and carrying out purification, thereby preparing the active double bond containing phosphorus-silicon organic or inorganic internal-hybridized activated monomer. The synthesized organic / inorganic internal-hybridized activated monomer is accurately controllable in elemental composition and can be applied to preparation of functional organic / inorganic hybridized polymers and flame-retardant, anticorrosive, heat-resistant and weather-resistantmodified materials.
Owner:XIAMEN UNIV
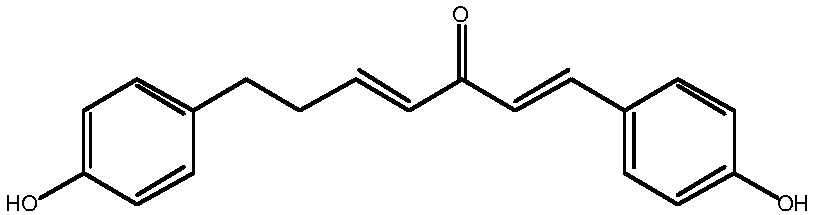
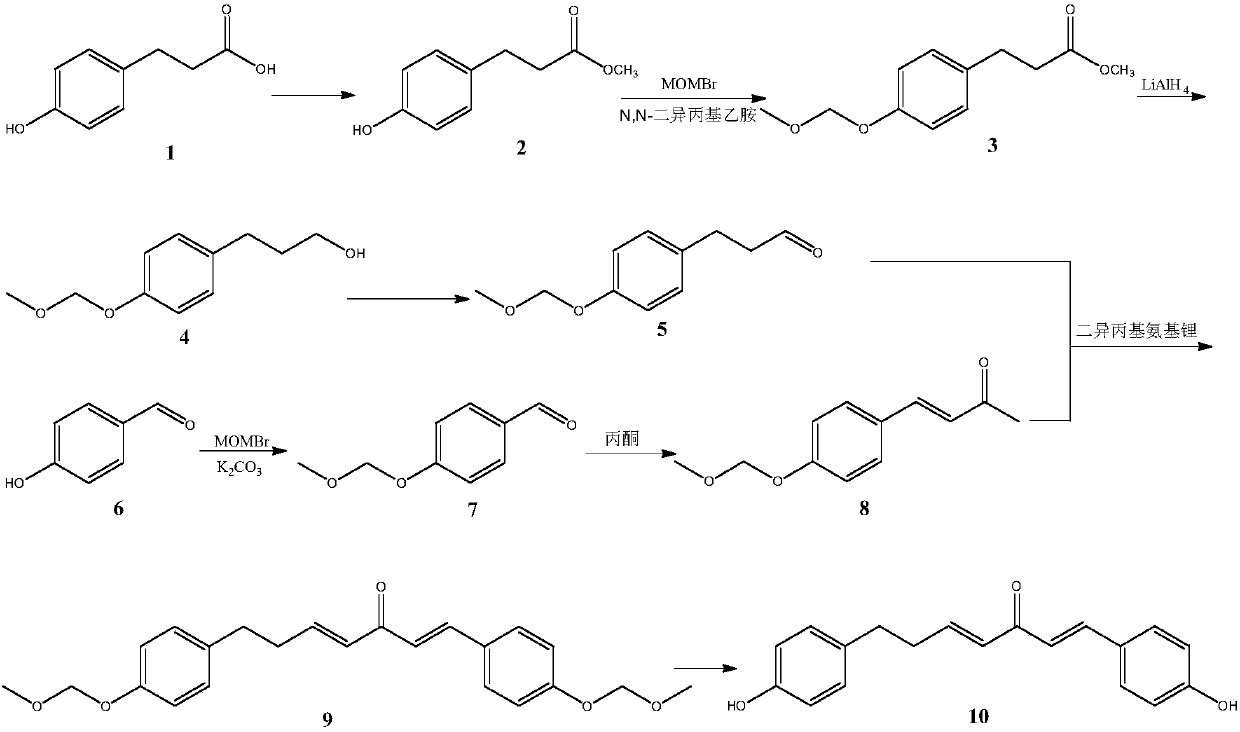
Method for synthesizing 1, 7-2-(4-hydroxy phenyl)-heptane-1, 4-diene-3-ketone
InactiveCN107721836ASimple methodEasy to operateOrganic compound preparationCarboxylic acid esters preparationPropanoic acidKetone
The invention relates to the field of pharmaceutical synthesis, in particular to a method for synthesizing 1,7-2-(4-hydroxy phenyl)-heptane-1,4-diene-3-ketone. The method, selecting 3-(4- hydroxy phenyl)propionic acid and 4-hydroxybenzaldehyde as raw materials, includes (1), subjecting the 3-(4-hydroxy phenyl)propionic acid to esterification, methyl protection, reduction and oxidation to obtain 3-(4-(methoxy methyl)phenyl)propionaldehyde; (2), subjecting the 4-hydroxybenzaldehyde to etherification and aldol reaction to obtain 4-(4-((methoxy methyl)phenyl)butyl-3-alkene-2-ketone; (3), subjecting the 4-(4-((methoxy methyl)phenyl) butyl-3-alkene-2-ketone and the 3-(4-(methoxy methyl)phenyl)propionaldehyde to aldol condensation, and allowing the reactor to react with hydrochloric acid to obtain 1, 7-2-(4-hydroxy phenyl)-heptane-1,4-diene-3-ketone. The method has the advantages of simplicity, easiness in operation, high yield and high purity.
Owner:SHENYANG PHARMA UNIVERSITY
Lubricating oil specially used for a biogas engine and a preparing method thereof
Lubricating oil specially used for a biogas engine and a preparing method thereof are disclosed. The lubricating oil includes phosphite, polyoxyethylene hydrogenated castor oil, phosphoric acid, 2(2-ethoxyethoxy)ethanol, sorbitol, 4-hydroxybenzaldehyde, isodehydro-costuslactone, aerosil, lupenyl palmitate, cross-linked polyvinylpolypyrrolidone, naphthenic oil, butter, a tetra(hydroxyethyl)ammonium swelling oily agent, succinimidyl succinate, boron nitride, glycerin, polyvinylsuccinimide, graphite and amino thio ester. The lubricating oil is developed specially for the biogas engine, and is prepared by adopting a unique formula and a unique processing process. Aiming at internal lubrication of the biogas engine, the lubricating oil ahs characteristics of high-temperature resistance, not liable volatilization and high adhesive force and has popularization and application value.
Owner:LONGYAN UNIV
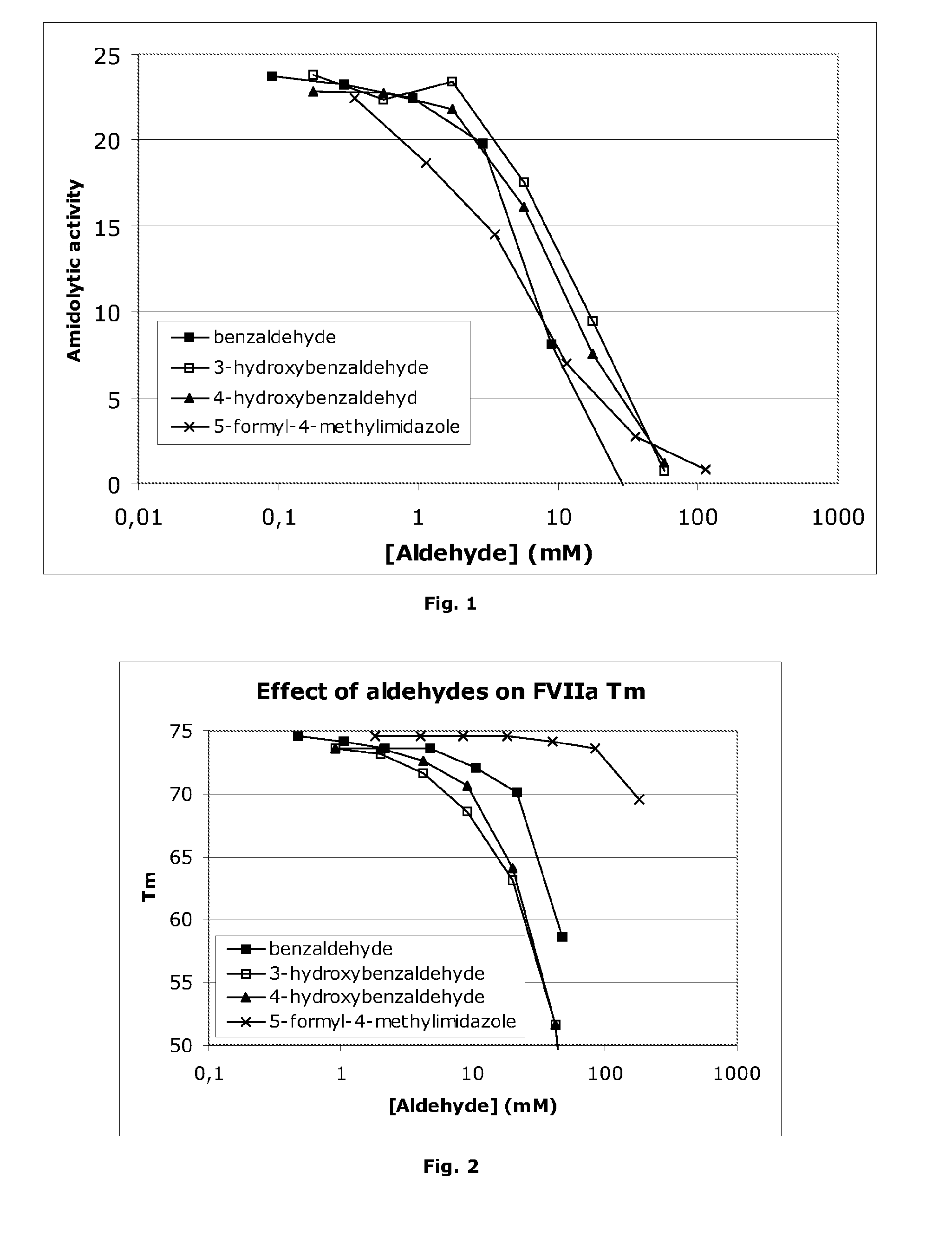
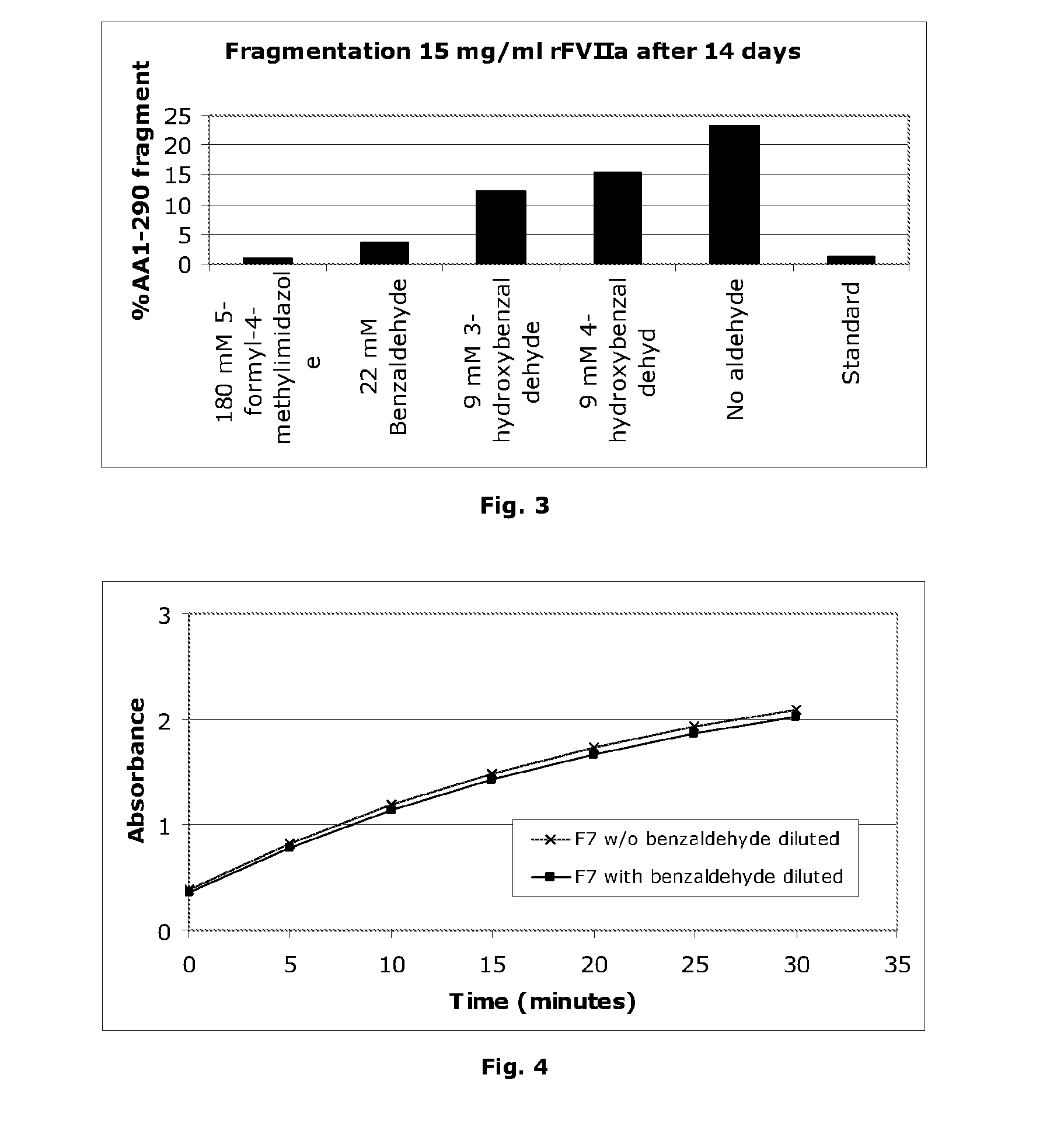
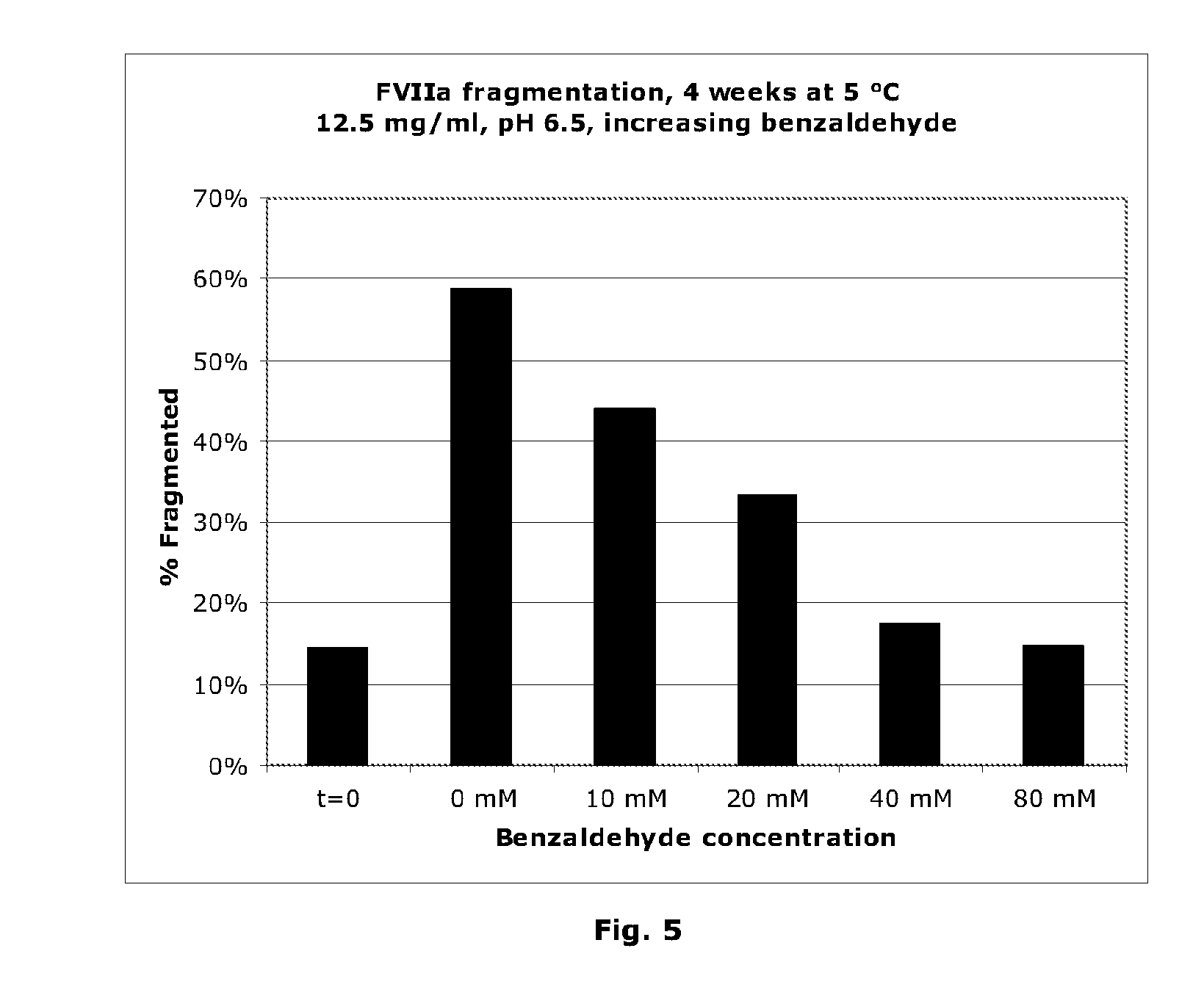
Stabilisation of Liquid-Formulated Factor VII(A) Polypeptides by Aldehyde-Containing Compounds
InactiveUS20100303786A1Improve stabilityPeptide/protein ingredientsPharmaceutical delivery mechanism3-HydroxybenzaldehydeBenzaldehyde
The present invention is directed to liquid, aqueous pharmaceutical compositions stabilised against chemical and / or physical degradation containing Factor VII polypeptides, and methods for preparing and using such compositions, as well as vials containing such compositions, and the use of such compositions in the treatment of a Factor VII-responsive syndrome. The main embodiment is represented by a liquid, aqueous pharmaceutical composition comprising at least 0.01 mg / mL of a Factor VII polypeptide (i); a buffering agent (ii) suitable for keeping pH in the range of from about 4.0 to about 9.0; and at least one stabilising agent (iii) comprising a R—CHO motif, e.g. Benzaldehyde, 3-hydroxybenzaldehyde, 4-hydroxybenzaldehyde, or 5-formyl-4-methylimidazole.
Owner:NOVO NORDISK AS
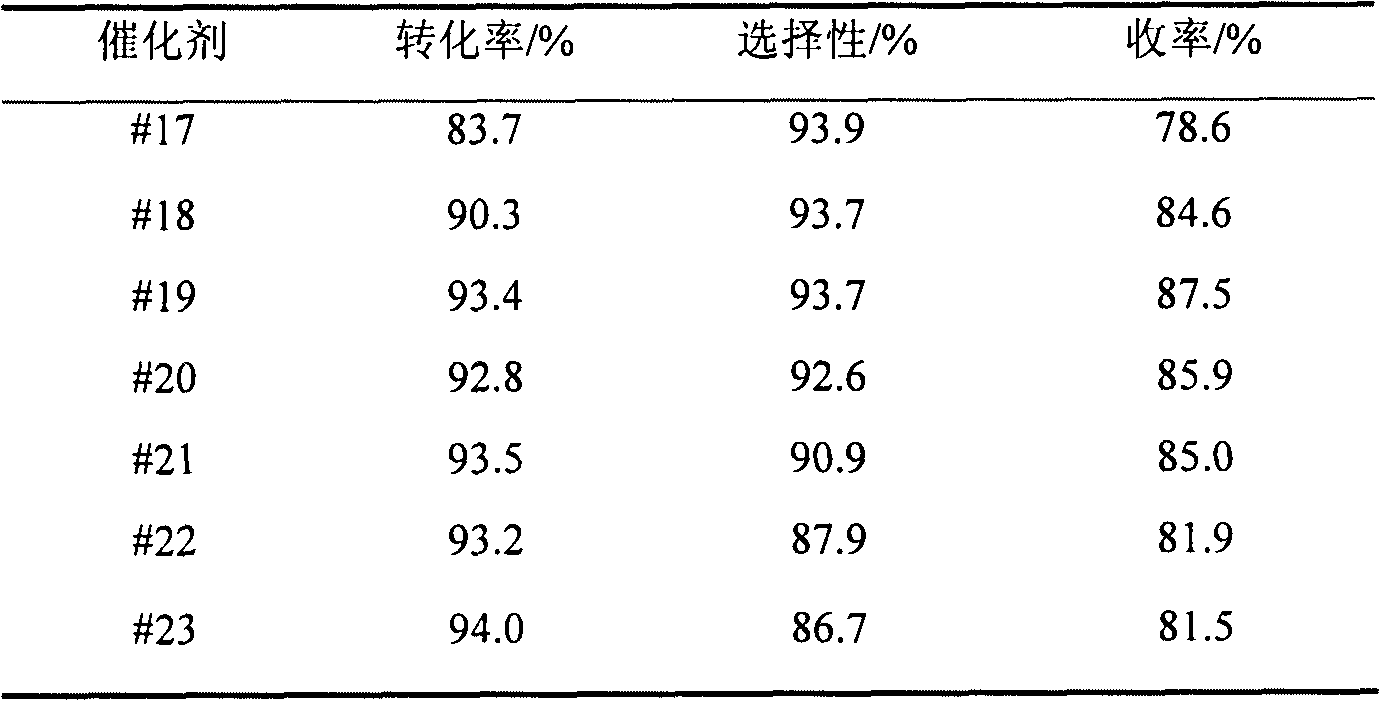
Preparation and application of multi-phase catalyst for use in synthesis of vanillin
The invention relates to preparation and an application of a (a)Co(b)Fe(x)M(y)O type multi-phase catalyst for catalyzing oxosynthesis of vanillin (3-methoxy-4-hydroxy benzaldehyde). The (a)Co(b)Fe(x)M(y)O type multi-phase catalyst is prepared by taking cobalt and iron as active ingredients, adding one or more of elements including copper, manganese, zirconium, cerium, niobium, nickel, bismuth, lanthanum and the like for serving as cocatalyst(s), taking an alkaline solution as a precipitant and adopting a coprecipitation method. The vanillin is prepared by the following steps of: dissolving 3-methoxy-4-hydroxy salicylic alcohol into a methanol solution of an alkali metal hydroxide; adding a catalyst; putting a reaction liquid into a high-pressure kettle; charging oxygen or air in a heating state; efficiently finishing an oxidation reaction; separating simply; and neutralizing. The transformation ratio of the 3-methoxy-4-hydroxy salicylic alcohol is over 94 percent, the selectivity of the vanillin is up to 93 percent, and the yield of the vanillin is up to 88 percent. The catalyst is easy to prepare, has low cost, is easy to separate after reacting, and can be used circularly after being simply treated; an high selectivity of the vanillin is kept while high transformation ratio of the 3-methoxy-4-hydroxy salicylic alcohol is ensured. The preparation has the advantages of high product yield, high purity, capability of directly recovering an alcohol solvent, small environmental pollution, low production cost and the like, and has a good application prospect in industrial synthesis of vanillin.
Owner:EAST CHINA UNIV OF SCI & TECH
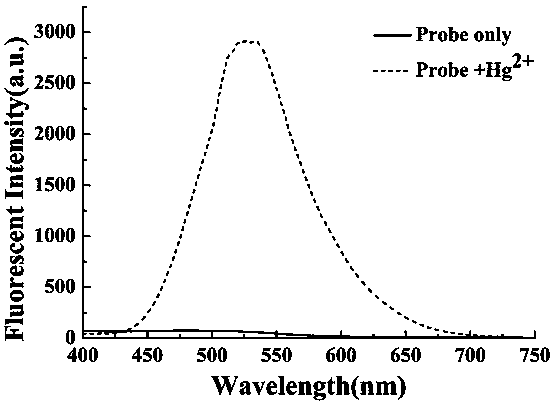
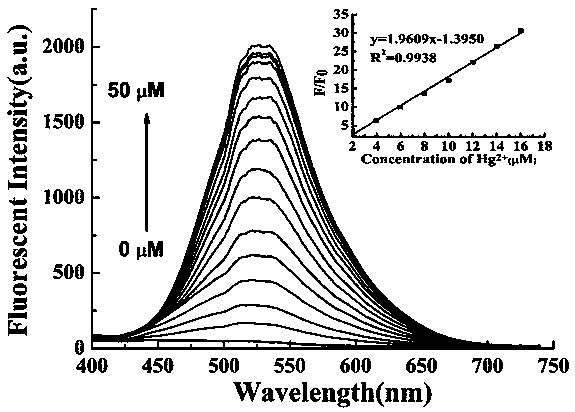
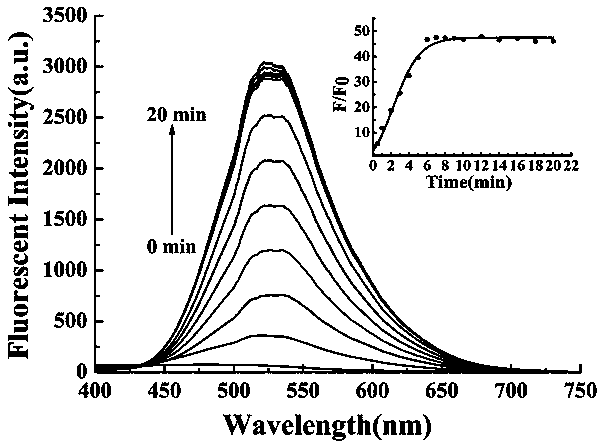
Preparation method and application of turn-on type mercury ion fluorescence probe
ActiveCN108863911ARealize detectionQuick responseOrganic chemistryFluorescence/phosphorescenceBenzoic acidIodised salt
The invention discloses a preparation method and application of a turn-on type mercury ion fluorescence probe. For the mercury ion fluorescence probe, the molecular formula is C21H18INO2S, and the preparation method comprises the following steps of (1) performing reaction on 4-hydroxybenzaldehyde and 4-methyipyridine to obtain 4-(4-hydroxy-diphenyl) pyridine, i.e. a compound 3; (2) performing reaction on the compound 3 and 2-(2-pyridyldithio) benzoic acid to obtain 4-[4-(2-pyridyldithio) benzoate] styrylpyridine, i.e. a compound 2; and (3) performing ionization on the compound 2 through CH3I to obtain 4-[4-(2-mercapto) benzoate] styryl-1-methylpyridinium iodide, i.e. a probe molecule 1. In a pure PBS buffer solution, the probe molecule performs reaction with mercury ions, and the concentration of the mercury ions can be detected by utilizing the change of the fluorescence strength. The probe provided by the invention is simple to prepare, rapid in response to the mercury ions, good inselectivity and high in sensitivity.
Owner:SHANXI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com