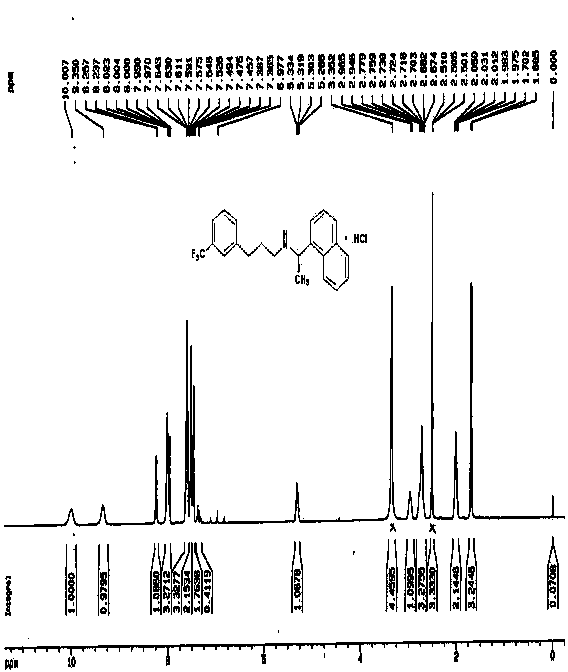
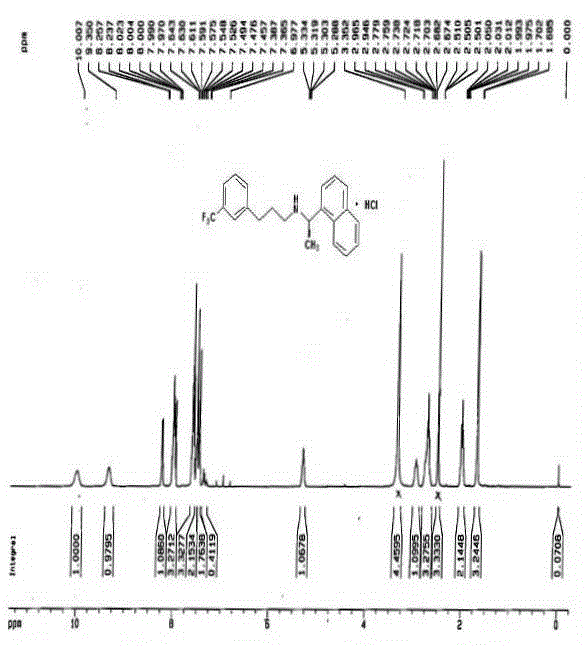
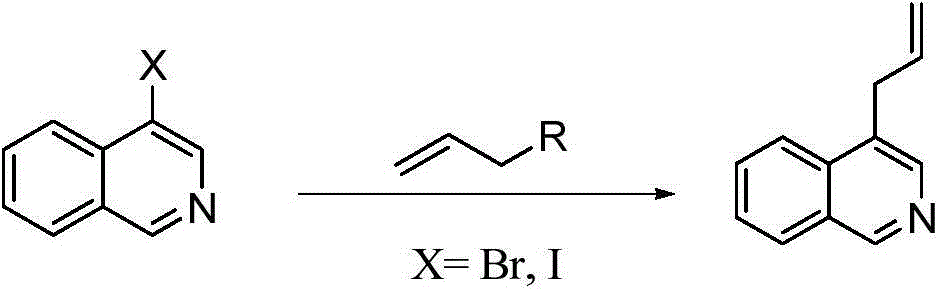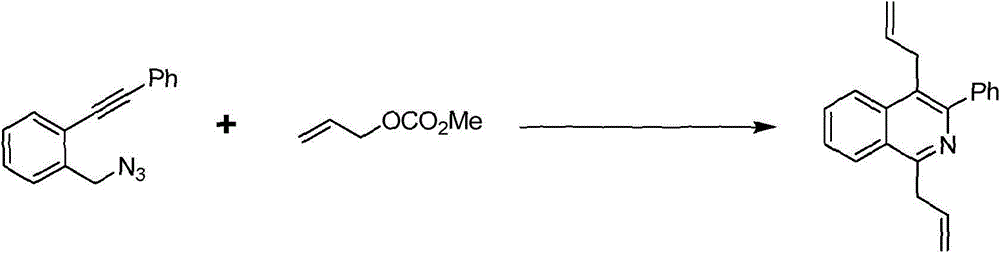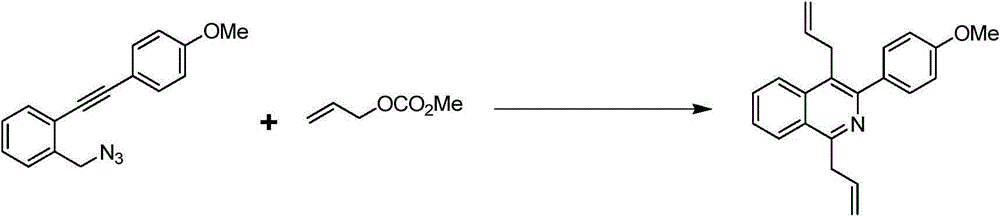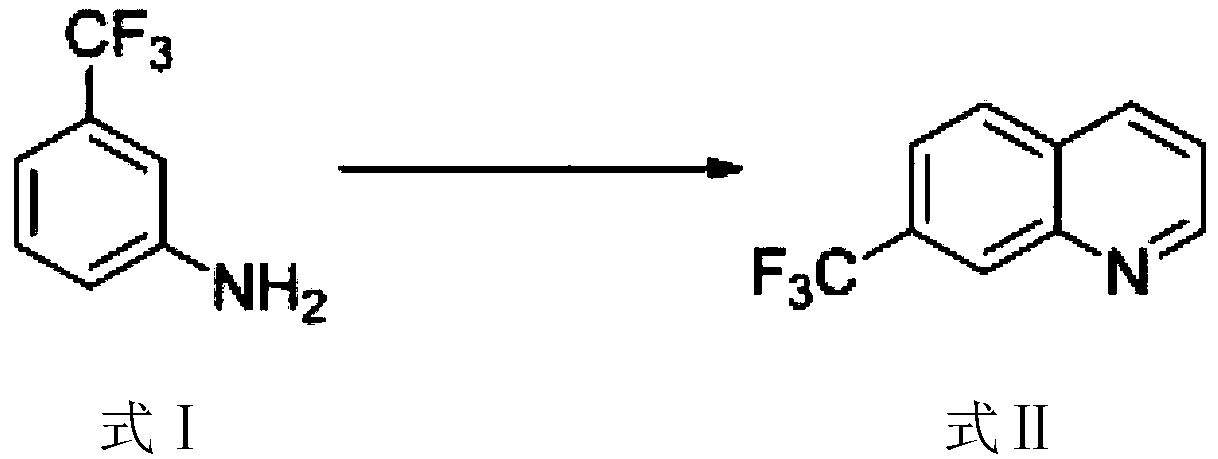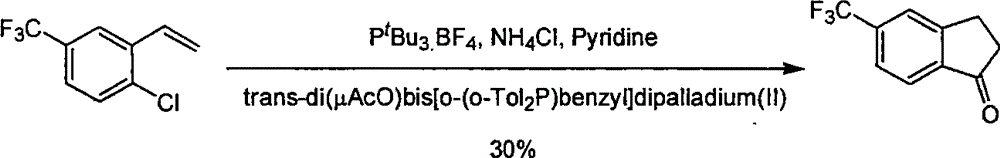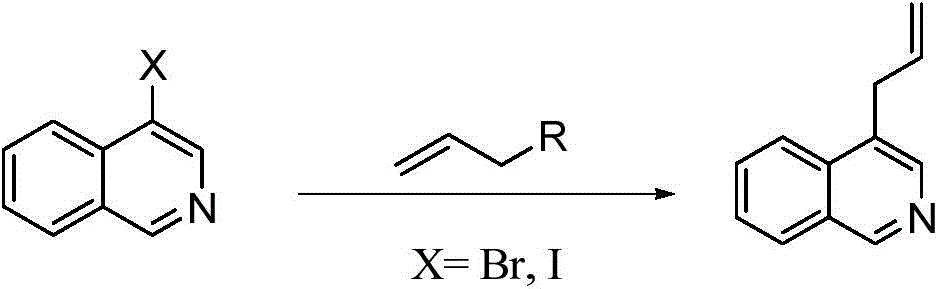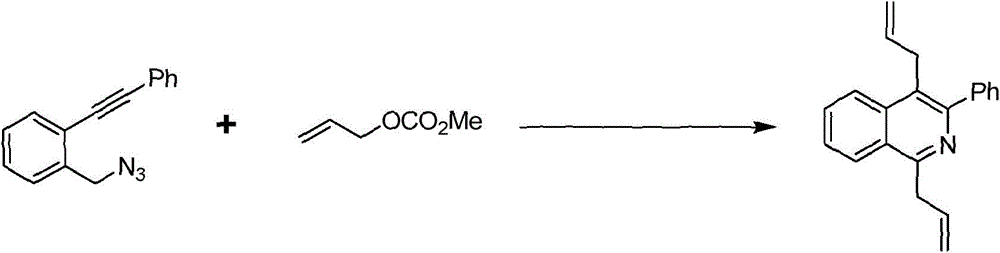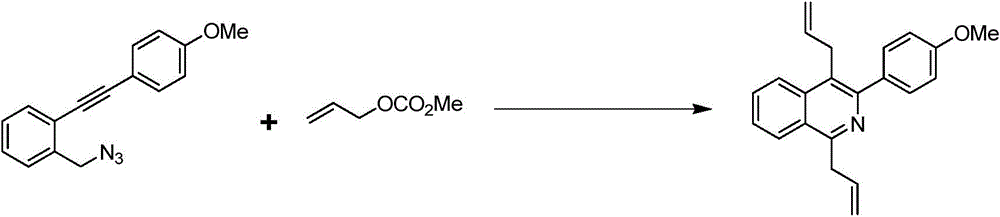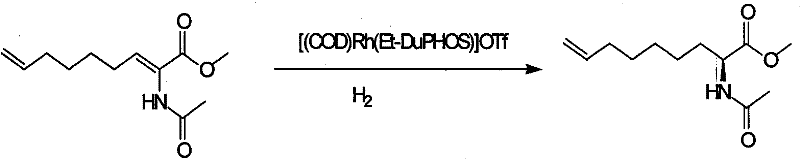Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44results about How to "Process selection is reasonable" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
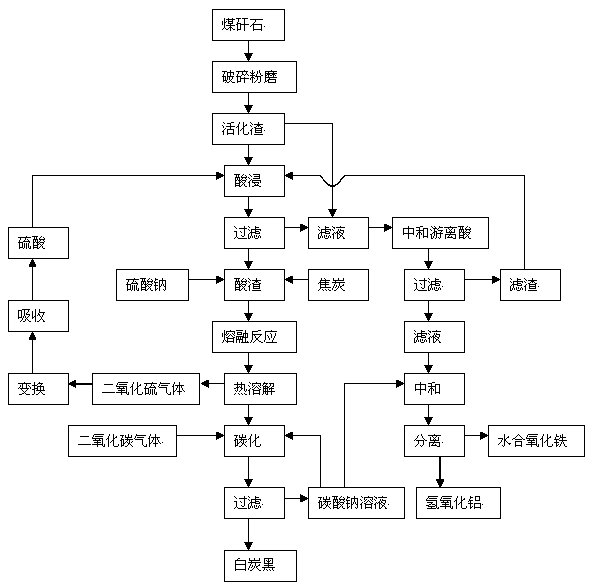
Comprehensive extraction method of ferro-silico-aluminum in gangue
ActiveCN102515279AGood slag activityMeet operating conditionsSilicaIron oxides/hydroxidesAluminium hydroxideSlag
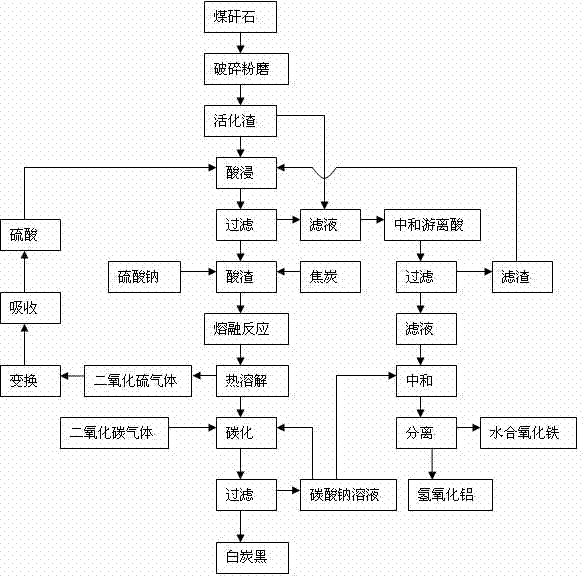
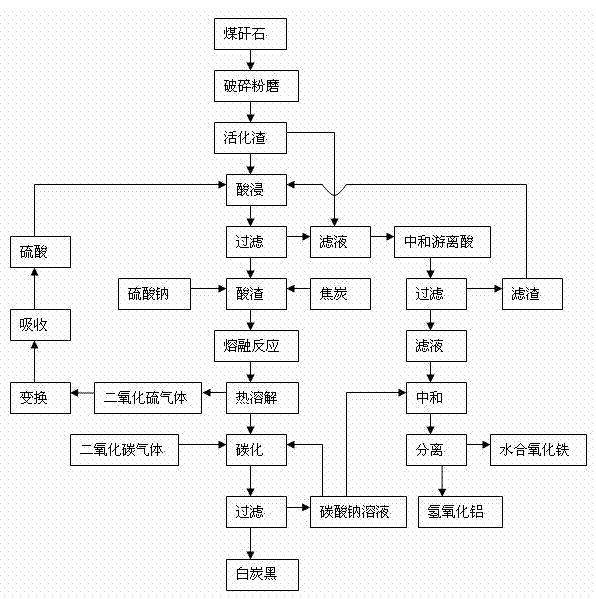
The invention discloses a comprehensive extraction method of ferro-silico-aluminum in gangue, comprising the following steps of: using gangue as a raw material, crushing, grinding, activating, carrying out acid leaching, filtering, neutralizing free acid in the acid leaching filtered solution by the use of active slag, filtering to obtain a neutralized acid leaching solution, adding a sodium carbonate solution into the neutralized acid leaching solution, adjusting pH value, separating iron and aluminium to obtain hydrous iron oxide, aluminium hydroxide and a by-product sodium sulphate, adding sodium sulphate and coke into the acid leaching slag which has undergone acid leaching and filtration, carrying out a high-temperature melt reaction to recover silicon so as to prepare soluble glass, simultaneously recovering sulfur dioxide to prepare sulfuric acid, reusing sulfuric acid for the acid leaching treatment, diluting the soluble glass by the use of a sodium carbonate solution, carrying out carborization to obtain white carbon black, and reusing the carbonating solution for iron-aluminium separation. The method provided by the invention has characteristics of wide application range of the raw material gangue, high comprehensive recovery rate, no output of by-products, less residue amount and the like, provides a novel technological process for high-efficiency recovery of ferro-silico-aluminum from gangue, and expands the ways of gangue application.
Owner:KUNMING UNIV OF SCI & TECH +1
Method for synthesizing and refining cinacalcet hydrochlorid
ActiveCN103739500AConvenient sourceLow costAmino compound purification/separationOrganic compound preparationEngineeringEnvironmental engineering
The invention explores a method for synthesizing and refining cinacalcet hydrochlorid, especially avoids toxic and expensive reaction reagents reported in literatures; the invention has the advantages of convenient sources of raw materials and reagents, low cost, little pollution to environment and simple operation, the invention is suitable for industrial production.
Owner:SINOPHARM A THINK PHARMA
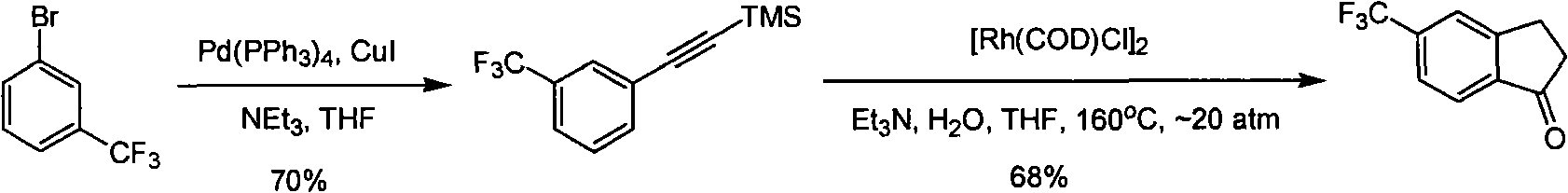
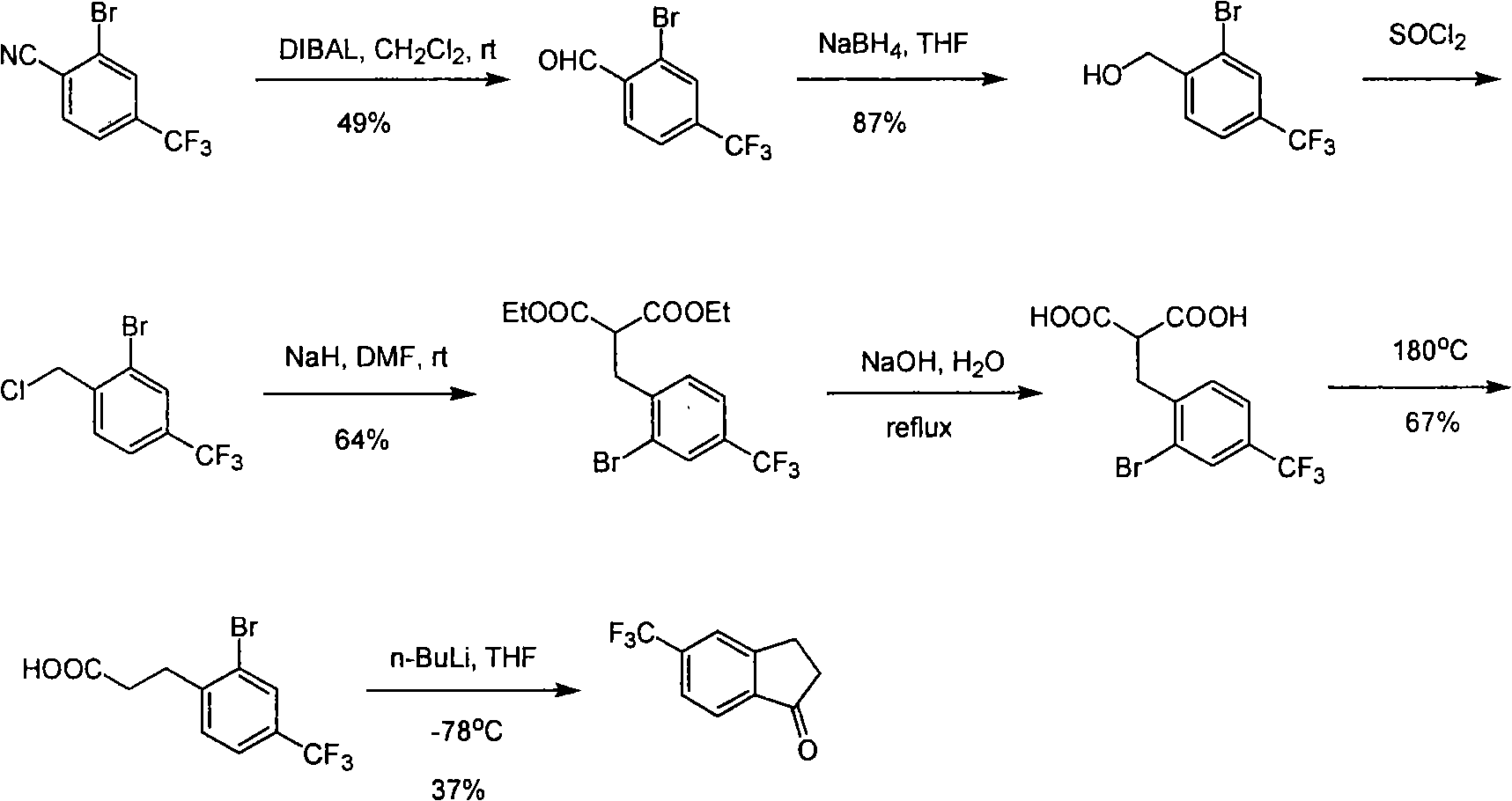
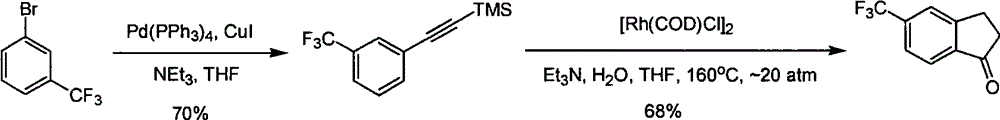
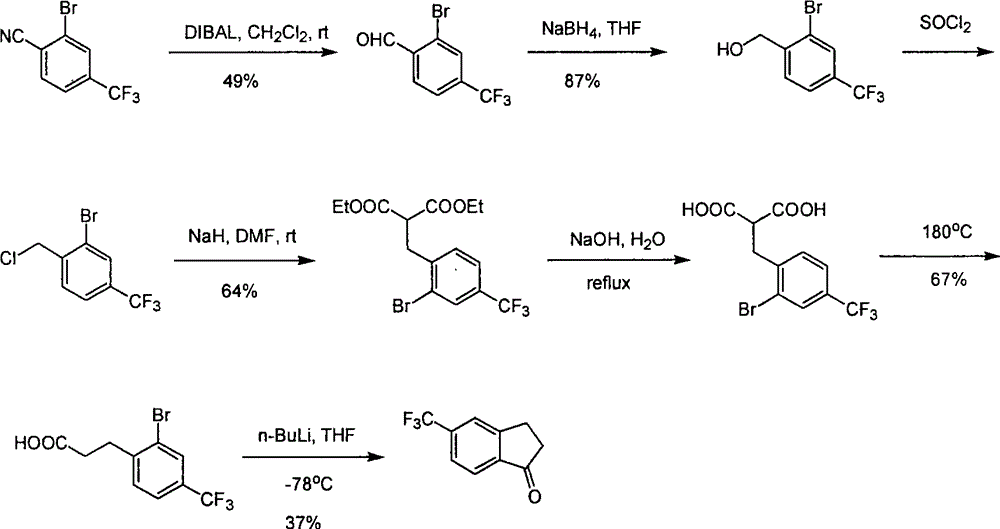
Process for synthesizing 5-trifluoromethyl-1-indene ketone
ActiveCN101293820AEasy to solveRealize large-scale productionCarbonyl compound preparation by condensationBenzaldehydeReaction temperature
The invention relates to a novel method for synthesizing 5-trifluoromethyl-1-indanone. The method comprises the following steps: adopting industrial m-trifluoromethyl benzaldehyde as raw material and preparing m-trifluoromethyl cinnamic acid by Knoevenagel condensation; hydrogenating to obtain m-trifluoromethyl phenylpropionic acid; and preparing 5-trifluoromethyl-1-indanone by intramolecular Friedel-Crafts acylation. The first step is carried out in the presence of malonic acid as organic reagent and pyridine or piperidine as catalyst under reflux at 100 DEG C. The second step is carried out in the presence of palladium / carbon or palladium hydroxide / carbon as catalyst and reaction solvent such as methanol, ethanol, ethyl acetate or tetrahydrofuran under a pressure of 40psi at room temperature. The third step is carried out with trifluoromethanesulfonic acid for closing rings at -20 DEG C-90 DEG C. The method provides a synthesis process for 5-trifluoromethyl-1-indanone which is simple and easy to be scaled, and solves the technical problems in prior art on relative long synthetic route, expensive catalyst or raw material, and severe reaction conditions, high cost, etc.
Owner:WUXI APPTEC (TIANJIN) CO LTD +1
Preparation method of 1,4-diallyl isoquinoline
The invention discloses a preparation method of 1,4-diallyl isoquinoline synthesized by using a palladium catalyst. The structural formula of 1,4-diallyl isoquinoline is shown in the specification, wherein R1 refers to one or more substituents connected to a benzene ring, and is selected from one of H, chlorine and -O-CH2-O-, and R2 is one of phenyl, p-methoxyphenyl, and 1-cyclohexenyl. In the process of preparing, 1-methyl azide-2-ethinyl-benzene shown in the specification is taken as a raw material, and reacted with allyl methyl carbonate in the presence of a Pd(PPh3)4 catalyst, an alkaline cocatalyst and an organic solvent, so that 1,4-diallyl isoquinoline is obtained. According to the invention, a 1,4-diallyl isoquinoline compound can be efficiently synthesized by using a one-pot method; the preparation method is high in conversion efficiency, short in reaction time and neutral in reaction conditions, and has no generated by-product; the method is low in raw material cost, simple in operation, low in pollution to the environment, and beneficial to industrialized production; and products are widely used, and can be used as intermediates for further synthesizing more complex compounds.
Owner:SHANGHAI JIAO TONG UNIV
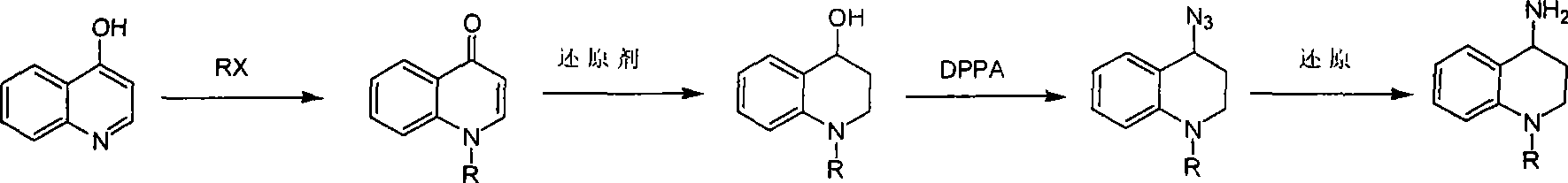
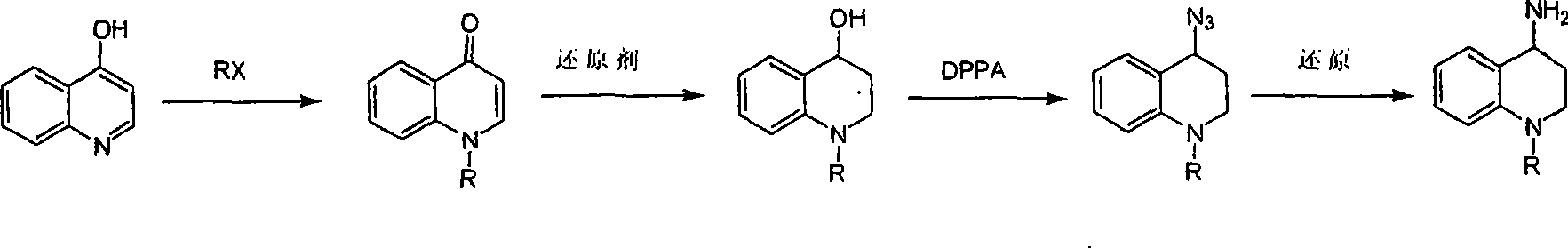
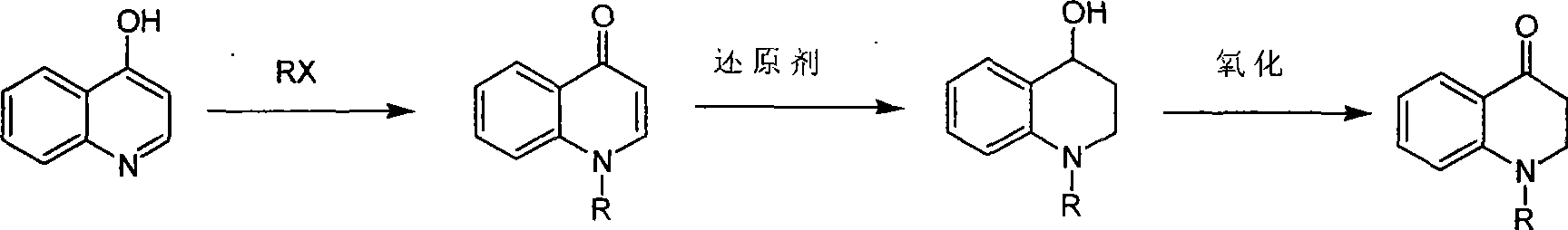
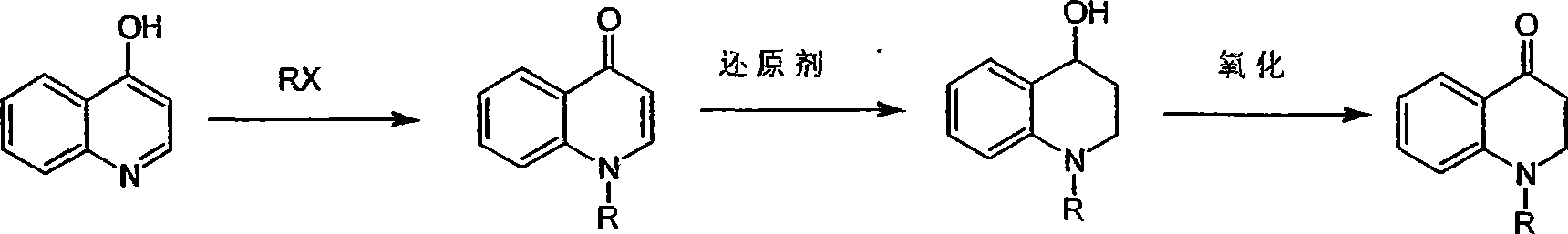
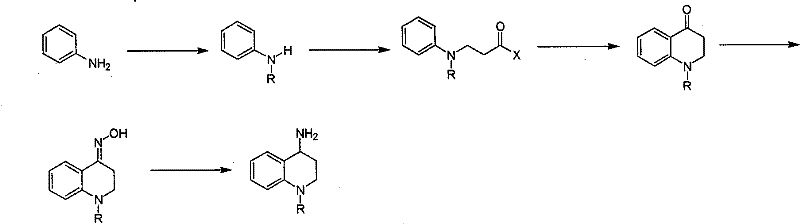
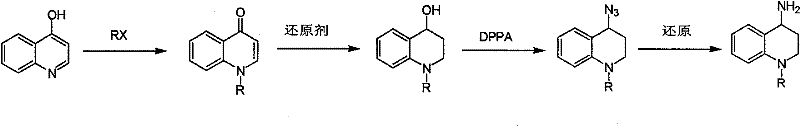
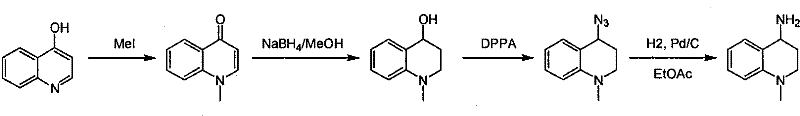
Synthesis of 1-R-4-amino-1,2,3,4-tetrahydroquinoline
ActiveCN101429160AProcess selection is reasonableRaw materials are easy to obtainOrganic chemistrySulfonateAlcohol
The invention relates to a method for synthesizing 1-R-4-amino-1, 2, 3, 4-tetrahydroquinoline, which mainly solves the technical problems that the prior synthesis method is difficult to amplify, long in route, inconvenient in reaction operation, and the like. The invention adopts a technical proposal that the method for synthesizing 1-R-4-amino-1, 2, 3, 4-tetrahydroquinoline is characterized by comprising the following steps: a. 4-hydroxyquinoline is taken as a raw material and reacts with alkyl halogenated matter, benzyl halogenated matter or alkyl sulphonic acid ester in solvent to form ketene; b. the ketene dissolves in the solvent, and a reducing agent is added to the solvent so as to obtain corresponding alcohol; c. corresponding alcohol of anhydrous tetrahydrofuran dissolved in the solvent is added to diphenoxyphosphinyl azide so as to be transformed into corresponding azide; and d. the obtained azide is dissolved in the solvent, and the 1-R-4-amino-1, 2, 3, 4-tetrahydroquinoline is obtained by a hydrogenation or chemical reduction method.
Owner:上海药明康德新药开发有限公司
A kind of synthetic method of 4-hydroxyl-8-bromoisoquinoline
The invention discloses a synthesis method of 4-hydroxy-8-bromoisoquinoline. The method comprises the following steps: mixing bromobenzylamine with a toluene solution, then adding p-toluenesulfonic acid and glyoxylic acid, heating, refluxing, dehydrating and condensing to generate a 2-bromobenzene imidoacetic acid crude product, adding polyphosphoric acid, simultaneously heating and stirring, pouring the product into water for filtering after the reaction is completed, washing filter cake with ethyl ether, then drying the filter cake. According to the synthesis method of 4-hydroxy-8-bromoisoquinoline, bromobenzylamine is used as the raw material; the synthesis route is simple; the process selection is reasonable; the raw material is simple and easily available; the operation and after-treatment are convenient; the total yield reaches up to 76%; the 4-hydroxy-8-bromoisoquinoline is easy to magnify and high in biological activity, can be used as a 5-hydroxytryptamine receptor, and has a strong effect of treating dementia and schizophrenia diseases.
Owner:SUZHOU KANGRUN PHARMA
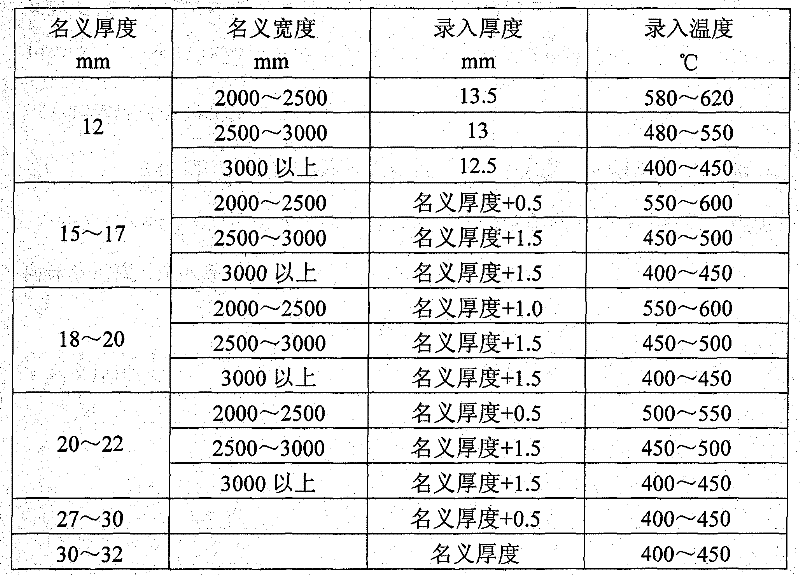
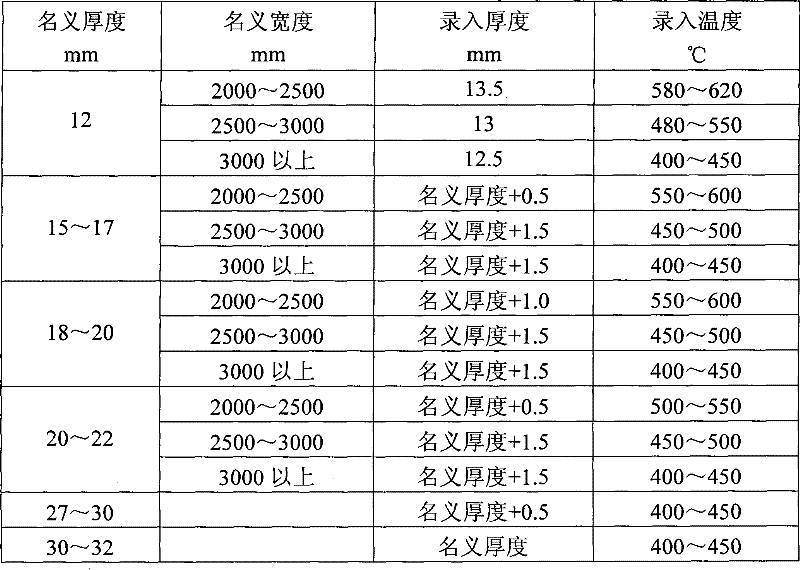
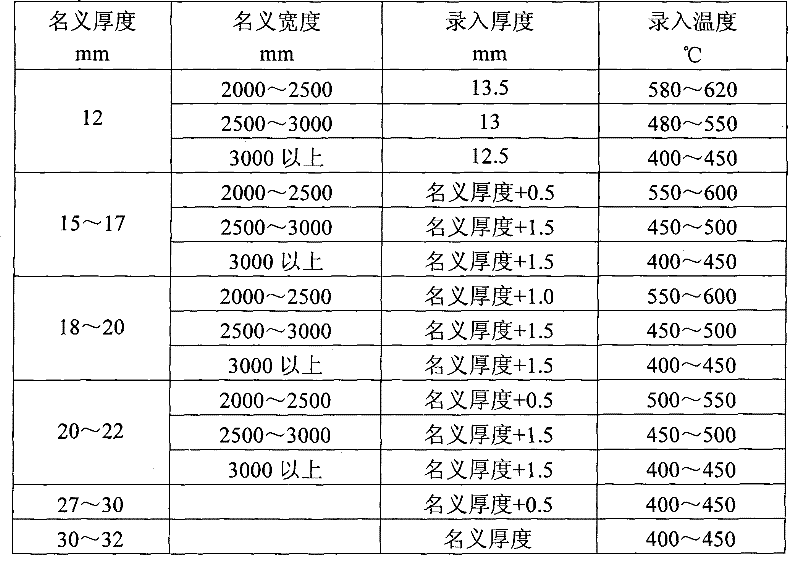
Method for straightening tank plate containing nickel and vanadium
The invention relates to a method for straightening a tank plate containing nickel and vanadium, which determines and controls recording temperature and roll bending manner and performances thrice straightening operations based on actual nominal thickness and nominal width of the steel plate and corresponding to the recording thickness input into the straightening model. The method of the invention has advantages of reasonable technique selection, scientific parameter determination and accuracy control method, the yield of the tank plate containing nickel and vanadium are raised from 80% to 97%, the integral time of delivery advances more than 10 days. Synthesis yield of the hot-rolled tank plate containing nickel and vanadium is enhanced, the re-straightening rate of the plate is reduced; but also the waste loss caused by disqualification of the plate is sufficiently decreased, and the economic benefit can reach 1.5 million yuan every year.
Owner:ANGANG STEEL CO LTD
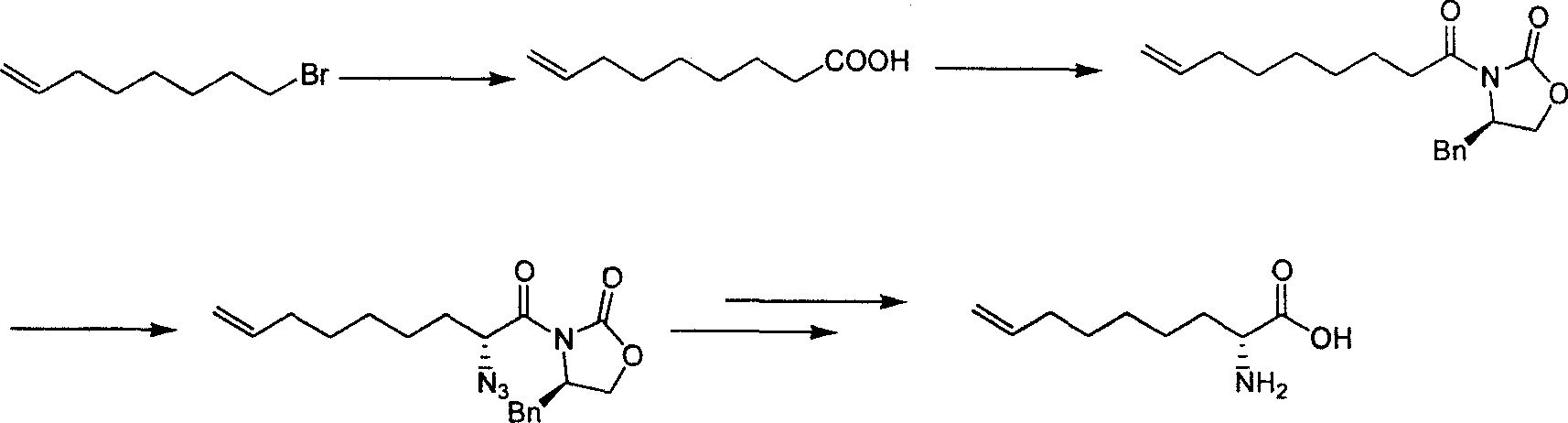
Method for practical synthesizing optically active 2 - amido - 8 - butenic acid
ActiveCN101092370AThe synthetic route is simpleProcess selection is reasonableOrganic compound preparationAmino-carboxyl compound preparationPresent methodGrignard reagent
This invention relates to a method for preparing 2-amino-8-nonenoic acid, especially optically active 2-amino-8-nonenoic acid. The method overcomes the problems of long synthesis route, low yield and expensive chiral catalyst or resolution reagent faced by the present method. The method comprises: attacking chiral pyroglutamate containing protecting groups with Grignard reagent of crotyl bromide to obtain chiral 2-amino-5-oxy-8- nonanoate, reducing to remove carbonyl and obtain chiral or racemized 2-amino-8-nonanoate, and hydrolyzing to obtain optically active 2-amino-8-nonenoic acid. The method in this invention has such advantages as simple route, reasonable process, and high yield, and is suitable for mass production.
Owner:上海药明康德新药开发有限公司
Synthetic method of 8-isoquinolinol
InactiveCN106966977AThe synthetic route is simpleProcess selection is reasonableOrganic chemistryLithium hydroxide monohydrateUrea
The invention discloses a synthetic method of 8-isoquinolinol, and belongs to the field of synthesis method of isoquinoline compounds. The synthetic method comprises following steps: 8-chloroisoquinoline, copper acetylacetonate, lithium hydroxide monohydrate, and ligand 1,3-bis(4-hydroxyl-2,6-dimethyl phenyl)urea are added into a mixed solution of dimethyl sulfoxide and water; under nitrogen protection, an obtained reaction solution is subjected to heating with stirring until reaction is completed, and pH value is adjusted to 5 with HCl after cooling; an obtained mixed liquid is extracted with ethyl acetate, an obtained organic phase is washed with saturated salt solution, is dried with anhydrous sodium sulfate, and then is subjected to spin drying; and an obtained crude product is subjected to column chromatography separation so as to obtain 8-isoquinolinol. According to the synthetic method, 8-chloroisoquinoline is taken as a raw material; synthesis route is simple; the synthetic method is reasonable; the raw materials are easily available; operation and subsequent treatment are convenient; total yield is as high as 72%; amplify production is convenient to realize; and large-scale production of 8-isoquinolinol can be realized.
Owner:XUZHOU MEDICAL UNIV +1
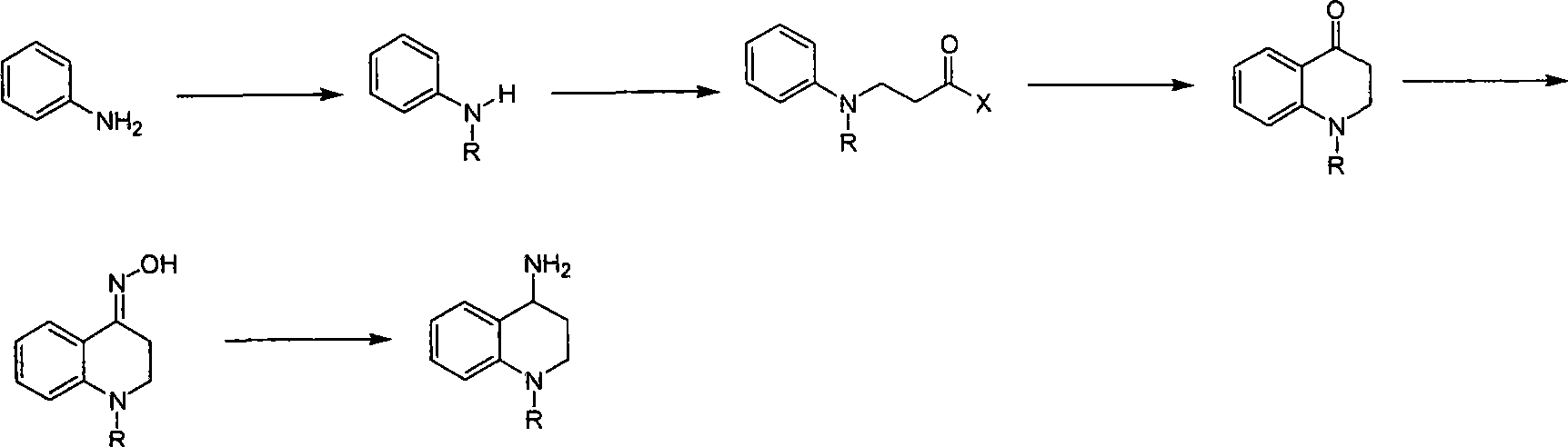
Synthesis of 1-R-2,3-dihydrogen-1H-quinoline-4-ketone
ActiveCN101429159AProcess selection is reasonableSimple ingredientsOrganic chemistrySolventQuinoline
The invention relates to a method for synthesizing 1-R-2, 3-dihydrogen-1H-quinoline-4-ketone, which mainly solves the technical problems that the prior synthesis method is difficult to amplify raw material, long in route, inconvenient in reaction operation, and the like. The method for synthesizing 1-R-2, 3-dihydrogen-1H-quinoline-4-ketone is characterized by comprising the following steps: a. 4-hydroxyquinoline is taken as a raw material and reacts with alkyl halogenated matter, benzyl halogenated matter or alkyl sulphonic acid ester in solvent to form ketene; b. the ketene dissolves in the solvent, and a reducing agent is added to the solvent so as to obtain corresponding alcohol; and c. oxidant is added to the corresponding alcohol dissolved in the solvent, so as to obtain the 1-R-2, 3-dihydrogen-1H-quinoline-4-ketone through oxidation.
Owner:上海药明康德新药开发有限公司
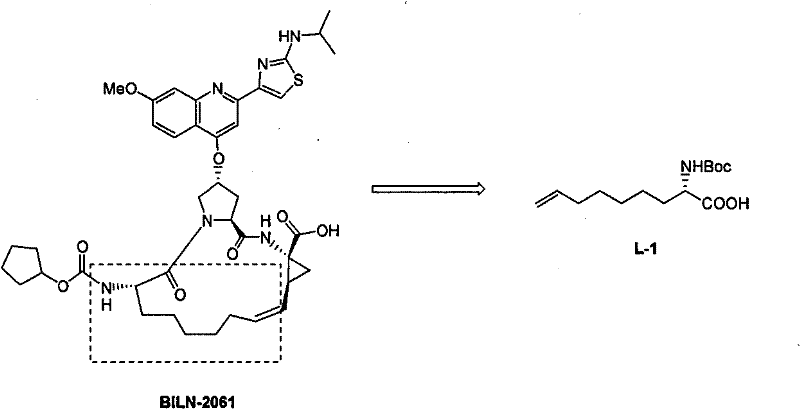
Preparing process of optically active long chain N-Boc-amino acid with end olefinic bond
InactiveCN1986520AConveniently preparedThe synthetic route is simpleOrganic compound preparationAntiviralsAlcoholGrignard reagent
The present invention relates to preparation process of optically active long chain N-Boc-amino acid with end olefinic bond, and the preparation process is superior to available process, which has long path, low yield and expensive chiral reagent. The preparation process includes attacking metylene radical of azirane derivative selectively with olefin halide Grignard reagent with end olefinic bond and opening ring to obtain beta-amino alcohol with protecting group, eliminating protecting hydroxyl group to obtain beta-amino alcohol, and oxidizing to obtain optically active long chain N-Boc-amino acid with end olefinic bond. The present invention is used in preparing anti-HCV medicine BILN2061.
Owner:上海药明康德新药开发有限公司
5-bromo-7-trifluoromethyl quinoline synthetic method
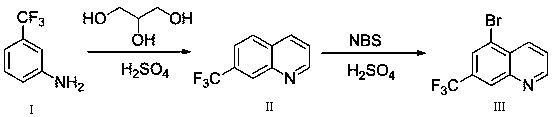
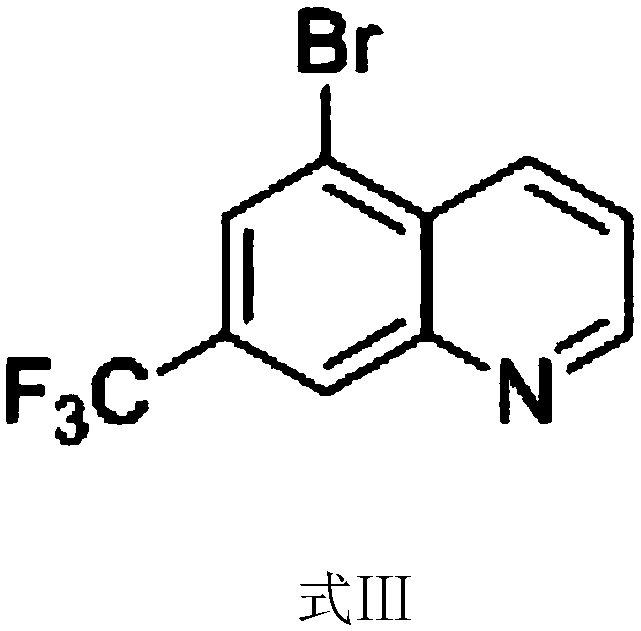
ActiveCN108409649AThe synthetic route is simpleProcess selection is reasonableOrganic chemistryTrifluoromethylQuinoline
The invention provides a 5-bromo-7-trifluoromethyl quinoline synthetic method. The 5-bromo-7-trifluoromethyl quinoline synthetic method comprises the following steps of by taking m-trifluoromethylaniline as a starting material, performing Skraup condensation to obtain 7-trifluoromethyl quinoline, and then performing reaction on the 7-trifluoromethyl quinoline and NBS to obtain 5-bromo-7-trifluoromethyl quinoline. According to the 5-bromo-7-trifluoromethyl quinoline synthetic method provided by the invention, a synthetic route of the method eliminates the disadvantages of incapability of purification, low yield and the like of a product in the existing synthetic technology, and the 5-bromo-7-trifluoromethyl quinoline synthetic method has the advantages of concision in synthetic route, reasonability in technology selection, low cost in raw materials, simpleness and availability in raw materials, convenience in operation and aftertreatment, high total yield and the like.
Owner:SUZHOU KANGRUN PHARMA
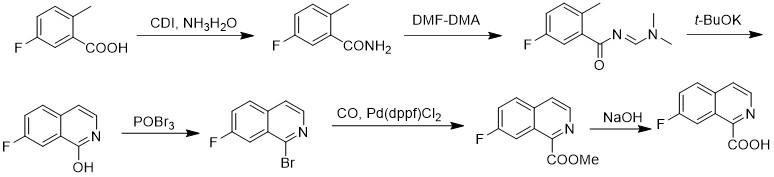
The synthetic method of 7-fluoroisoquinoline-1-carboxylic acid
The invention discloses a method for synthesizing 7-fluoroisoquinoline-1-carboxylic acid. The method uses 5-fluoro-2-methylbenzoic acid as a raw material to obtain 5-fluoroisoquinoline-1-carboxylic acid through condensation with N,N-carbonyldiimidazole ‑Fluoro‑2‑methylbenzamide reacted with N,N‑dimethylformamide dimethyl acetal (E) ‑N‑((dimethylamino)methylene)‑5‑fluoro‑2‑methylbenzamide, followed by cyclization in potassium tert-butoxide to give 7‑fluoroisoquinoline‑1‑alcohol, which is then reacted with tribromo Oxon reaction generates 1-bromo 7-fluoroisoquinoline, and then under carbon monoxide conditions, 7-fluoroisoquinoline-1-carboxylate methyl ester is obtained, and finally hydrolyzed in aqueous sodium hydroxide solution to obtain 7-fluoroisoquinoline- 1‑Carboxylic acid. The method has simple synthesis route, reasonable process selection, low cost of raw materials, simple and easy-to-obtain raw materials, simple and safe operation, no use of highly toxic reagents, convenient post-treatment, high total yield, easy scale-up, and large-scale production.
Owner:SUZHOU KANGRUN PHARMA
Comprehensive extraction method of ferro-silico-aluminum in gangue
ActiveCN102515279BGood slag activityMeet operating conditionsSilicaIron oxides/hydroxidesAluminium hydroxideSlag
The invention discloses a comprehensive extraction method of ferro-silico-aluminum in gangue, comprising the following steps of: using gangue as a raw material, crushing, grinding, activating, carrying out acid leaching, filtering, neutralizing free acid in the acid leaching filtered solution by the use of active slag, filtering to obtain a neutralized acid leaching solution, adding a sodium carbonate solution into the neutralized acid leaching solution, adjusting pH value, separating iron and aluminium to obtain hydrous iron oxide, aluminium hydroxide and a by-product sodium sulphate, adding sodium sulphate and coke into the acid leaching slag which has undergone acid leaching and filtration, carrying out a high-temperature melt reaction to recover silicon so as to prepare soluble glass, simultaneously recovering sulfur dioxide to prepare sulfuric acid, reusing sulfuric acid for the acid leaching treatment, diluting the soluble glass by the use of a sodium carbonate solution, carrying out carborization to obtain white carbon black, and reusing the carbonating solution for iron-aluminium separation. The method provided by the invention has characteristics of wide application range of the raw material gangue, high comprehensive recovery rate, no output of by-products, less residue amount and the like, provides a novel technological process for high-efficiency recovery of ferro-silico-aluminum from gangue, and expands the ways of gangue application.
Owner:KUNMING UNIV OF SCI & TECH +1
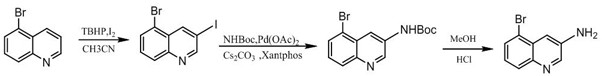
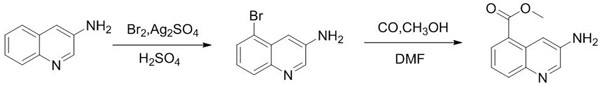
Synthesis method of 3-amino-5-bromoquinoline
ActiveCN112375037ALow costThe synthetic route is simpleOrganic chemistryBiochemical engineeringQuinoline
The invention discloses a synthesis method of 3-amino-5-bromoquinoline, which comprises the following steps of: by using 5-bromoquinoline as a raw material, carrying out non-metal catalytic oxidationreaction on the 5-bromoquinoline, TBHP and iodine to obtain 5-bromo-3-iodoquinoline, and carrying out palladium catalytic coupling and hydrolysis on the 5-bromo-3-iodoquinoline and NH2Boc to obtain the 3-amino-5-bromoquinoline. The synthesis method is simple in synthesis route, reasonable in process selection, low in raw material cost, simple and easily available in raw materials, convenient in operation and post-treatment, high in total yield, free of highly toxic reagents, easy in amplification and capable of realizing large-scale production.
Owner:SUZHOU KANGRUN PHARMA
A kind of synthetic and refining method of cinacalcet hydrochloride
ActiveCN103739500BShort reaction stepsHigh yieldAmino compound purification/separationOrganic compound preparationCinacalcet HydrochlorideCinacalcet
Owner:SINOPHARM A THINK PHARMA
Process for synthesizing 5-trifluoromethyl-1-indene ketone
ActiveCN101293820BEasy to solveRealize large-scale productionCarbonyl compound preparation by condensationBenzaldehydeReaction temperature
The invention relates to a novel method for synthesizing 5-trifluoromethyl-1-indanone. The method comprises the following steps: adopting industrial m-trifluoromethyl benzaldehyde as raw material and preparing m-trifluoromethyl cinnamic acid by Knoevenagel condensation; hydrogenating to obtain m-trifluoromethyl phenylpropionic acid; and preparing 5-trifluoromethyl-1-indanone by intramolecular Friedel-Crafts acylation. The first step is carried out in the presence of malonic acid as organic reagent and pyridine or piperidine as catalyst under reflux at 100 DEG C. The second step is carried out in the presence of palladium / carbon or palladium hydroxide / carbon as catalyst and reaction solvent such as methanol, ethanol, ethyl acetate or tetrahydrofuran under a pressure of 40psi at room temperature. The third step is carried out with trifluoromethanesulfonic acid for closing rings at -20 DEG C-90 DEG C. The method provides a synthesis process for 5-trifluoromethyl-1-indanone which is simple and easy to be scaled, and solves the technical problems in prior art on relative long synthetic route, expensive catalyst or raw material, and severe reaction conditions, high cost, etc.
Owner:WUXI APPTEC (TIANJIN) CO LTD +1
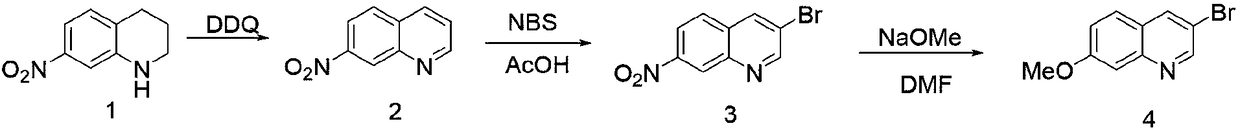
Synthetic method of 3-bromo-7-methoxyl quinoline
The invention provides a synthetic method of 3-bromo-7-methoxyl quinoline. The synthetic method of 3-bromo-7-methoxyl quinoline comprises the following steps: 1) adding a solvent into 7-nitryl-1,2,3,4-tetrahydroquinoline, adding DDQ and stirring the mixture to react to obtain 7-nitryl quinoline, wherein the molar ratio of 7-nitryl-1,2,3,4-tetrahydroquinoline and DDQ is 1: (1-5); 2) adding the solvent into 7-nitryl quinoline, heating the mixture, adding N-bromo-succinimide to react to obtain 3-bromo-7-nitryl quinoline in an insulating manner; and 3) dissolving 3-bromo-7-nitryl quinoline in thesolvent, adding sodium methylate, and heating the mixture to stir and react to obtain 3-bromo-7-methoxyl quinoline. The synthetic method of 3-bromo-7-methoxyl quinoline is concise in synthetic route,reasonable in process selection, low in raw material cost, simple and easy, convenient to operate and post-treat, high in total yield and easy to amplify.
Owner:SUZHOU KANGRUN PHARMA
The preparation method of 1,4-diallylisoquinoline
The invention discloses a preparation method of 1,4-diallyl isoquinoline synthesized by using a palladium catalyst. The structural formula of 1,4-diallyl isoquinoline is shown in the specification, wherein R1 refers to one or more substituents connected to a benzene ring, and is selected from one of H, chlorine and -O-CH2-O-, and R2 is one of phenyl, p-methoxyphenyl, and 1-cyclohexenyl. In the process of preparing, 1-methyl azide-2-ethinyl-benzene shown in the specification is taken as a raw material, and reacted with allyl methyl carbonate in the presence of a Pd(PPh3)4 catalyst, an alkaline cocatalyst and an organic solvent, so that 1,4-diallyl isoquinoline is obtained. According to the invention, a 1,4-diallyl isoquinoline compound can be efficiently synthesized by using a one-pot method; the preparation method is high in conversion efficiency, short in reaction time and neutral in reaction conditions, and has no generated by-product; the method is low in raw material cost, simple in operation, low in pollution to the environment, and beneficial to industrialized production; and products are widely used, and can be used as intermediates for further synthesizing more complex compounds.
Owner:SHANGHAI JIAO TONG UNIV
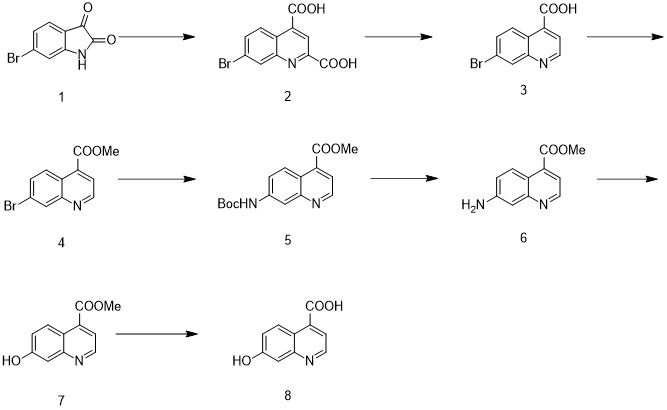
The synthetic method of 7-hydroxyquinoline-4-carboxylic acid
ActiveCN112500341BThe synthetic route is simpleProcess selection is reasonableOrganic chemistryTert-Butyloxycarbonyl protecting groupQuinoline
The invention discloses a method for synthesizing 7-hydroxyquinoline-4-carboxylic acid, which uses 6-bromoisatin as a raw material to react with pyruvic acid to obtain 7-bromoquinoline-2,4-carboxylic acid, and Nitrobenzene reacts to obtain 7-bromoquinoline-4-carboxylic acid, then reacts 7-bromoquinoline-4-carboxylic acid methyl ester with methanol, and reacts with NH2Boc to obtain 7-((tert-butoxycarbonyl)amino)quinoline ‑4‑carboxylate methyl ester, remove amino protection in hydrochloric acid and methanol solution to obtain 7‑aminoquinoline‑4‑carboxylate methyl ester, diazotization reaction occurs in concentrated sulfuric acid to generate 7‑hydroxyquinoline‑4‑ Methyl carboxylate, and finally hydrolyzed in aqueous sodium hydroxide solution to obtain 7-hydroxyquinoline-4-carboxylic acid. The synthesis method uses 6-bromoisatin as a raw material, the synthetic route is simple, the process selection is reasonable, the raw material cost is low, the raw material is simple and easy to obtain, the operation and post-treatment are convenient, the total yield is high, no highly toxic reagent is used, and it is easy to scale up and can be used. for mass production.
Owner:SUZHOU KANGRUN PHARMA
A kind of synthetic method of 3-bromo-7-hydroxyquinoline
ActiveCN108484495BLow costThe synthetic route is simpleOrganic chemistryTrifluoromethanesulfonic anhydrideHydrolysis
The invention provides a method for synthesizing 3-bromo-7-hydroxyquinoline. The synthesis method of 3-bromo-7-hydroxyquinoline of the present invention comprises the following steps: 1) dissolving 7-hydroxyquinoline in a solvent, adding trifluoromethanesulfonic anhydride, and reacting at low temperature to obtain quinoline-7-trifluoroquinoline mesylate, wherein the molar ratio of the 7-hydroxyquinoline to the trifluoromethanesulfonic anhydride is 1:(1~1.5); 2) the quinoline-7-trifluoroform obtained in step 1) The sulfonate is dissolved in a solvent, and N-bromosuccinimide is added to obtain 3-bromoquinoline-7-trifluoromethanesulfonate after the reaction; 3) the 3-bromoquinoline-7-trifluoromethanesulfonate obtained in step 2) Hydrolysis of the 7-triflate under basic conditions affords 3-bromo-7-hydroxyquinoline. The synthesis method of 3-bromo-7-hydroxyquinoline of the present invention has stable raw materials, non-toxicity, simple synthesis process and high yield.
Owner:SUZHOU KANGRUN PHARMA
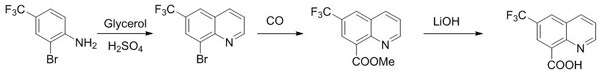
The synthetic method of 6-(trifluoromethyl)quinoline-8-carboxylic acid
ActiveCN112028826BThe synthetic route is simpleProcess selection is reasonableOrganic chemistryMethylanilineLithium hydroxide
The invention discloses a synthesis method of 6-(trifluoromethyl)quinoline-8-carboxylic acid, which uses 2-bromo-4-(trifluoromethyl)aniline as raw material to obtain 8-bromo ‑6‑(trifluoromethyl)quinoline, 6‑(trifluoromethyl)quinoline‑8‑carboxylate methyl ester is obtained under pressure of carbon monoxide, and finally hydrolyzed in aqueous lithium hydroxide solution to obtain 6‑(trifluoromethyl) fluoromethyl) quinoline‑8‑carboxylic acid. The synthesis method of the invention has simple synthesis route, reasonable process selection, low raw material cost, simple and easy to obtain raw material, convenient operation and post-treatment, high total yield, easy scale-up and large-scale production.
Owner:SUZHOU KANGRUN PHARMA
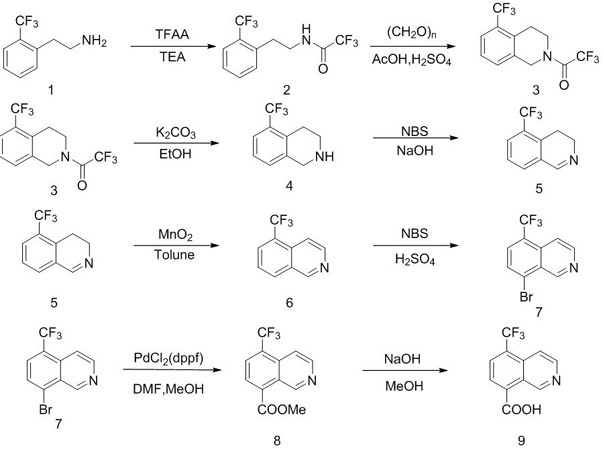
Synthesis method of 6-(trifluoromethyl) isoquinoline-5-ol
ActiveCN112094229AThe synthetic route is simpleProcess selection is reasonableOrganic chemistryPhenethylaminesTrifluoromethyl
The invention discloses a synthesis method of 6- (trifluoromethyl) isoquinoline-5-ol, which comprises the following steps of by using p-trifluoromethyl phenylethylamine as a raw material, carrying outamino protection, cyclization, hydrolysis, bromination, debromination alkene insertion and dehydroaromatization on the p-trifluoromethyl phenylethylamine and TFAA to obtain 7-trifluoromethyl isoquinoline, and carrying out 5-site bromination on the 7-trifluoromethyl isoquinoline and NBS to generate 5-bromine- 7-trifluoromethyl isoquinoline, and carrying out methoxylation and demethylation with sodium methoxide to obtain 6-(trifluoromethyl) isoquinoline-5-alcohol. The method is simple in synthetic route, reasonable in process selection, low in raw material cost, simple and easily available in raw materials, convenient to operate and post-treat, high in total yield, free of highly toxic reagents and easy to amplify, and can be used for large-scale production.
Owner:SUZHOU KANGRUN PHARMA
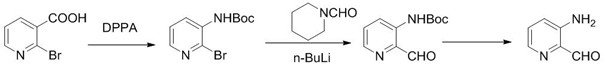
Synthesis method of 3-aminopyridine formaldehyde
The invention discloses a synthesis method of 3-aminopyridine formaldehyde, which comprises the following steps of: carrying out a cutius rearrangement reaction on 2-bromonicotinic acid serving as a raw material to obtain tert-butyl (2-bromopyridine-3-yl) carbamate, carrying out a Grignard reaction to obtain tert-butyl (2-formyl pyridine-3-yl) carbamate, and finally carrying out a hydrolysis reaction to obtain 3-aminopyridine formaldehyde. The synthesis method overcomes the defects of expensive raw materials, low yield, use of microwave reaction and other conditions, difficulty in amplification and the like in the existing synthesis process, and has the advantages of simple synthesis route, reasonable process selection, low raw material cost, simple and easily available raw materials, convenience in operation and post-treatment, high total yield, no use of highly toxic reagents, easiness in amplification, and realization of mass production.
Owner:SUZHOU KANGRUN PHARMA
Synthesis method of 3-aminoquinoline-5-carboxylic acid methyl ester
ActiveCN112142661AThe synthetic route is simpleProcess selection is reasonableOrganic chemistryBiochemical engineeringQuinoline
The invention discloses a synthesis method of 3-aminoquinoline-5-carboxylic acid methyl ester. The method comprises the following steps: carrying out a bromination reaction on 3-aminoquinoline servingas a raw material and bromine to obtain 3-amino-5-bromoquinoline, and carrying out a carbonyl insertion reaction to obtain the 3-aminoquinoline-5-carboxylic acid methyl ester. The method is simple insynthetic route, reasonable in process selection, simple and easily available in raw materials, convenient to operate and post-treat, relatively high in total yield and easy to amplify, and can be used for large-scale production.
Owner:SUZHOU KANGRUN PHARMA
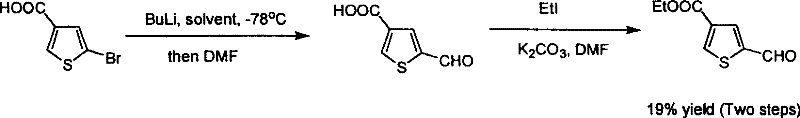
Industrialized preparation method for 5 - formoxyl - 3 - ester thiohenic acid
ActiveCN101092411BAvoid using effectsAvoid reactionOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSynthesis methodsPalladium catalyst
This invention relates to an industrial production method for 5-formyl-3-thiophenecarboxylate. The method comprises: performing carbonylation reaction on 4-bromo-thiophene-2-carbaldehyde and alcohol in the presence of Pd catalyst and reaction promoter to obtain 5-formyl-3-thiophenecarboxylate in one step. The method has such advantages as simple route, reasonable process, no need for organic metal reagent, no need for low-temperature reaction, and high synthesis efficiency, and is suitable for mass production. Pd catalyst can be recovered and recycled, which can reduce the cost. The method solves the problems of long synthesis route, dew raw materials, low reaction yield, rigorous reaction conditions and organic metal reagent faced by the present synthesis method.
Owner:SHANGHAI STA PHARMA R&D CO LTD
Synthesis method of 1-R-4-amino-1,2,3,4-tetrahydroquinoline
ActiveCN101429160BProcess selection is reasonableRaw materials are easy to obtainOrganic chemistryAlcoholSynthesis methods
The present invention relates to a method for synthesizing 1-R-4-amino-1, 2, 3, 4-tetrahydroquinoline, which mainly solves the technical problems that the prior synthesis method is difficult to amplify, long in route, inconvenient in reaction operation, and the like. The invention adopts a technical proposal that the method for synthesizing 1-R-4-amino-1, 2, 3, 4-tetrahydroquinoline is characterized by comprising the following steps: a. 4-hydroxyquinoline is taken as a raw material and reacts with alkyl halogenated matter or benzyl halogenated matter in solvent to form ketene; b. the ketene dissolves in the solvent, and a reducing agent is added to the solvent so as to obtain corresponding alcohol; c. corresponding alcohol of anhydrous tetrahydrofuran dissolved in the solvent is added to diphenoxyphosphinyl azide so as to be transformed into corresponding azide; and d. the obtained azide is dissolved in the solvent, and the 1-R-4-amino-1, 2, 3, 4-tetrahydroquinoline is obtained by a hydrogenation or adding triphenyl phosphine chemical reduction method.
Owner:上海药明康德新药开发有限公司
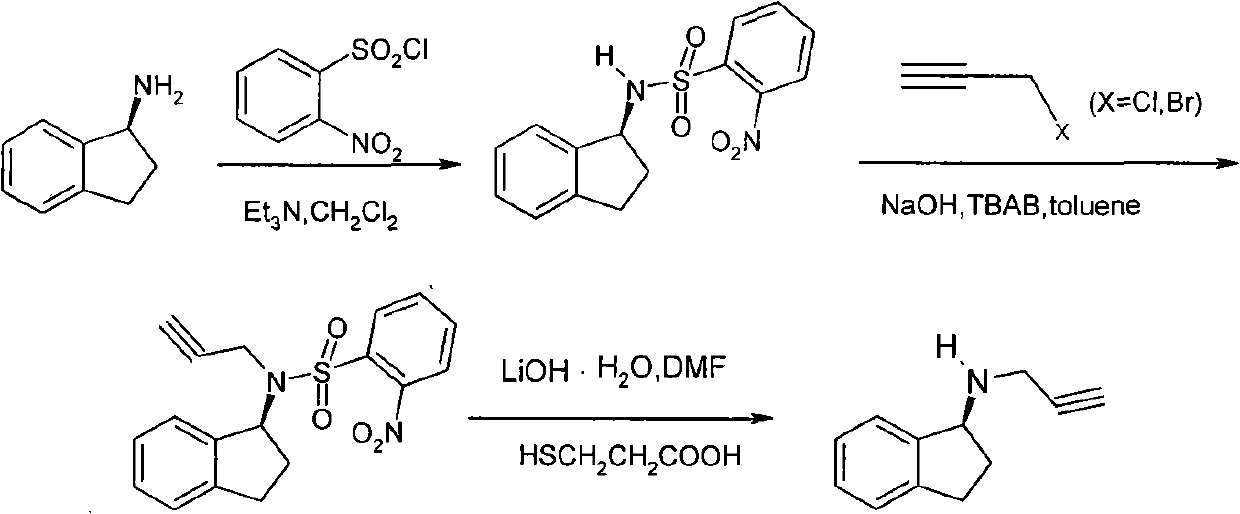
Method for preparing rasagiline mesylate
ActiveCN102010353BGood choiceHigh yieldOrganic compound preparationSulfonic acids salts preparationMedronic acidRasagiline Mesylate
The invention relates to a new preparation method for rasagiline mesylate for a Parkinson's disease resistant medicament, and belongs to the technical field of rasagiline mesylate preparation. The method comprises the following steps of: introducing a chiral auxiliary agent into 1-indenone (II) serving as a raw material, and performing reduction reaction to obtain a compound (III); then reacting the obtained compound (III) and 3-propiolic halide to obtain a compound (IV); and finally, dripping methylsulfonic acid into the obtained compound (IV) at a certain temperature in a certain solvent toobtain rasagiline mesylate (I). The method has the advantages of chiral synthesis, unique reaction route, good selectivity, high yield and a few reaction steps, and is simple and convenient to operate. The raw materials used for the reaction route are cheap and easily obtained, the cost is low, the optical purity is high, and the method is suitable for industrialized production. The method solvesthe problems of high cost due to the adoption of a key intermediate R-1-indamine, low reaction yield, long reaction steps and disadvantage on industrialized production in the conventional rasagiline mesylate synthesis process.
Owner:NENTER & CO
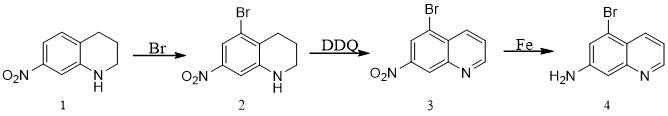
Synthesis method of 7-amino-5-bromoquinoline
ActiveCN112574106ALow costProcess selection is reasonableOrganic chemistryBiochemical engineeringQuinoline
The invention discloses a synthesis method of 7-amino-5-bromoquinoline, which comprises the following steps: carrying out a bromination reaction on 7-nitro-1, 2, 3, 4-tetrahydroquinoline serving as araw material to obtain 5-bromine-7-nitro-1, 2, 3, 4-tetrahydroquinoline, carrying out a dehydrogenation reaction on the 5-bromine- 7-nitro 1, 2, 3, 4-tetrahydroquinoline and DDQ to obtain 5-bromine-7-nitro quinoline, and carrying out iron powder reduction reaction to obtain 7-amino-5-bromoquinoline. The method provides a new synthetic route for synthesis of 7-amino-5-bromoquinoline, and the synthetic route is simple, reasonable in process selection, low in raw material cost, simple and easily available in raw materials, convenient to operate and post-treat, high in total yield, free of highlytoxic reagents, easy to amplify and suitable for large-scale production.
Owner:SUZHOU KANGRUN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com