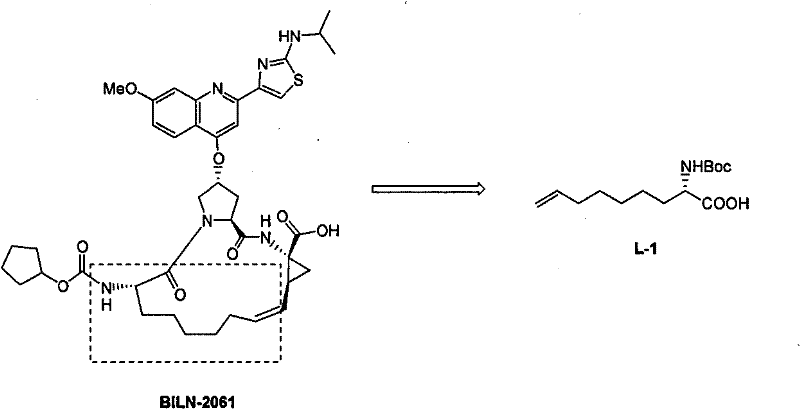
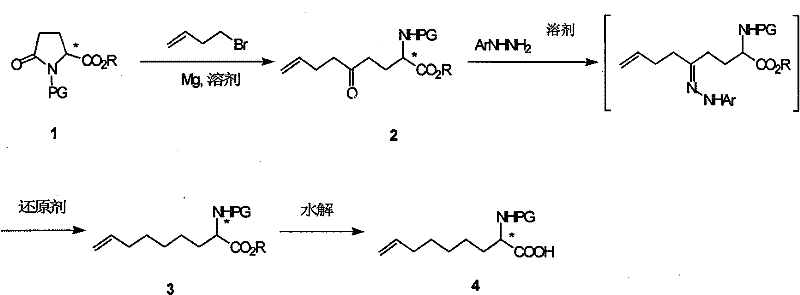
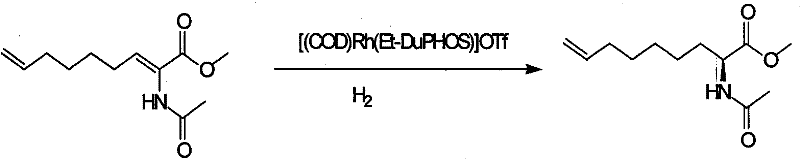Method for practical synthesizing optically active 2 - amido - 8 - butenic acid
A synthetic method and optically active technology, which is applied in chemical instruments and methods, organic chemistry, bulk chemical production, etc., can solve the problems of use price and high cost, and achieve the effects of reducing costs, improving synthesis efficiency, and succinct synthesis routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Synthesis of (S)-N-tert-butoxycarbonyl-2-amino-8-nonenoic acid
[0025] The first step: the synthesis of (S)-N-tert-butoxycarbonyl-2-amino-5-oxo-8-nonenoic acid ethyl ester
[0026]
[0027] In a 3L three-neck flask equipped with a spherical condenser and a dropping funnel, add magnesium chips (51g, 2.09mol) and a small amount of iodine particles, and heat under a nitrogen stream to drive away the humid air. After 10 minutes, the system is sealed and protected by N2. Then inject anhydrous tetrahydrofuran solution (1.5L) of 1-butyl bromide (177g, 1.31mol) into the dropping funnel, and add 100mL solution at a time, after the reaction is triggered, slowly add it again, keep the reaction temperature at 60- between 70°C. After the dropwise addition, wait for the temperature of the reaction solution to drop to room temperature, and heat it with a hot air blower for half an hour to make the reaction fully. A solution of (S)-N-tert-butoxycarbonyl-pyroglutamic acid ethyl es...
Embodiment 2
[0035] Synthesis of (R)-N-tert-butoxycarbonyl-2-amino-8-nonenoic acid
[0036] The first step: the synthesis of (R)-N-tert-butoxycarbonyl-2-amino-5-oxo-8-nonenoic acid ethyl ester
[0037]
[0038] In a 3L three-neck flask equipped with a spherical condenser and a dropping funnel, add magnesium chips (3.6g, 0.15mol) and a small amount of iodine particles, heat under a nitrogen stream to drive off the humid air, and after 10 minutes, seal the system and protect it with nitrogen. , then inject anhydrous tetrahydrofuran solution (150mL) of 1-butyl bromide (13.5g, 1.00mol) into the dropping funnel, and add 100mL solution at a time, after the reaction triggers, slowly add it again, keeping the reaction temperature at 60 Between -70°C. After the dropwise addition, wait for the temperature of the reaction solution to drop to room temperature, and heat it with a hot air blower for half an hour to make the reaction fully. A solution of (R)-N-tert-butoxycarbonyl-pyroglutamic acid eth...
Embodiment 3
[0043] Synthesis of (S)-N-tert-butoxycarbonyl-2-amino-8-nonenoic acid ethyl ester
[0044]
[0045] Add p-toluenesulfonylhydrazide (20.5g, 0.11mol) to (S)-N-tert-butoxycarbonyl-2-amino-5-oxo-8-nonenoic acid ethyl ester (31.3g, 0.10mol) in acetic acid ( 400 mL) solution, stirred at room temperature for one hour, then added sodium borohydride (19 g, 0.50 mol) in batches, and continued to stir for 12-24 hours after the addition was complete. After the reaction was detected by TLC, it was quenched by adding water and extracted with ethyl acetate. The organic phases were combined, washed successively with saturated aqueous sodium bicarbonate solution and saturated saline solution, filtered, the filtrate was concentrated under reduced pressure, and the crude product was purified by column chromatography to obtain (S)-N-tert-butoxycarbonyl-2-amino-8-nonene Acetate ethyl ester (16.8 g, 56% yield). MS(E / Z): 300(M+H + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com