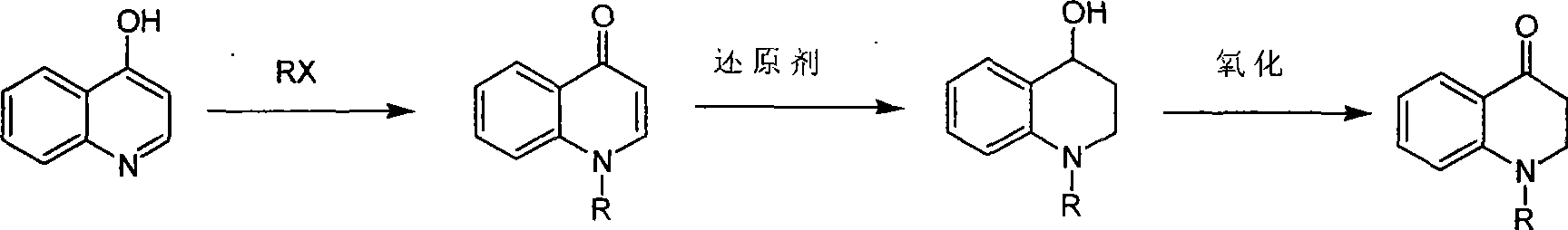
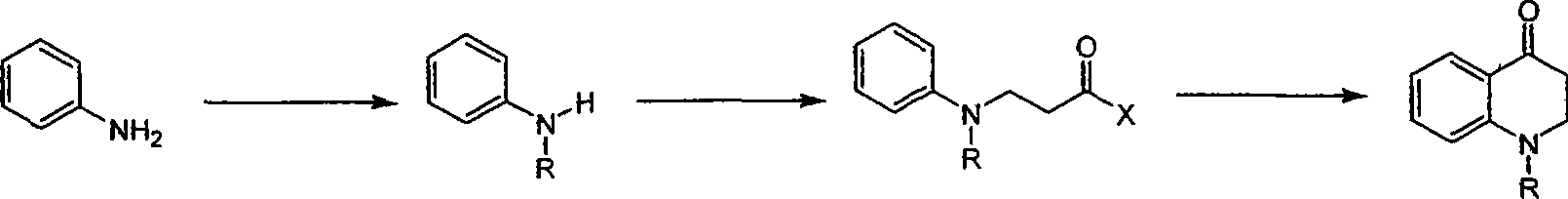
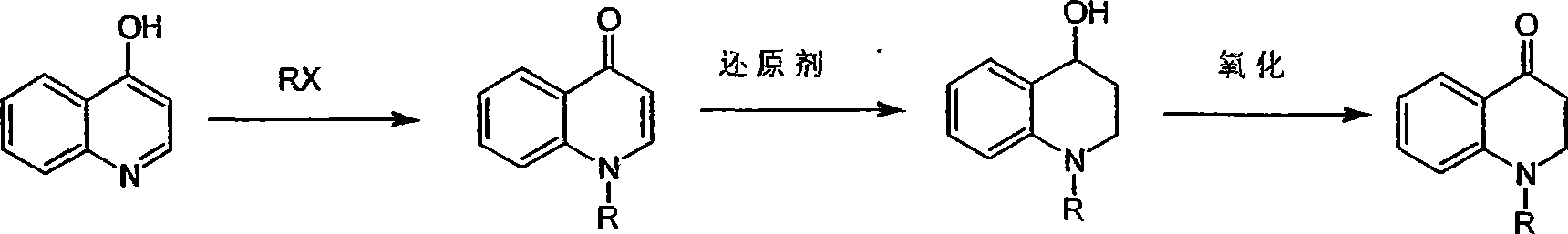
Synthesis of 1-R-2,3-dihydrogen-1H-quinoline-4-ketone
A synthesis method and 1-R-2 technology, applied in organic chemistry and other directions, can solve the problems of inconvenient reaction operation, difficult amplification of raw materials, long route, etc., and achieve the effects of simple raw materials, easy amplification and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012]
[0013] 1. Synthesis of 1-methyl-1H-quinolin-4-one
[0014] At room temperature, iodomethane (11.8g, 82.8mmol) was slowly added dropwise to 4-hydroxyquinoline (4g, 27.6mmol) and K 2 CO 3 (7.6g, 55.2mmol) in acetonitrile solution. After dropping, heat to reflux overnight. Then the reaction solution was spin-dried, and 200 mL of ethyl acetate was added to the residue, stirred for 0.5 hours and filtered, and the filtrate was spin-dried to obtain 1-methyl-1H-quinolin-4-one (2.8 g, yield 64%).
[0015] 1 H-NMR (400MHz, DMSO): δ 8.14(d, J=7.6Hz, 1H), 7.94(d, J=7.6Hz, 1H), 7.72(t, J=13.2Hz, 1H), 7.63(d, J=8.4Hz, 1H), 7.37(t, J=14.4Hz, 1H), 6.01(d, J=11.6Hz, 2H), 3.78(s, 3H).
[0016] 2. Synthesis of 1-methyl-4-hydroxy-1,2,3,4-tetrahydroquinoline
[0017] Dissolve 1-methyl-1H-quinolin-4-one (1g, 6.3mmol) in methanol, add NaBH under ice-bath cooling 4 (1.9 g, 50.3 mmol), then stirred overnight at room temperature. After the reaction solution was quenched with ice wat...
Embodiment 2
[0023]
[0024] 1. Synthesis of 1-ethyl-1H-quinolin-4-one
[0025] At room temperature, iodoethane (12.9g, 82.8mmol) was slowly added dropwise to 4-hydroxyquinoline (4g, 27.6mmol) and K 2 CO 3 (7.6g, 55.2mmol) in ethanol solution. After dropping, heat to reflux overnight. Then the reaction solution was spin-dried, and 200 mL of ethyl acetate was added to the residue, stirred for 0.5 hours and filtered, and the filtrate was spin-dried to obtain 1-ethyl-1H-quinolin-4-one (2.86 g, yield 60%).
[0026] 1 H-NMR (400MHz, DMSO): δ 8.17 (d, J = 8Hz, 1H), 7.99 (d, J = 7.6Hz, 1H), 7.72 (m, J = 3.6Hz, 2H), 7.36 (m, J =15.6Hz, 1H), 6.04(d, J=7.6Hz, 1H), 4.26(q, J=21.2Hz, 2H), 1.32(t, 3H).2.1-ethyl-4-hydroxyl-1,2 , the synthesis of 3,4-tetrahydroquinoline
[0027] Dissolve 1-ethyl-1H-quinolin-4-one (1g, 5.8mmol) in ethanol, add KBH under ice-bath cooling 4 (2.49g, 46.2mmol), then stirred overnight at room temperature. After the reaction solution was quenched with ice water, the ...
Embodiment 3
[0033]
[0034] 1. Synthesis of 1-benzyl-1H-quinolin-4-one
[0035] At room temperature, benzyl bromide (7g, 41.3mmol) was slowly added dropwise to 4-hydroxyquinoline (2g, 13.8mmol) and K 2 CO 3(3.8g, 27.6mmol) in tetrahydrofuran solution. After dropping, heat to reflux overnight. Then the reaction solution was spin-dried, and 200 mL of ethyl acetate was added to the residue, stirred for 0.5 hours and filtered, and the filtrate was spin-dried to obtain 1-benzyl-1H-quinolin-4-one (1.0 g, yield 31%).
[0036] 1 H-NMR (400MHz, DMSO): δ 8.21(q, J=41.6Hz, 2H), 7.59(m, J=31.2Hz, 2H), 7.35-7.20(m, 6H), 6.14(d, J=7.6 Hz, 1H), 5.52(s, 2H).
[0037] 2. Synthesis of 1-benzyl-4-hydroxy-1,2,3,4-tetrahydroquinoline
[0038] 1-Benzyl-1H-quinolin-4-one (1 g, 4.3 mmol) was dissolved in anhydrous THF, and lithium aluminum hydride (0.65 g, 17 mmol) was added under ice-cooling, followed by stirring overnight at room temperature. After the reaction solution was quenched with ice water, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com