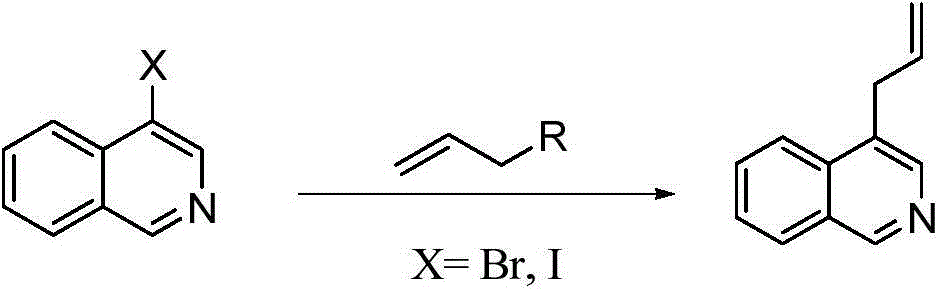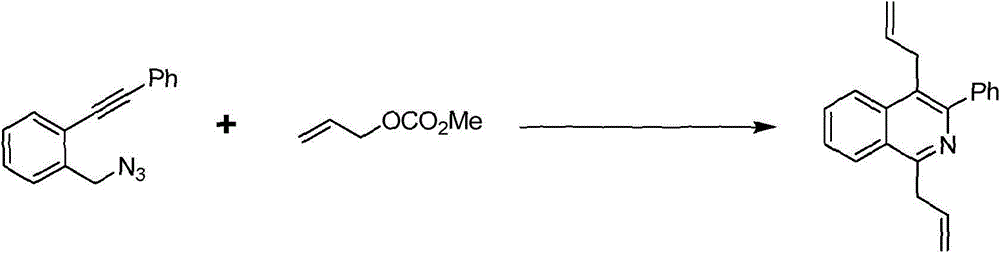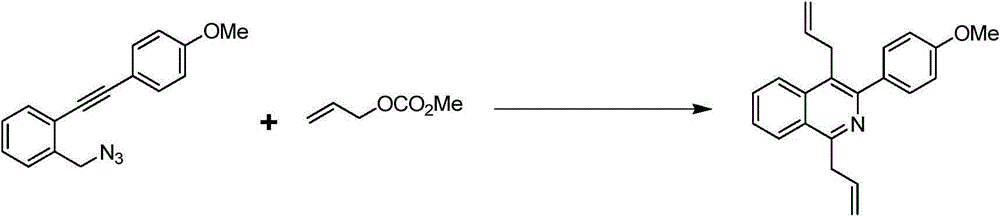Preparation method of 1,4-diallyl isoquinoline
A technology of diallylisoquinoline and cyclohexenyl, which is applied in the field of 1, can solve the problems of single starting material and application limitation, and achieve the effects of short reaction time, wide range and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 1, the synthesis of 4-diallyl-3-phenylisoquinoline
[0019] The synthetic route of 1,4-diallyl-3-phenylisoquinoline is shown in the following formula:
[0020]
[0021] At room temperature, 1-(azidomethyl)-2-(phenylethynyl)benzene (11.7mg, 0.05mmol), potassium phosphate (53.1mg, 0.25mmol), Pd(PPh 3 ) 4 (2.9mg, 0.0025mmol) was put into a 5ml reaction kettle with magnetic stirring function. After completion, fill the reactor with argon and cover it with a lid. DMF (1 ml) and allyl methcarbonate (28.4 μL, 0.25 mmol) were sequentially injected into the autoclave using a syringe. The reaction kettle was stirred at room temperature for ten minutes, then heated to 100°C and continued to stir, and was taken out after 4 hours. After-treatment of the reactant, first pass the solid-liquid mixture through the column to obtain the liquid, then use water and ethyl acetate to extract the obtained liquid, combine the organic layers, dry and spin dry, and the residu...
Embodiment 2
[0023] Example 2 Synthesis of 1,4-diallyl-3-p-methoxyphenylisoquinoline
[0024] The synthetic route of 1,4-diallyl-3-p-methoxyphenylisoquinoline is shown in the following formula:
[0025]
[0026] At room temperature, 1-(azidomethyl)-2-((4-methoxyphenyl)ethynyl)benzene (13.2mg, 0.05mmol), potassium phosphate (53.1mg, 0.25mmol), Pd(PPh 3 ) 4 (3.8mg, 0.0033mmol) was put into a 5ml reaction kettle with magnetic stirring function. After completion, fill the reactor with argon and cover it with a lid. DMF (1 ml) and allyl methcarbonate (28.4 μL, 0.25 mmol) were sequentially injected into the autoclave using a syringe. The reaction kettle was stirred at room temperature for ten minutes, then heated to 100°C and continued to stir, and was taken out after 4 hours. After-treatment of the reactant, first pass the solid-liquid mixture through the column to obtain the liquid, then use water and ethyl acetate to extract the obtained liquid, combine the organic layers, dry and sp...
Embodiment 3
[0028] Example 3 Synthesis of 1,4-diallyl-7-chloro-3-phenylisoquinoline
[0029] The synthetic route of 1,4-diallyl-7-chloro-3-phenylisoquinoline is shown in the following formula:
[0030]
[0031] At room temperature, 2-(azidomethyl)-4-chloro-1-(phenylethynyl)benzene (12.4mg, 0.05mmol), potassium phosphate (53.1mg, 0.25mmol), Pd(PPh 3 ) 4 (2.9mg, 0.0025mmol) was put into a 5ml reaction kettle with magnetic stirring function. After completion, fill the reactor with argon and cover it with a lid. DMF (1 ml) and allyl methcarbonate (28.4 μL, 0.25 mmol) were sequentially injected into the autoclave using a syringe. The reaction kettle was stirred at room temperature for ten minutes, then heated to 100°C and continued to stir, and was taken out after 4 hours. After-treatment of the reactant, first pass the solid-liquid mixture through the column to obtain the liquid, then use water and ethyl acetate to extract the obtained liquid, combine the organic layers, dry and spin d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com