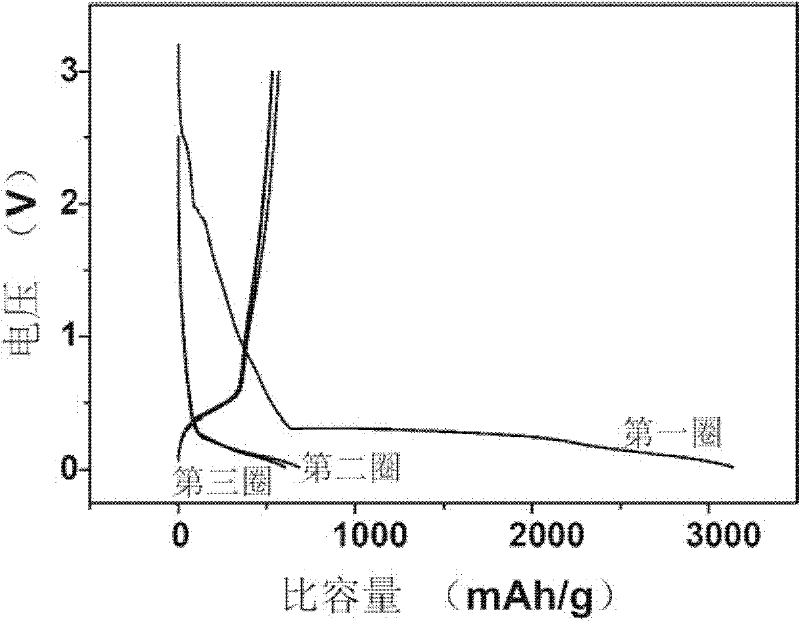
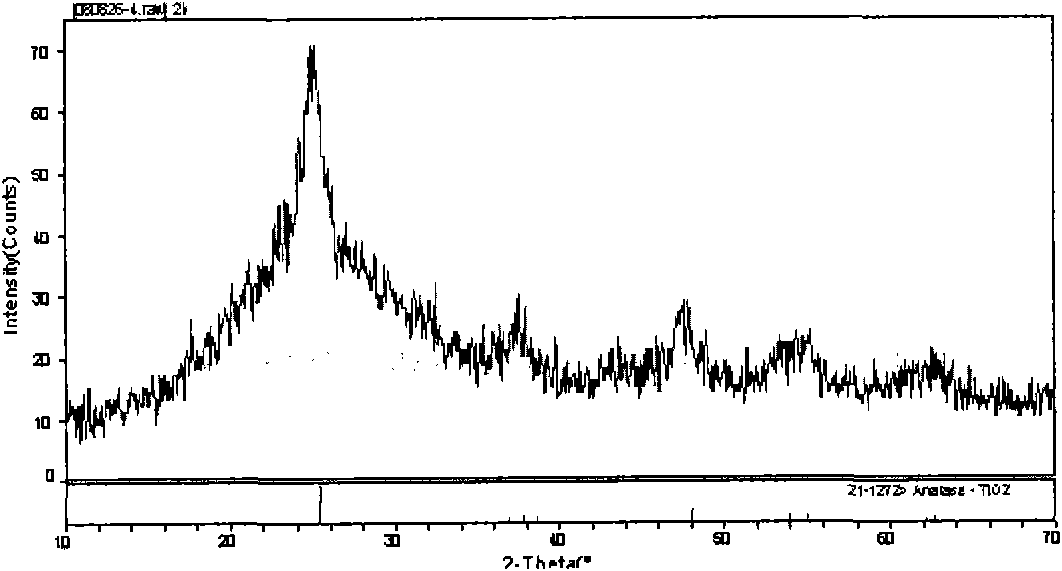
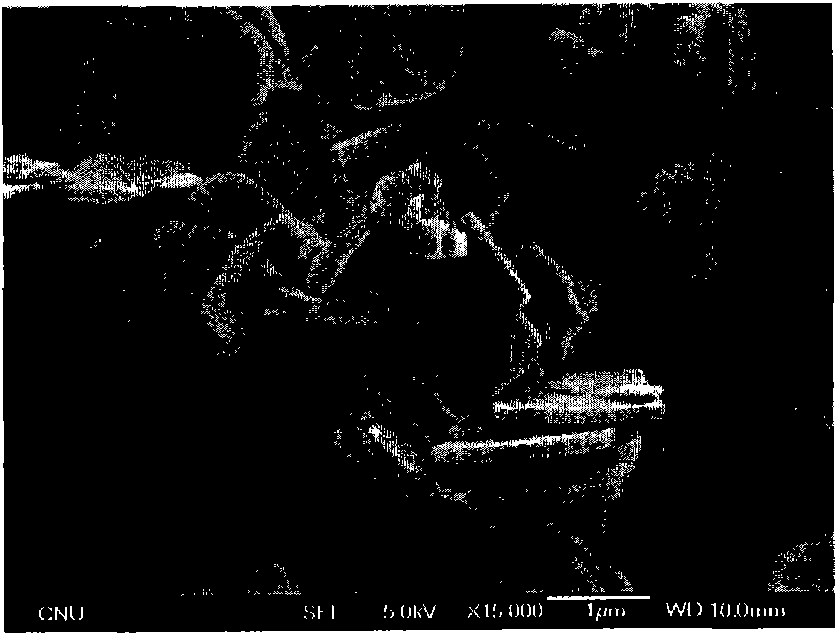
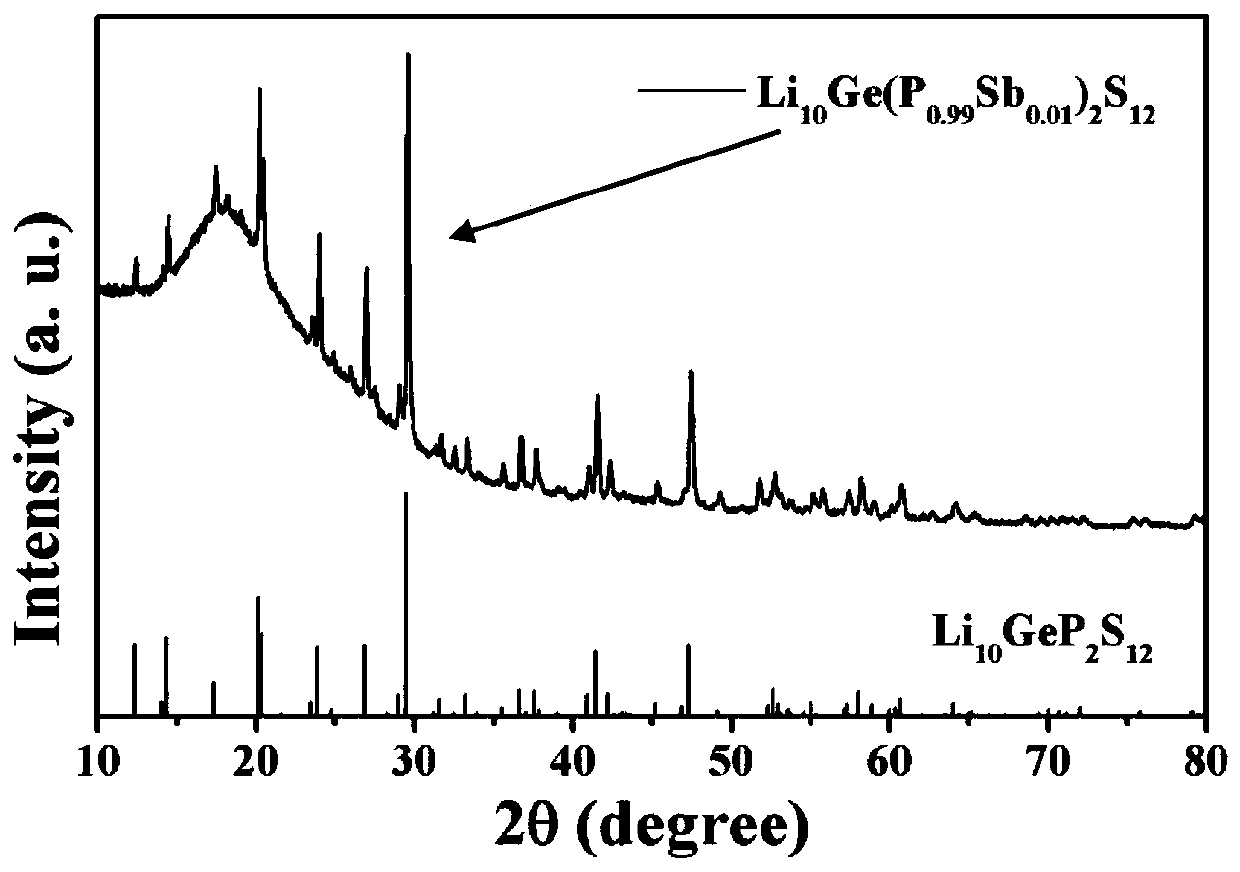
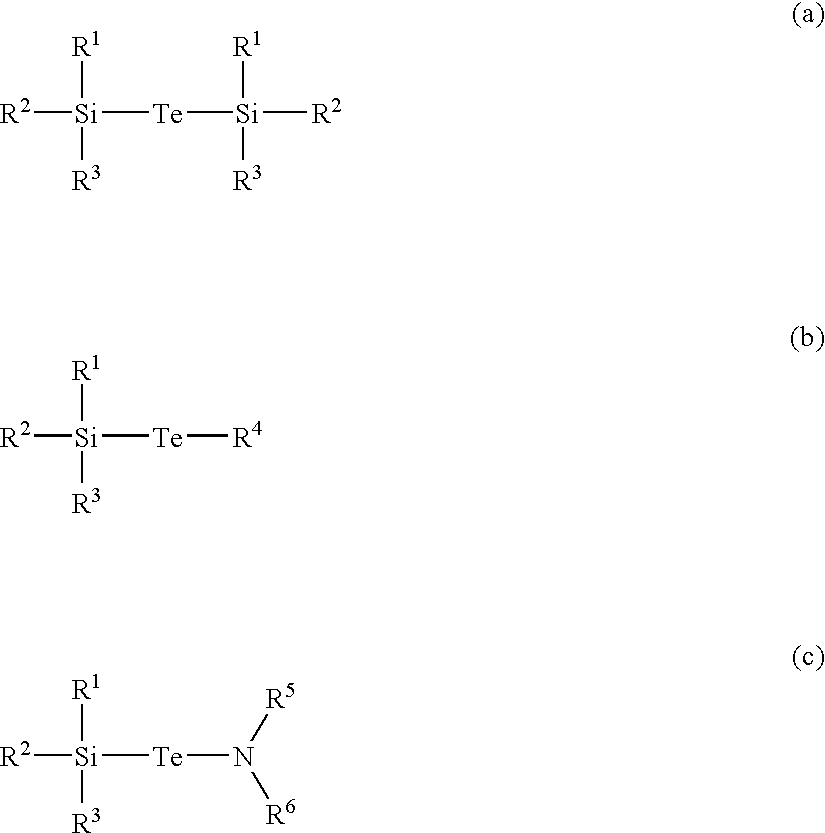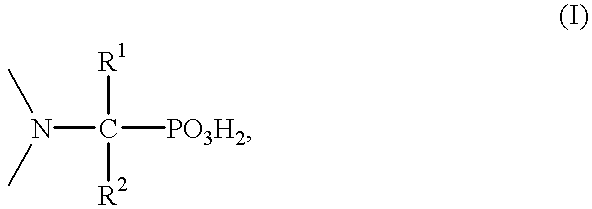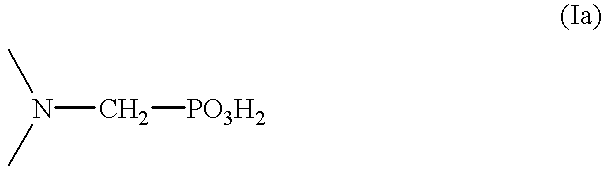Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
257results about "Germanium compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
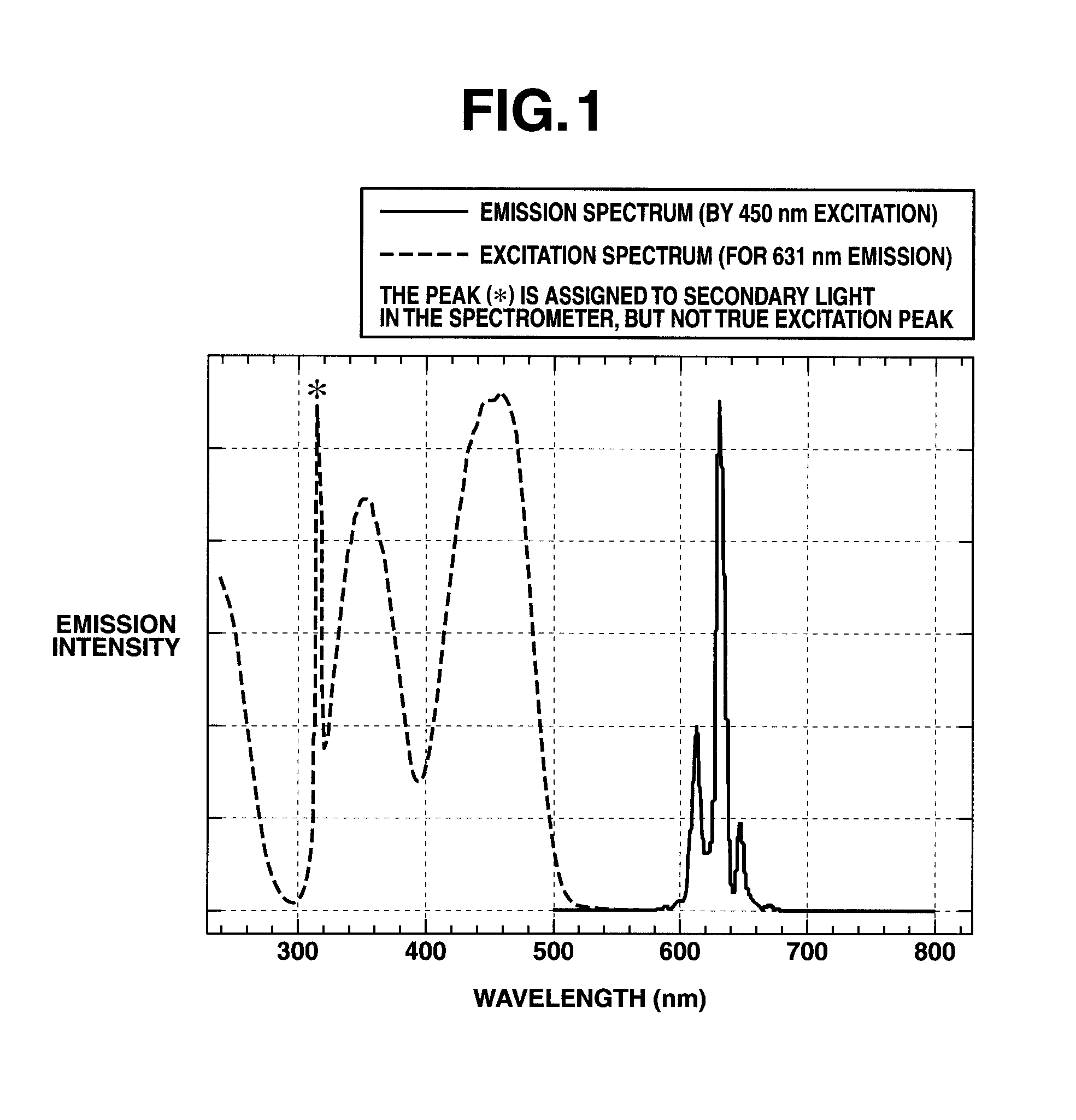
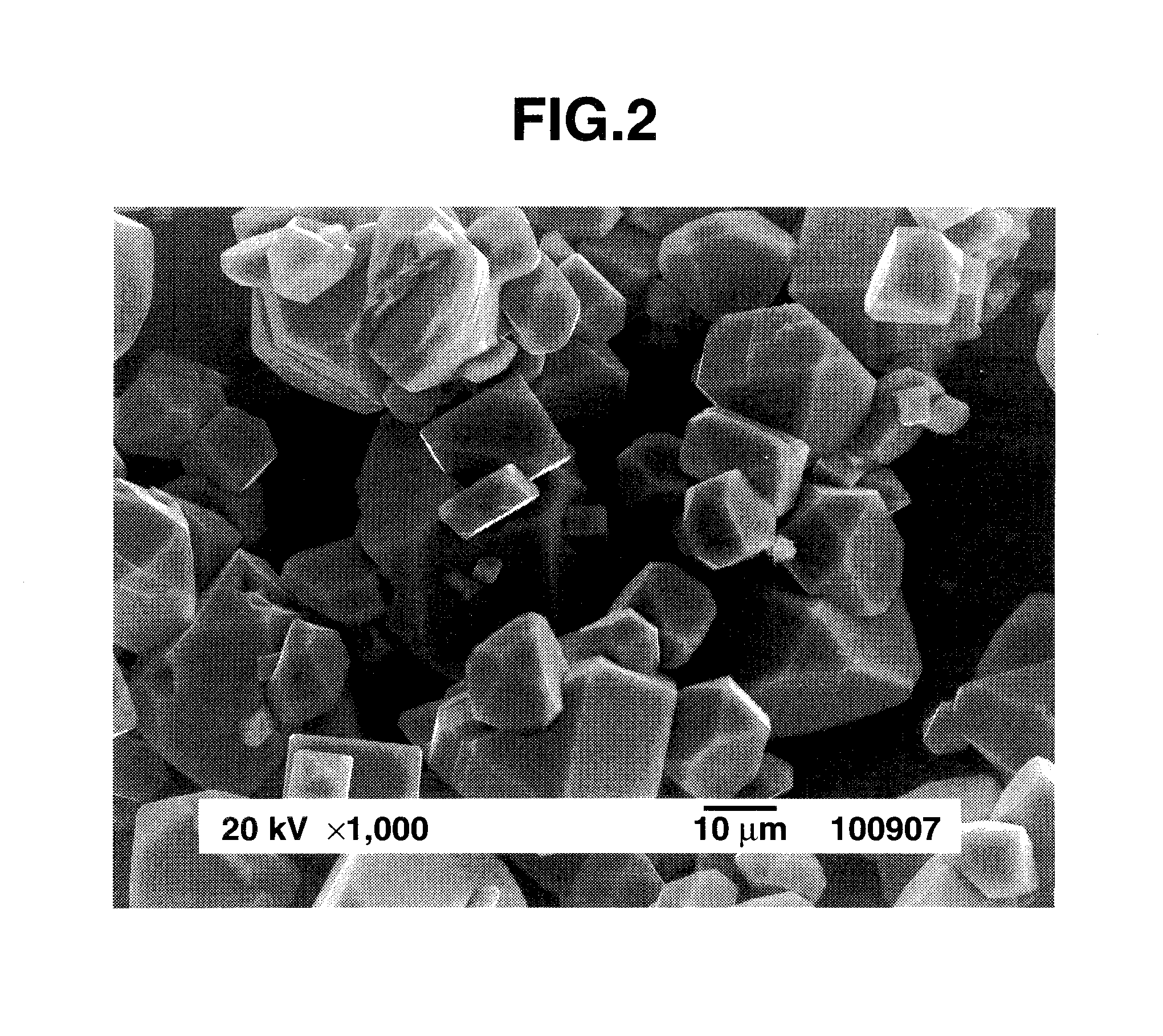
Preparation of complex fluoride and complex fluoride phosphor
ActiveUS20120256125A1Uniform sizeSatisfactory emissive propertyTin compoundsSilicon halogen compoundsPhysical chemistryFluoride
A complex fluoride A2MF6 wherein M is a tetravalent element Si, Ti, Zr, Hf, Ge or Sn, A is an alkali metal Li, Na, K, Rb or Cs is prepared by providing a first solution containing a fluoride of M, providing a second solution containing a compound of A and / or the compound of A in solid form, mixing the first solution with the second solution and / or the solid for reacting the fluoride of M with the compound of A, and recovering the resulting solid product via solid-liquid separation.
Owner:SHIN ETSU CHEM IND CO LTD
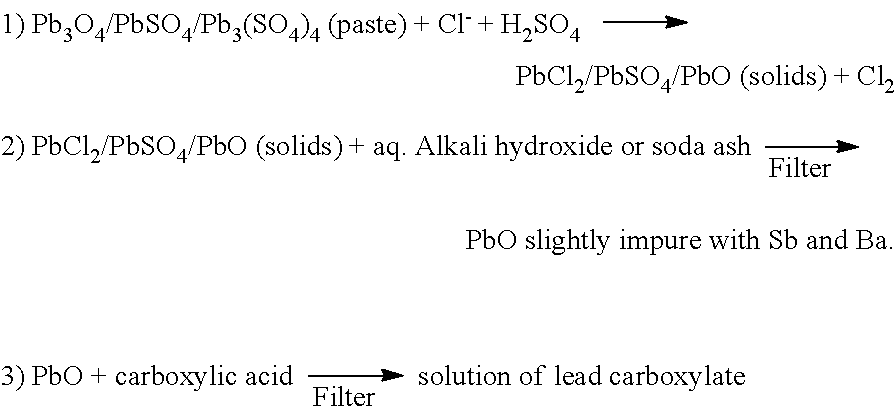
Process for recovering lead oxides from exhausted batteries
ActiveUS7507496B1Speed up the processObtained inexpensivelySolvent extractionPrimary cell maintainance/servicingLead dioxideLead oxide
A process for recovering lead oxides from the spent paste of exhausted lead acid batteries. The process provides heating the spent paste with an alkali hydroxide solution at elevated temperatures prior to calcinations. Calcination is at various temperatures so that either lead mono-oxide, lead dioxide or red lead is obtained as the principal product. There is also provided the use of the lead oxide to prepare the paste for positive and negative electrodes or other lead compounds.
Owner:RETRIEV TECH +1
Stabilization method for lead projectile impact area
Owner:FORRESTER KEITH E
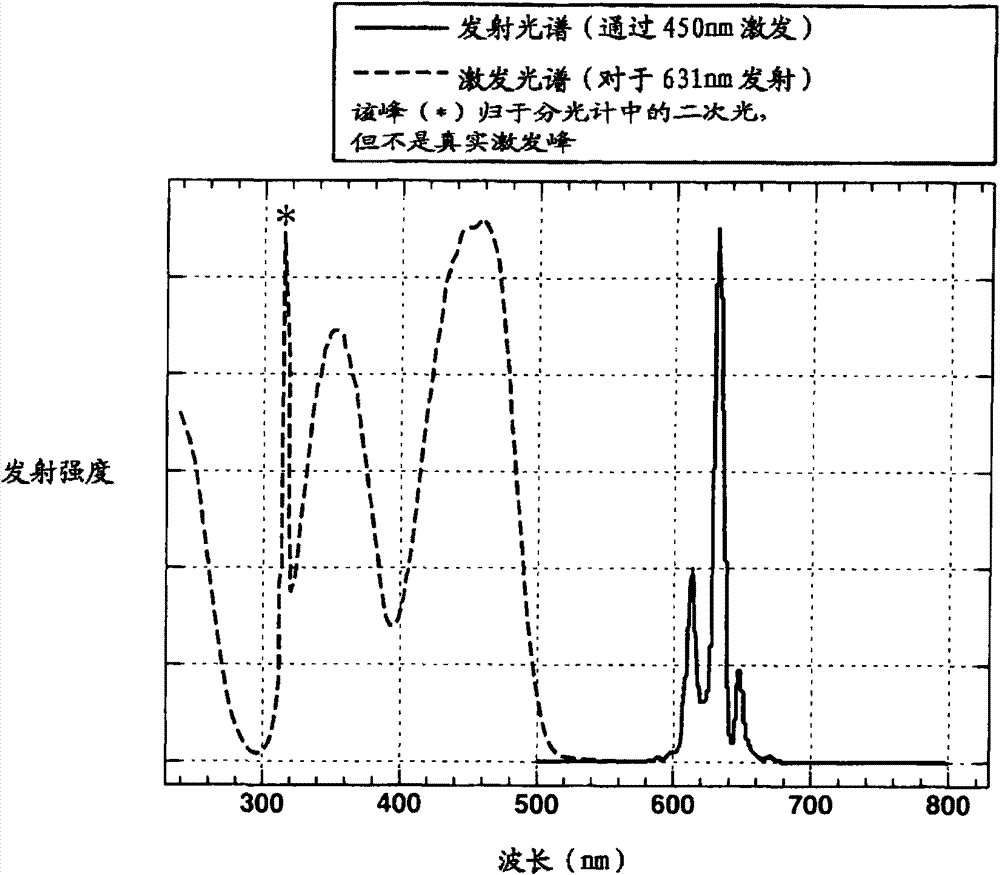
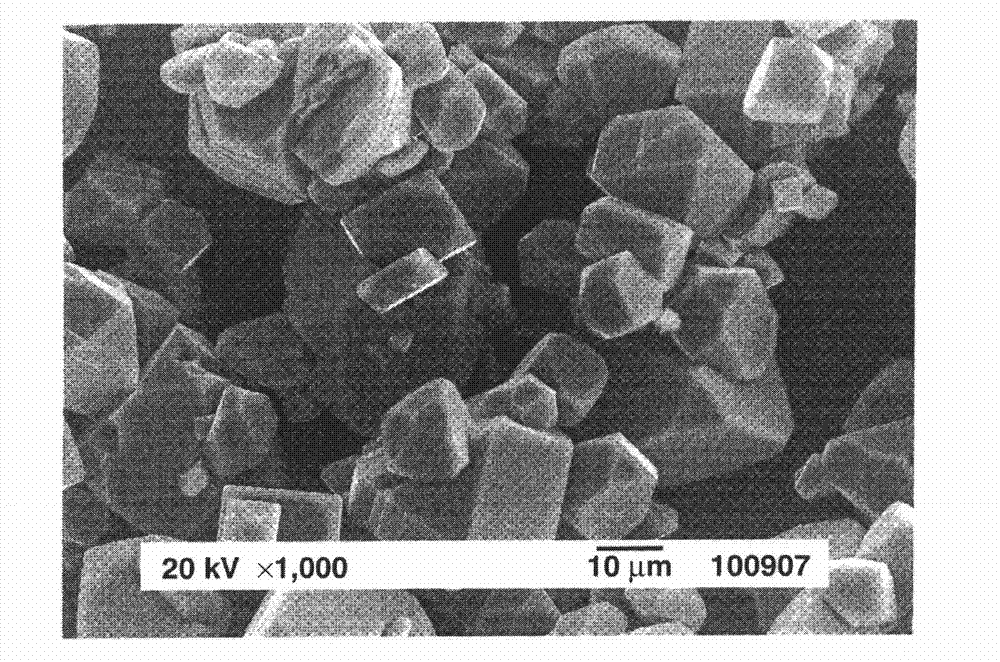
Preparation of complex fluoride and complex fluoride phosphor
ActiveCN102732249AHigh yieldSatisfied with launch performanceTin compoundsFluoride preparationPhosphorFluoride
A complex fluoride A 2 MF 6 wherein M is a tetravalent element Si, Ti, Zr, Hf, Ge or Sn, A is an alkali metal Li, Na, K, Rb or Cs is prepared by providing a first solution containing a fluoride of M, providing a second solution containing a compound of A and / or the compound of A in solid form, mixing the first solution with the second solution and / or the solid for reacting the fluoride of M with the compound of A, and recovering the resulting solid product via solid-liquid separation.
Owner:SHIN ETSU CHEM CO LTD
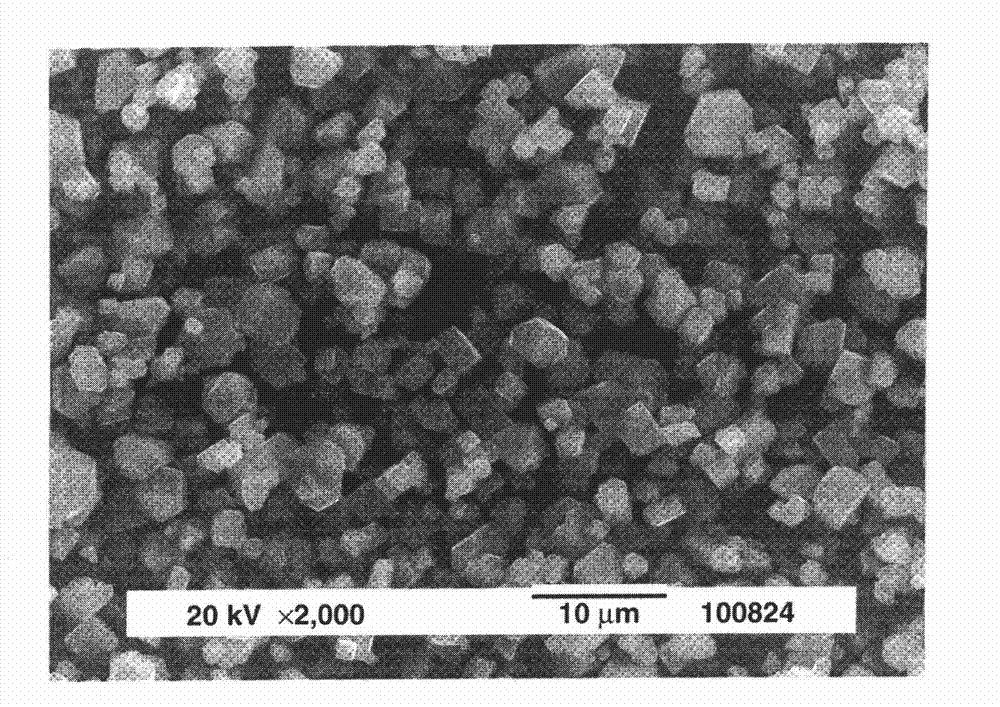
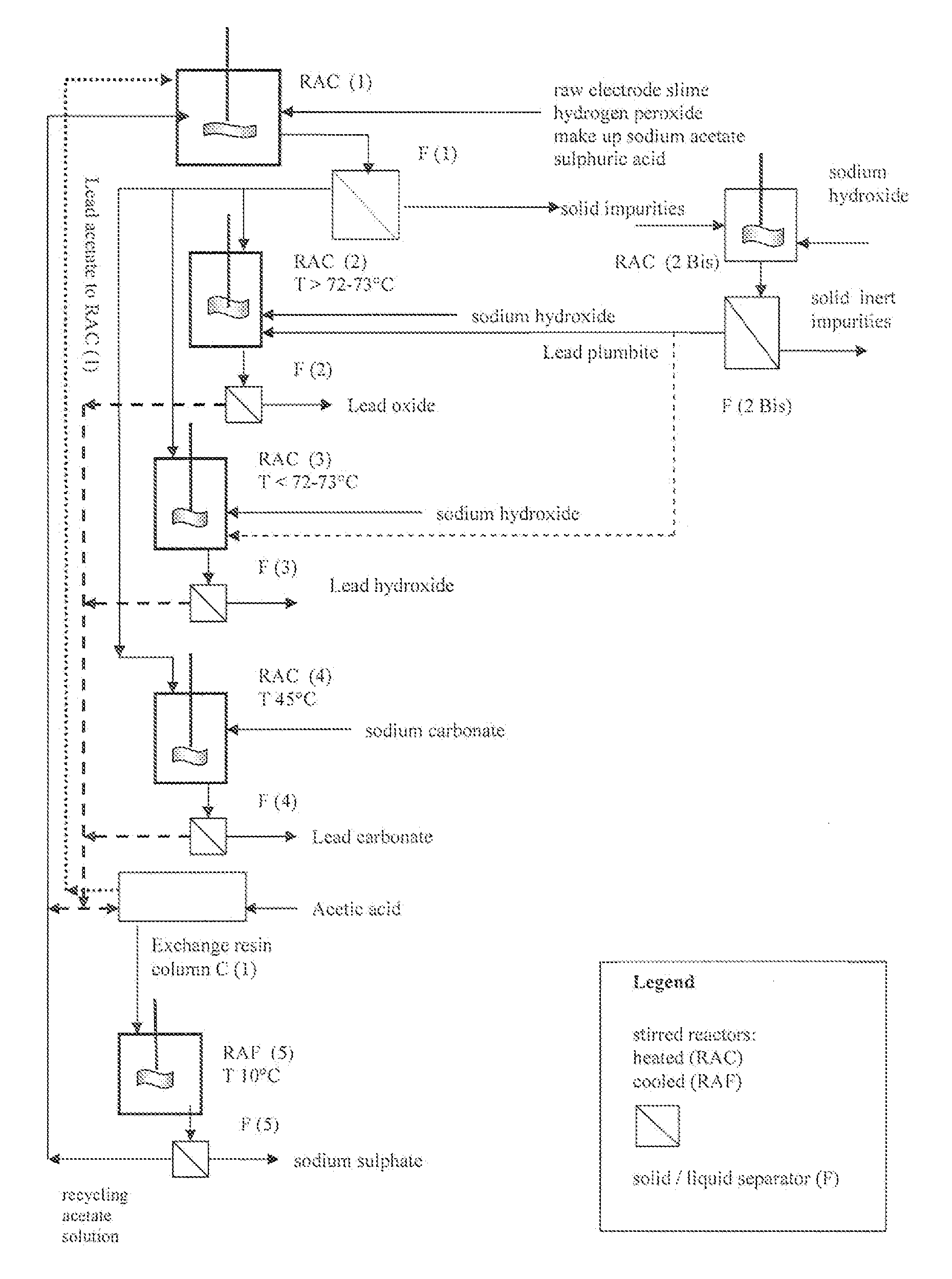
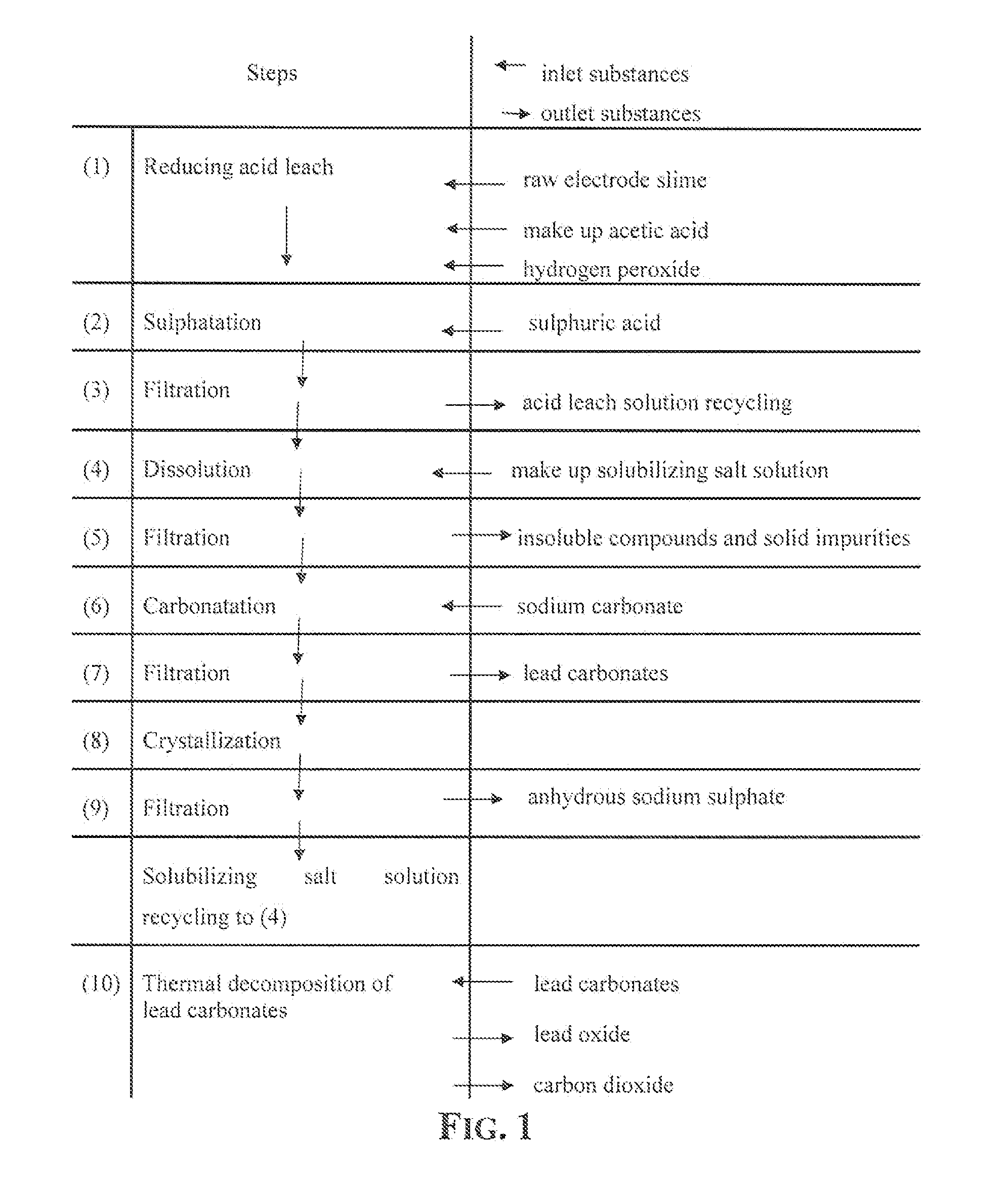
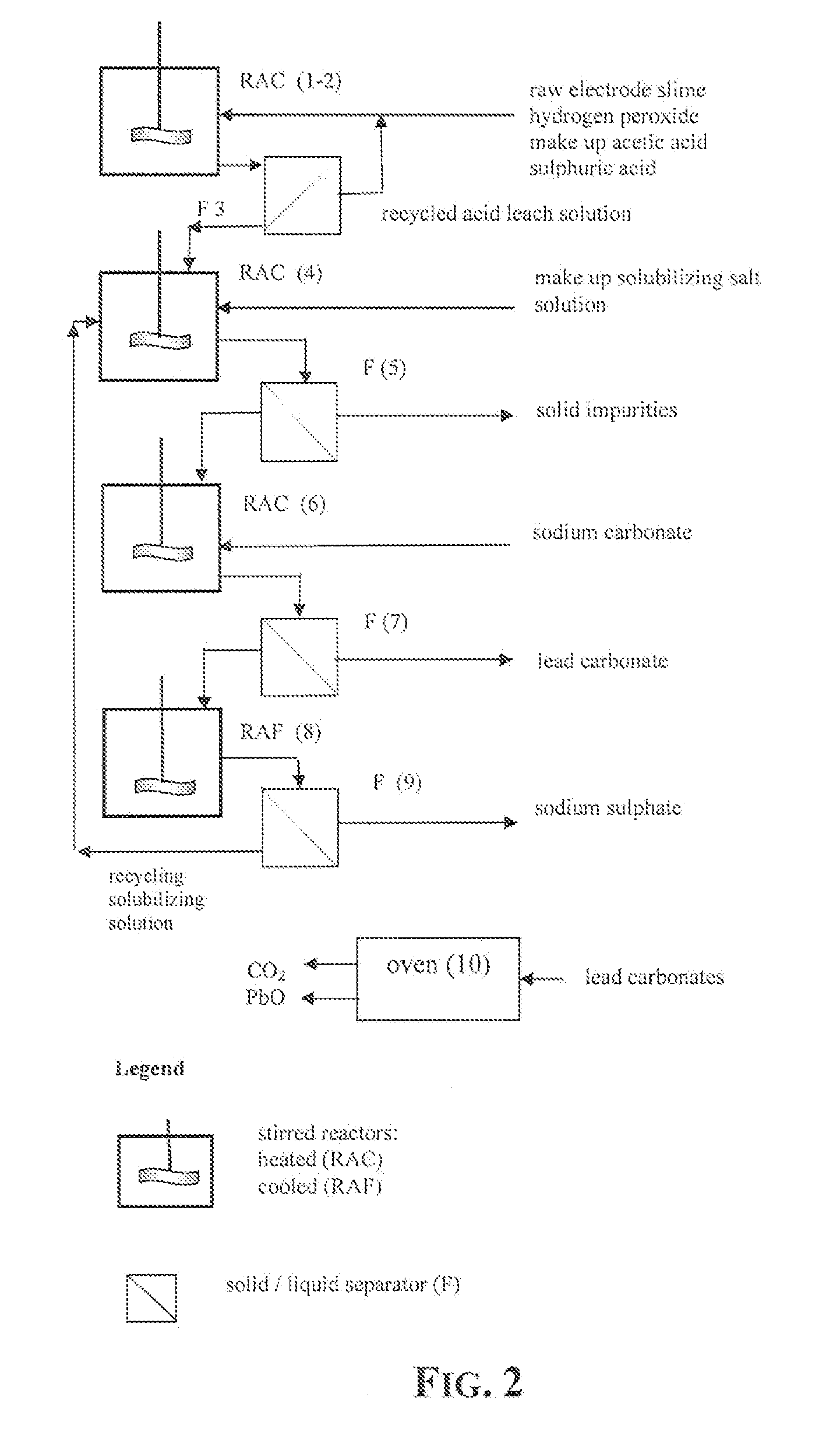
Reclaiming of lead in form of high purity lead compound from recovered electrode paste slime of dismissed lead batteries and/or of lead minerals
An outstandingly low environmental impact wet process recovers the lead content of an electrode slime and / or of lead minerals in the valuable form of high purity lead oxide or compound convertible to highly pure lead oxide by heat treatment in oven at relatively low temperature, perfectly suited for making active electrode pastes of new batteries or other uses. The process basically comprises the following treatments:a) suspending the impure lead containing material in an aqueous bath containing at least a lead oxide dissolving acid;b) reducing any insoluble lead dioxide to lead oxide by introducing in the suspension either hydrogen peroxide, a sulphite or sulphurous anhydride;c) converting all dissolved lead oxide to lead sulphate in the aqueous bath;d) obtaining a solution of lead sulphate obtained in an aqueous solution containing an acetate salt;e) precipitating and separating a purified lead compound in the form of either carbonate / oxycarbonate or of oxide / or hydroxide by adding to said acetate salt solution a carbonate salt or a hydroxide of the same cation of said acetate salt, respectively.Exemplary flow sheets according to several alternative embodiments and related processing plant diagrams are disclosed.
Owner:MILLBROOK LEAD RECYCLING TECH
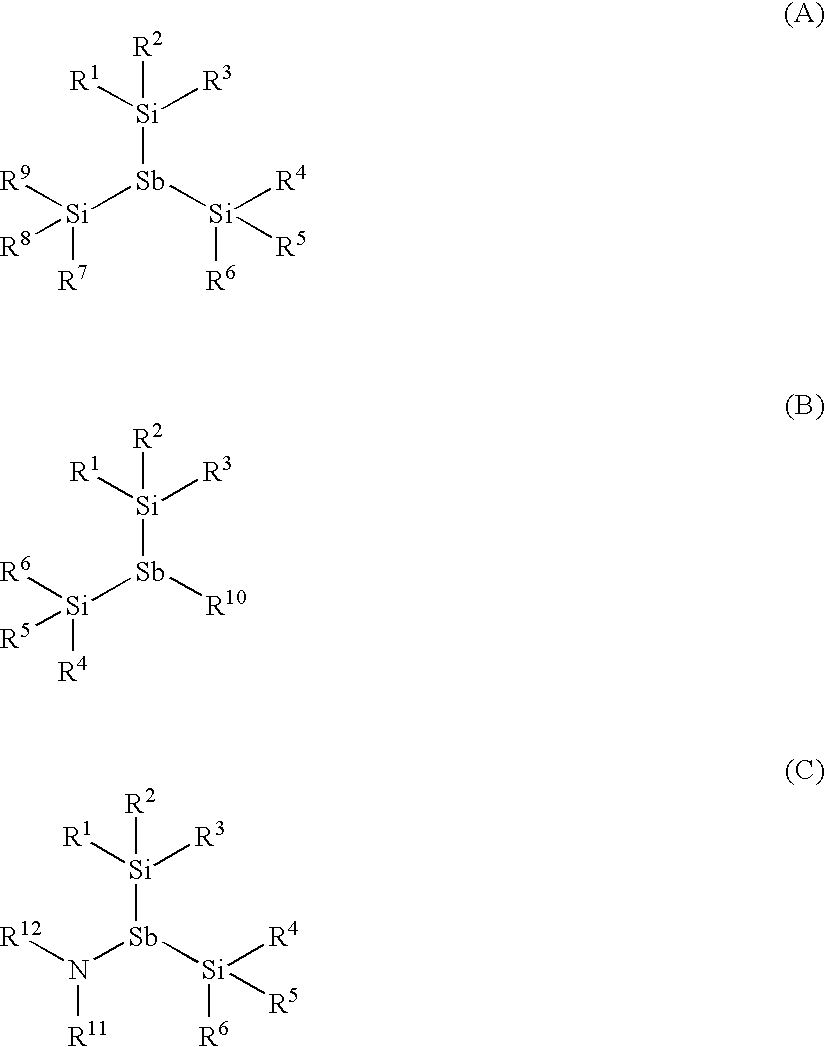
Antimony Precursors for GST Films in ALD/CVD Processes
The present invention is a process of making a germanium-antimony-tellurium alloy film using a process selected from the group consisting of atomic layer deposition and chemical vapor deposition, wherein a silylantimony precursor is used as a source of antimony for the alloy film. Novel silylantimony compounds are also disclosed.
Owner:VERSUM MATERIALS US LLC
Metal oxide nanostructures with hierarchical morphology
InactiveUS7294417B2Reduce synthetic pressureStable emissionsMaterial nanotechnologyOxide/hydroxide preparationNanostructureMetal
The present invention relates generally to metal oxide materials with varied symmetrical nanostructure morphologies. In particular, the present invention provides metal oxide materials comprising one or more metallic oxides with three-dimensionally ordered nanostructural morphologies, including hierarchical morphologies. The present invention also provides methods for producing such metal oxide materials.
Owner:TRUSTEES OF BOSTON COLLEGE THE
Recovery of high purity lead oxide from lead acid battery paste
There is provided a process for recovering high purity litharge PbO from spent lead acid battery paste at low temperatures and the further preparation of highly pure lead oxides and Pb(OH)2.
Owner:RETRIEV TECH
Electrode active material for secondary battery and method for preparing the same
ActiveUS20110311875A1Minimizing dead volumeHigh-capacity batteryMaterial nanotechnologyGallium/indium/thallium compoundsParticulatesLithium oxide
The disclosure relates to an electrode active material including: (a) first particulate of a metal (or metalloid) oxide alloyable with lithium; and (b) second particulate of an oxide containing lithium and the same metal (or metalloid) as that of the metal (or metalloid) oxide, and to a secondary battery including the electrode active material. When the electrode active material is used as an anode active material, reduced amounts of an irreversible phase such as a lithium oxide or a lithium metal oxide are produced during initial charge-discharge of a battery since lithium is already contained in the second particulate before the initial charge-discharge, and thus a dead volume on the side of the cathode can be minimized and a high-capacity battery can be fabricated.
Owner:LG ENERGY SOLUTION LTD
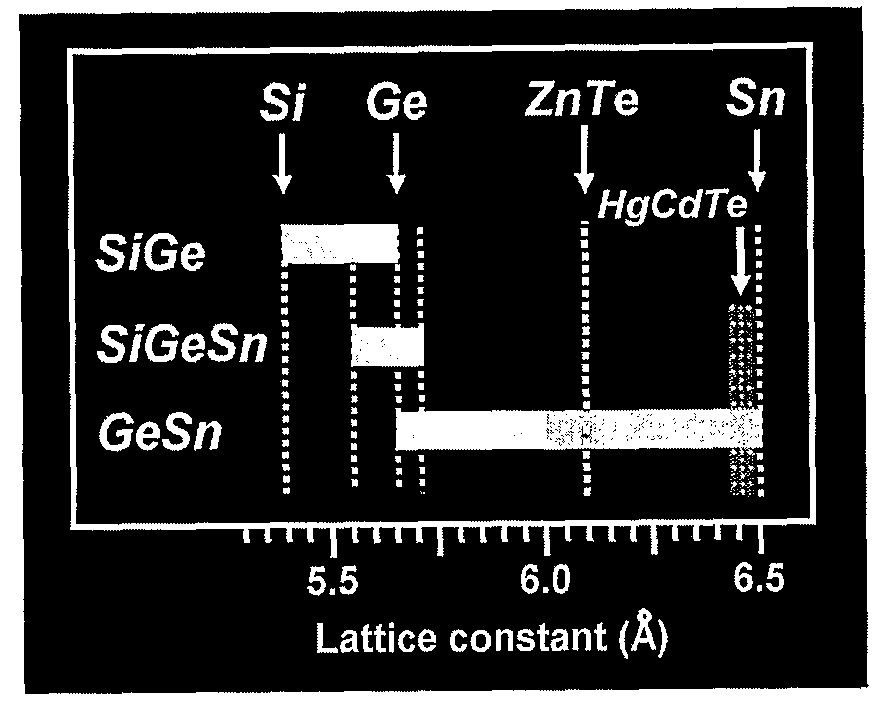
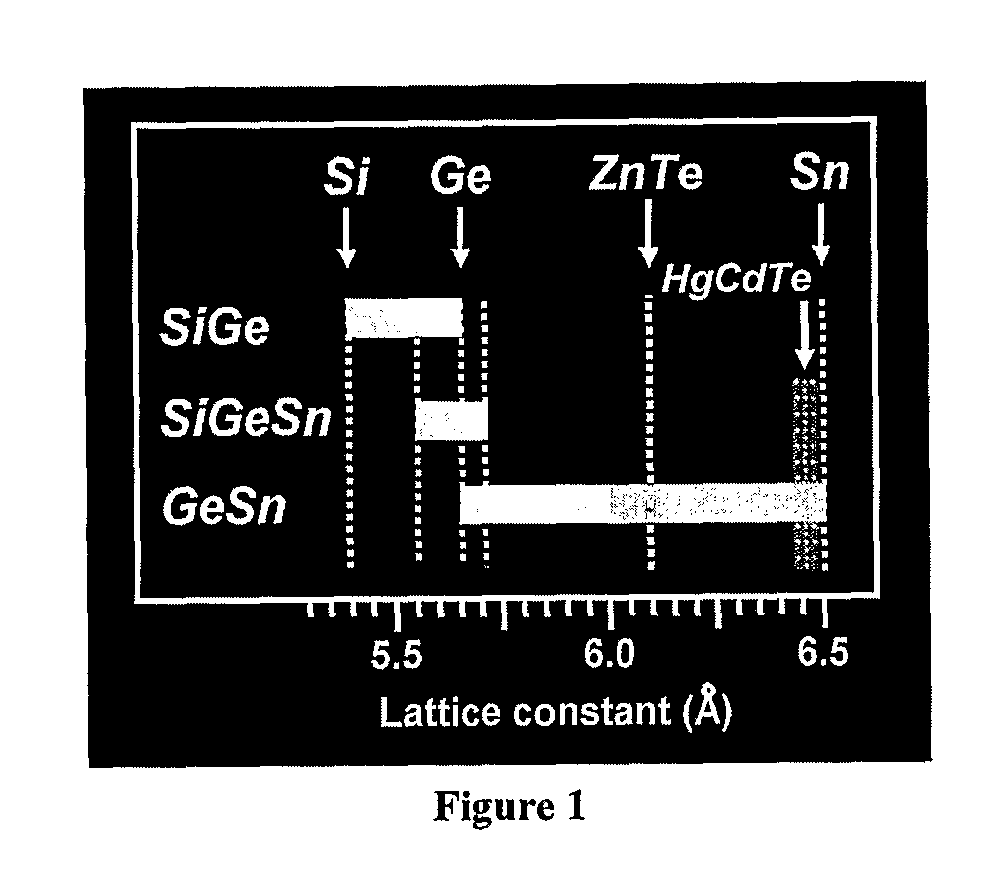
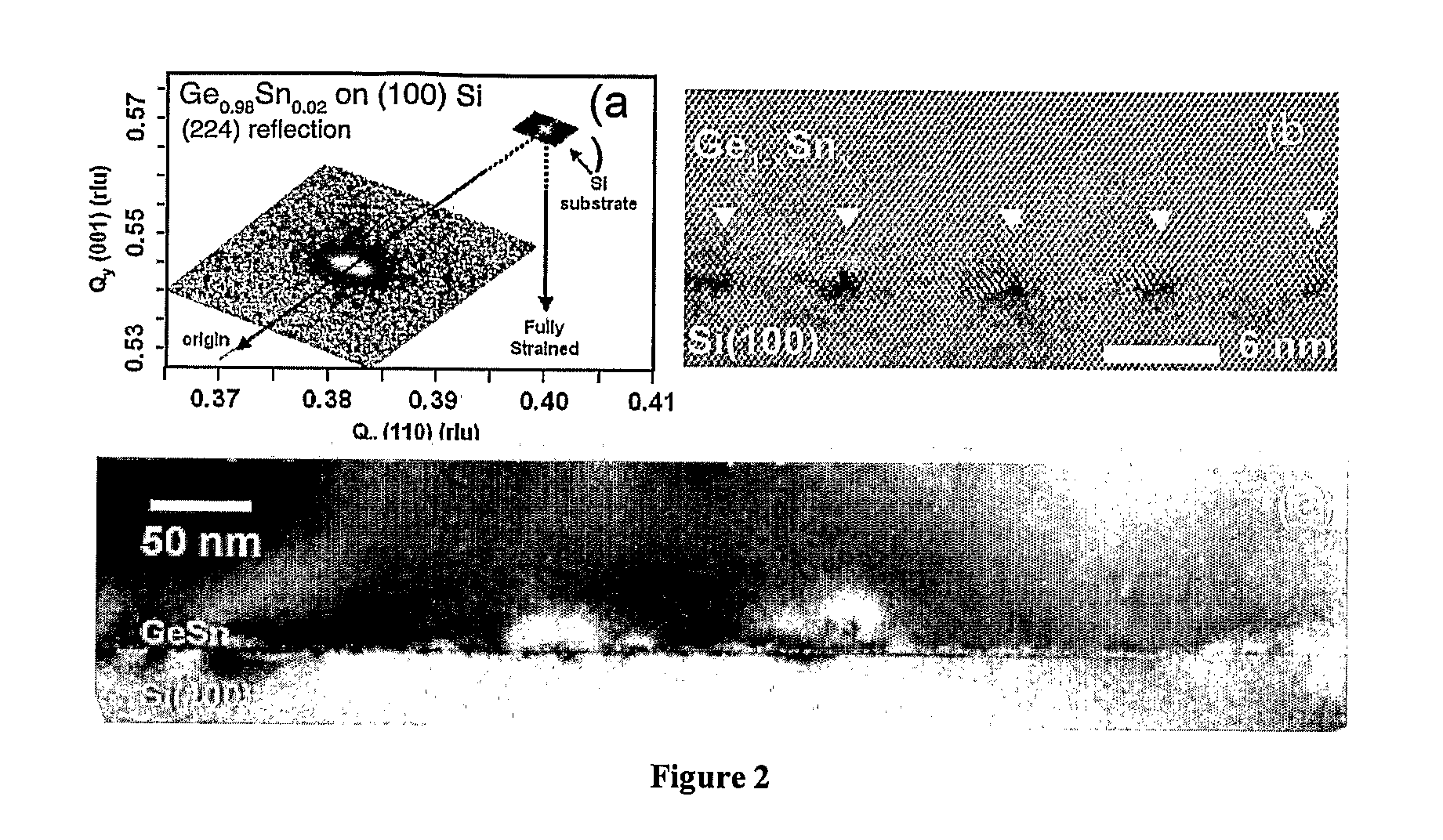
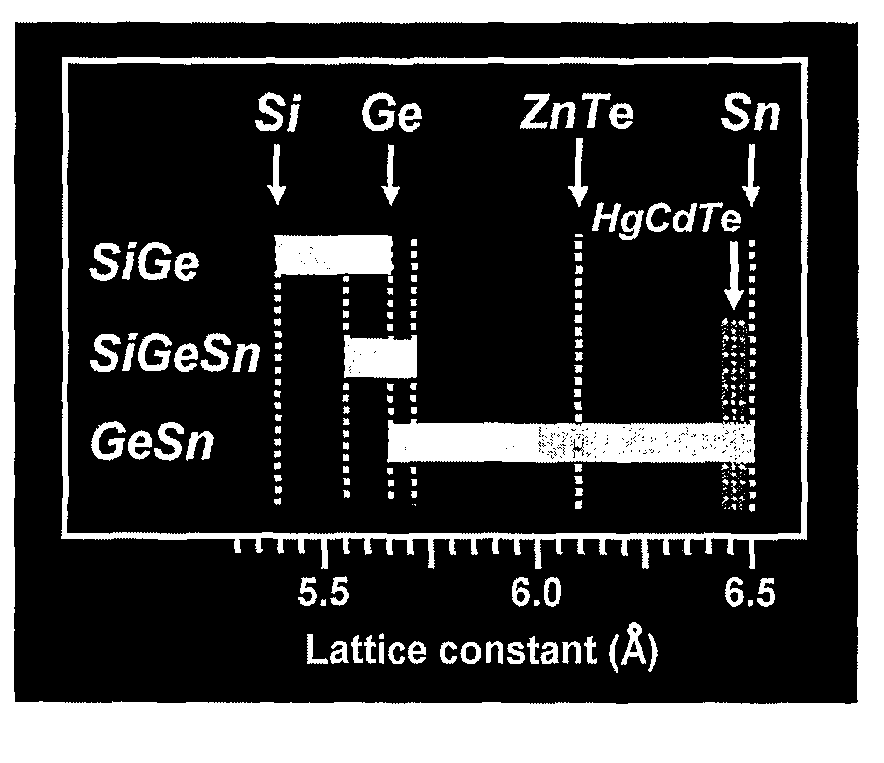
Novel Gesisn-Based Compounds, Templates, and Semiconductor Structures
InactiveUS20080187768A1Polycrystalline material growthSemiconductor/solid-state device manufacturingSemiconductor structureActive layer
The present invention provides novel compounds of the formula Gei-x-ySixSny, wherein 0.01<y<0.11, and 0.26<x<0.35, and semiconductor structures comprising such compounds. The present invention also provides novel semiconductor structures comprising silicon substrates, an SiGe buffer layer, and a Group III-V or II-VI active layer. The present invention also provides novel semiconductor structures comprising silicon substrates, an SiGe buffer layer, an SiGeSn template layer, and an SiGe, Ge, Group III-V, or Group II-VI active layer.
Owner:ARIZONA STATE UNIVERSITY
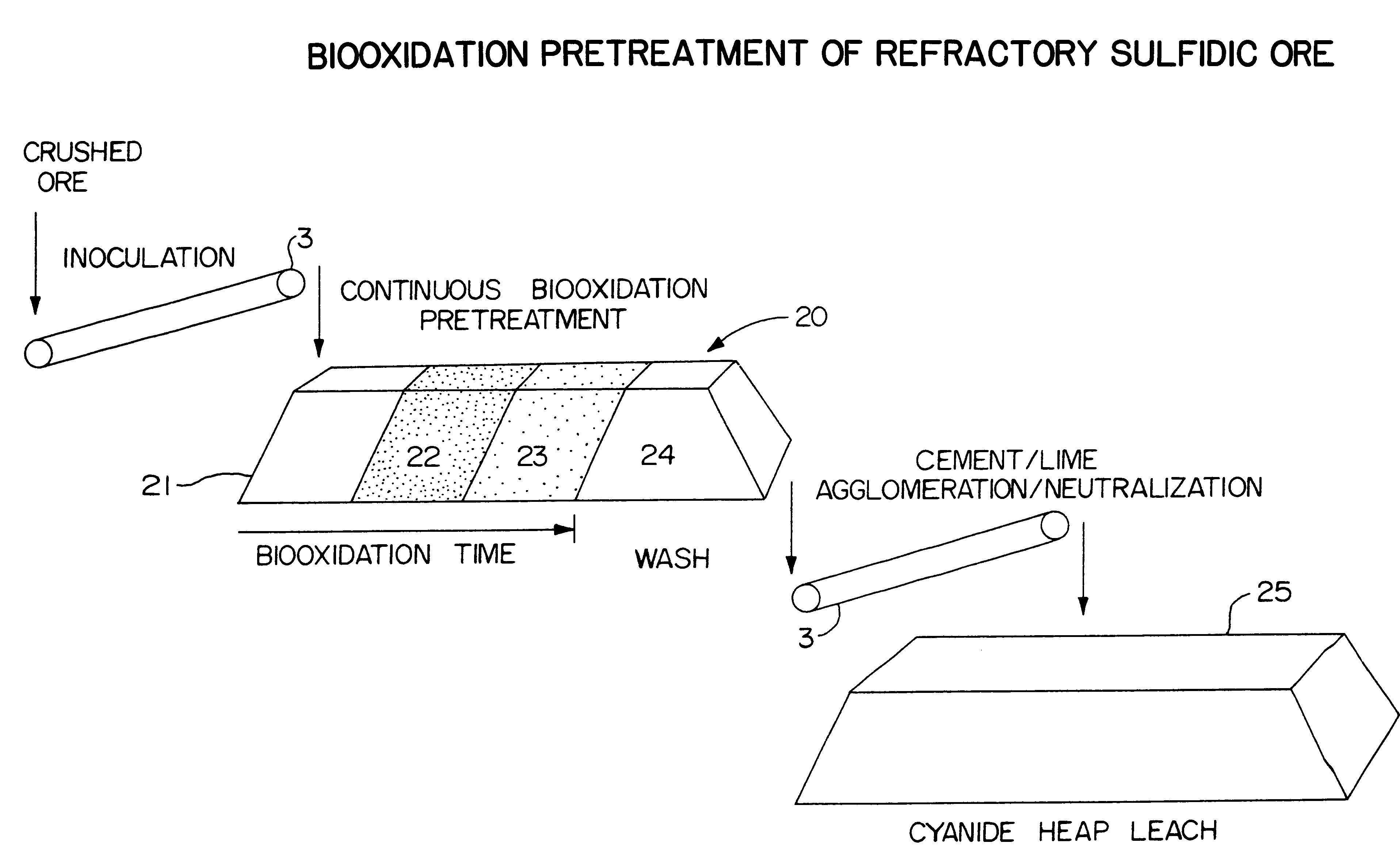
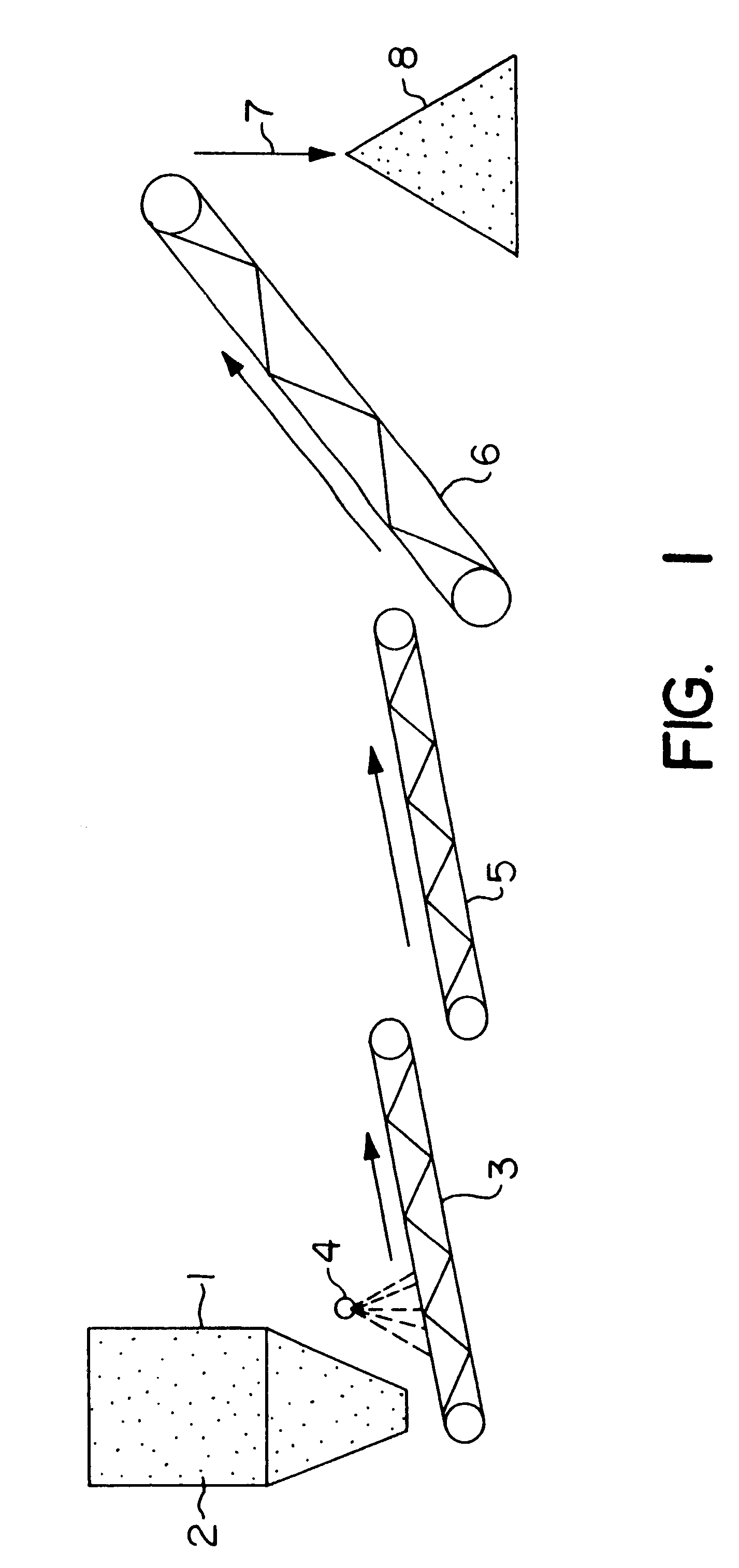
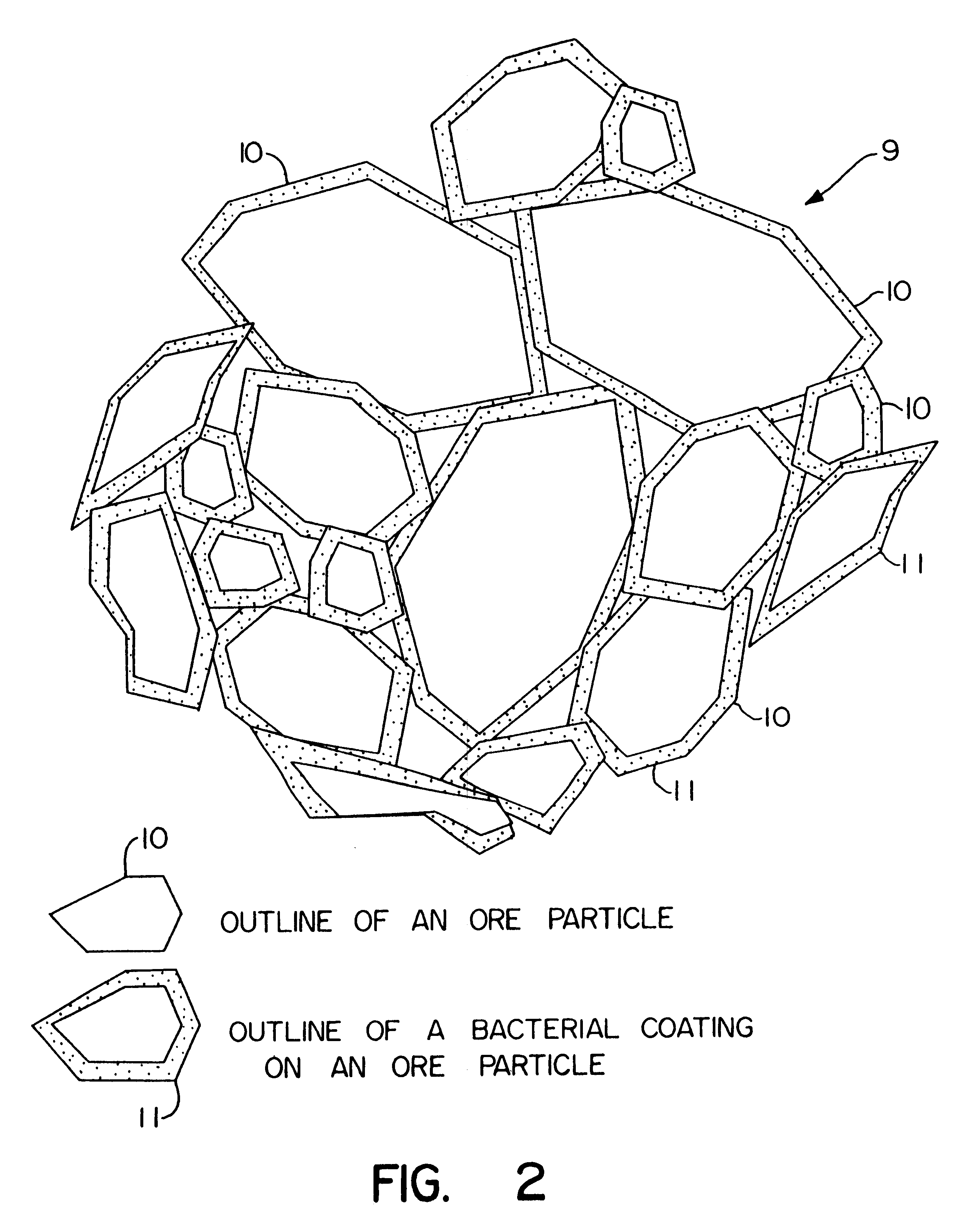
Biooxidation process for recovery of metal values from sulfur-containing ore materials
InactiveUS6383458B1Increase ratingsIncrease attractivenessTransuranic element compoundsSolvent extractionParticulatesPartial oxidation
A process for the recovery of one or more metal values from a metal ore material comprising those of one or more values and a matrix material having a sulfur content wherein the sulfur is present in an oxidation-reduction state of zero or less comprisinga. forming particulates from particles of said ore and an inoculate comprising bacteria capable of at least partially oxidizing the sulfur content;b. forming a heap of said particulates;c. biooxidizing the sulfur content andd. recovering those one or more metal values.
Owner:NEWMONT USA LTD
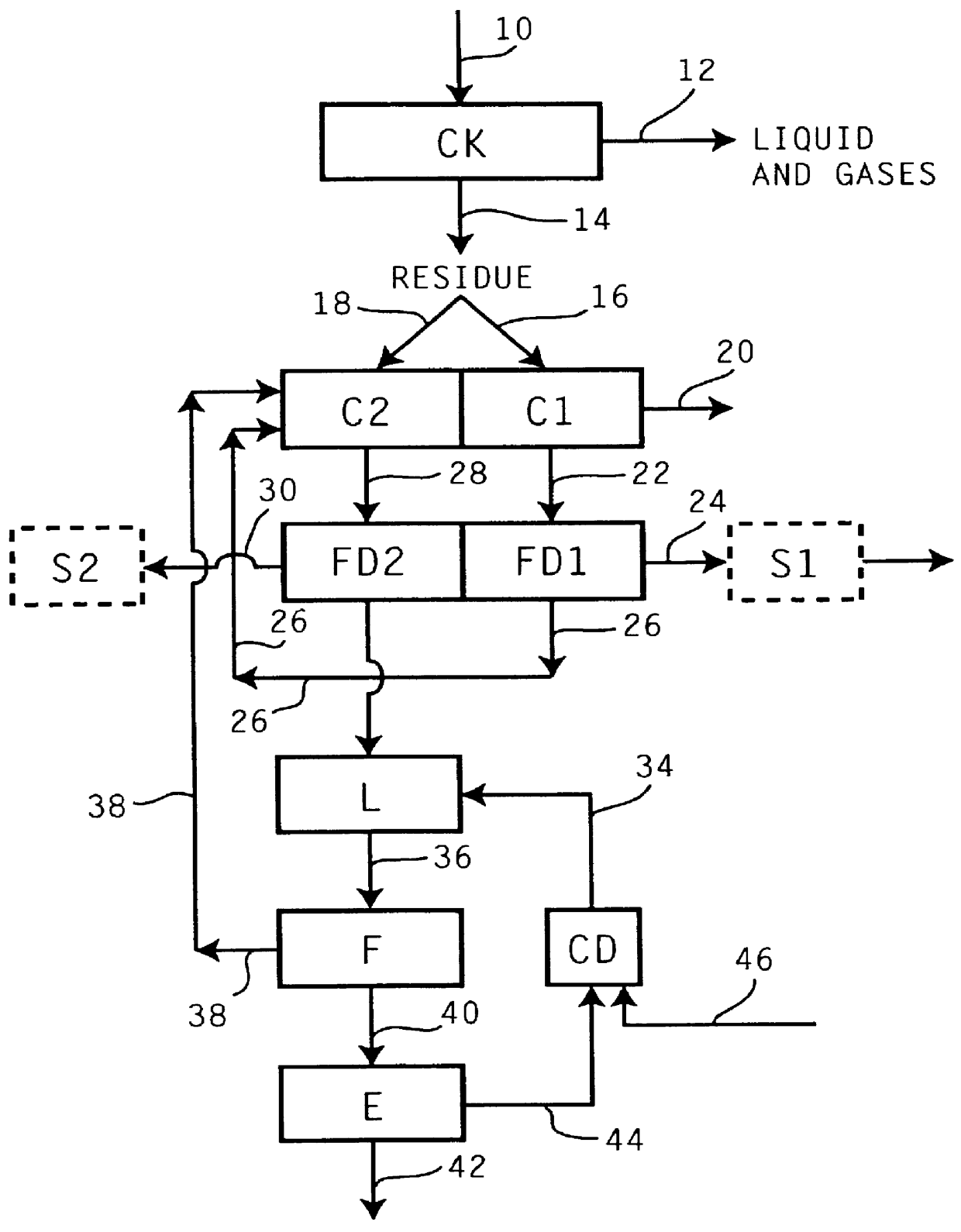
Recovery of the transition metal component of catalyst used in heavy feed hydroconversion
The invention relates to a process for recovering the transition metal component of catalysts used in the hydroconversion of heavy hydrocarbonaceous materials. In accordance with the invention, a slurry of a transition metal catalyst and hydrocarbon is catalytically desulfurized resulting in a desulfurized product and a solid residue containing the transition metal. The transition metal may be recovered by coking the residue and then dividing the coker residue into two portions are combusted with the flue dust from the first combustion zone being conducted to the second combustion zone. The flue dust from the second combustion zone is treated with ammonia and ammonium carbonate in order to obtain ammonium molybdate.
Owner:EXXON RES & ENG CO
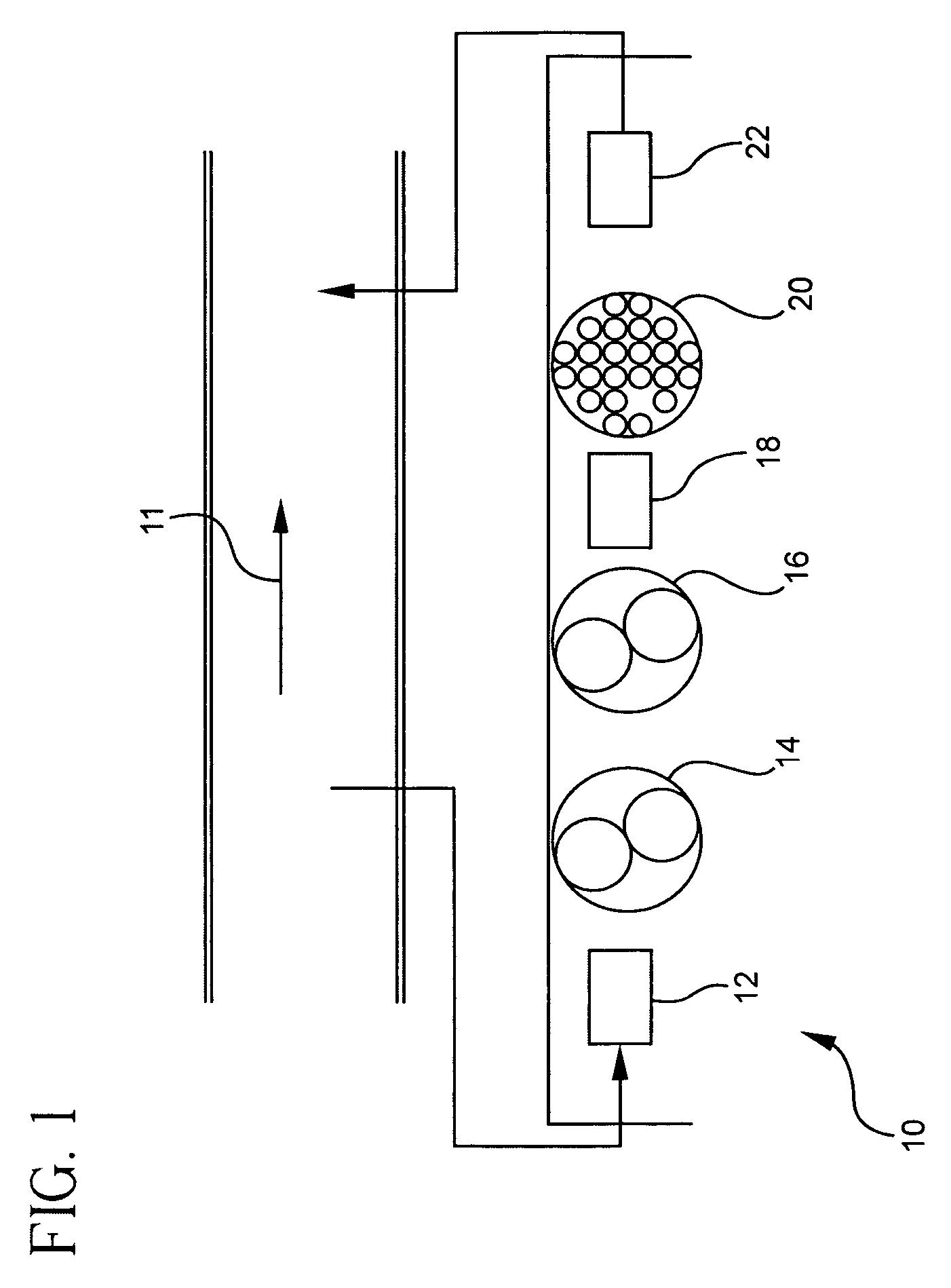
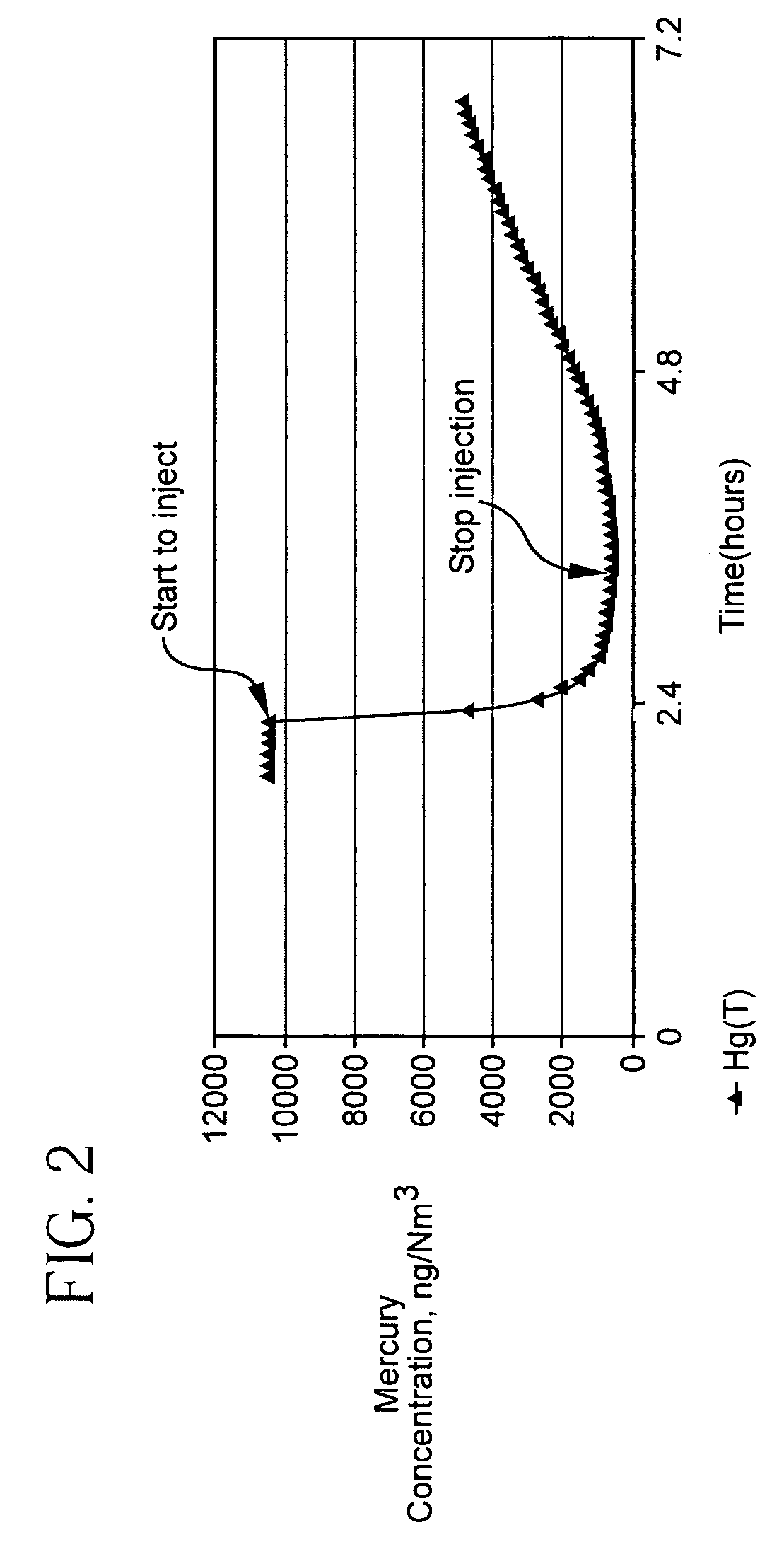
Pollutant emission control sorbents and methods of manufacture
Sorbents for removal of mercury and other pollutants from gas streams, such as a flue gas stream from coal-fired utility plants, and methods for their manufacture and use are disclosed. The methods include mixing fly ash particles with a sulfide salt and a metal salt to form a metal sulfide on the outer surface of the fly ash particles.
Owner:BASF CATALYSTS LLC
Lead recycling
ActiveUS20100040938A1Simple methodOrganic chemistryPrimary cell maintainance/servicingCITRATE ESTERLead oxide
The present invention describes a method of recycling lead from lead containing waste, the method comprising the steps of mixing the battery paste with aqueous citric acid solution so as to generate lead citrate; isolating lead citrate from the aqueous solution; and converting the lead citrate to lead and / or lead oxide.
Owner:CAMBRIDGE ENTERPRISE LTD
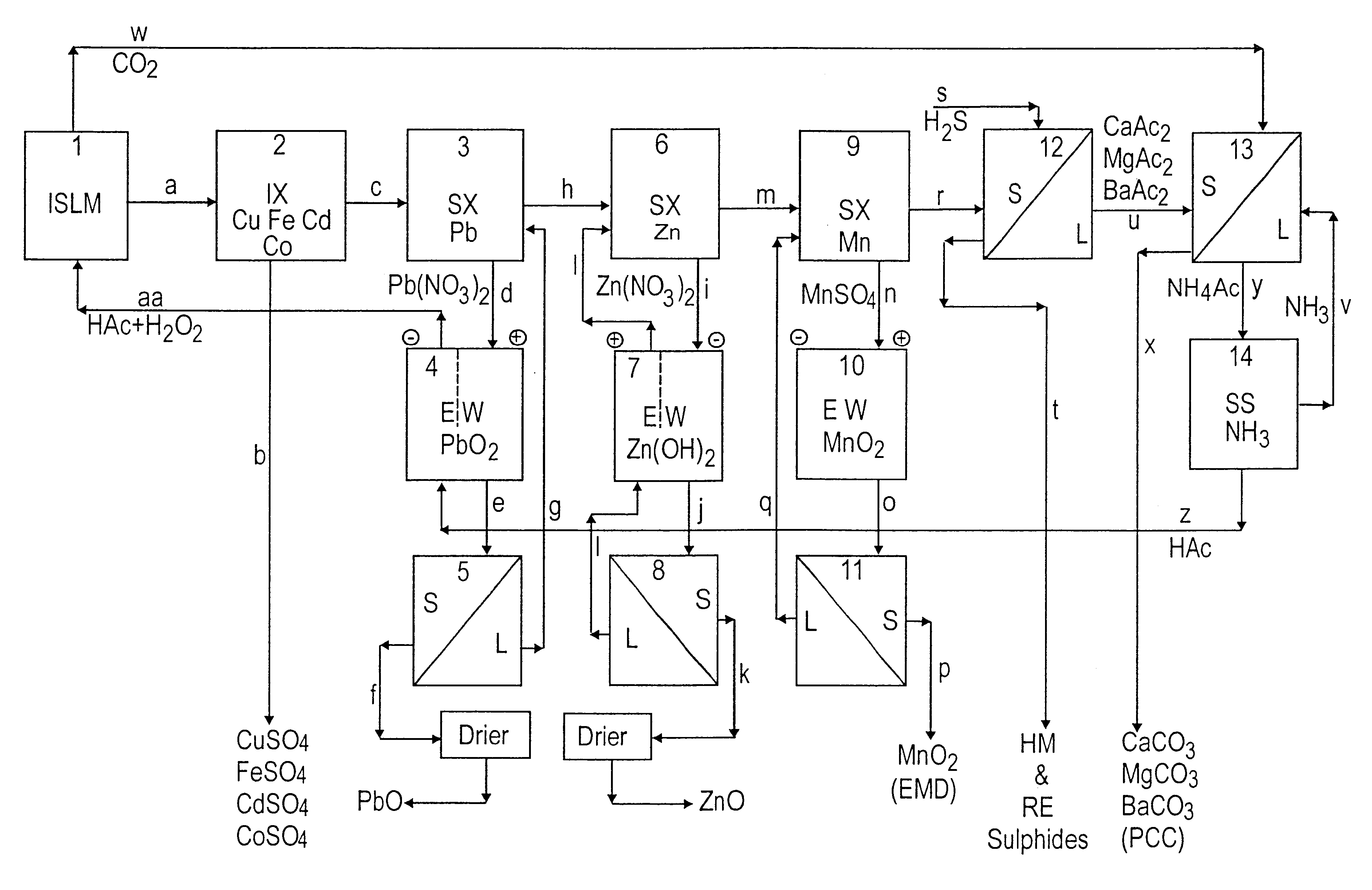
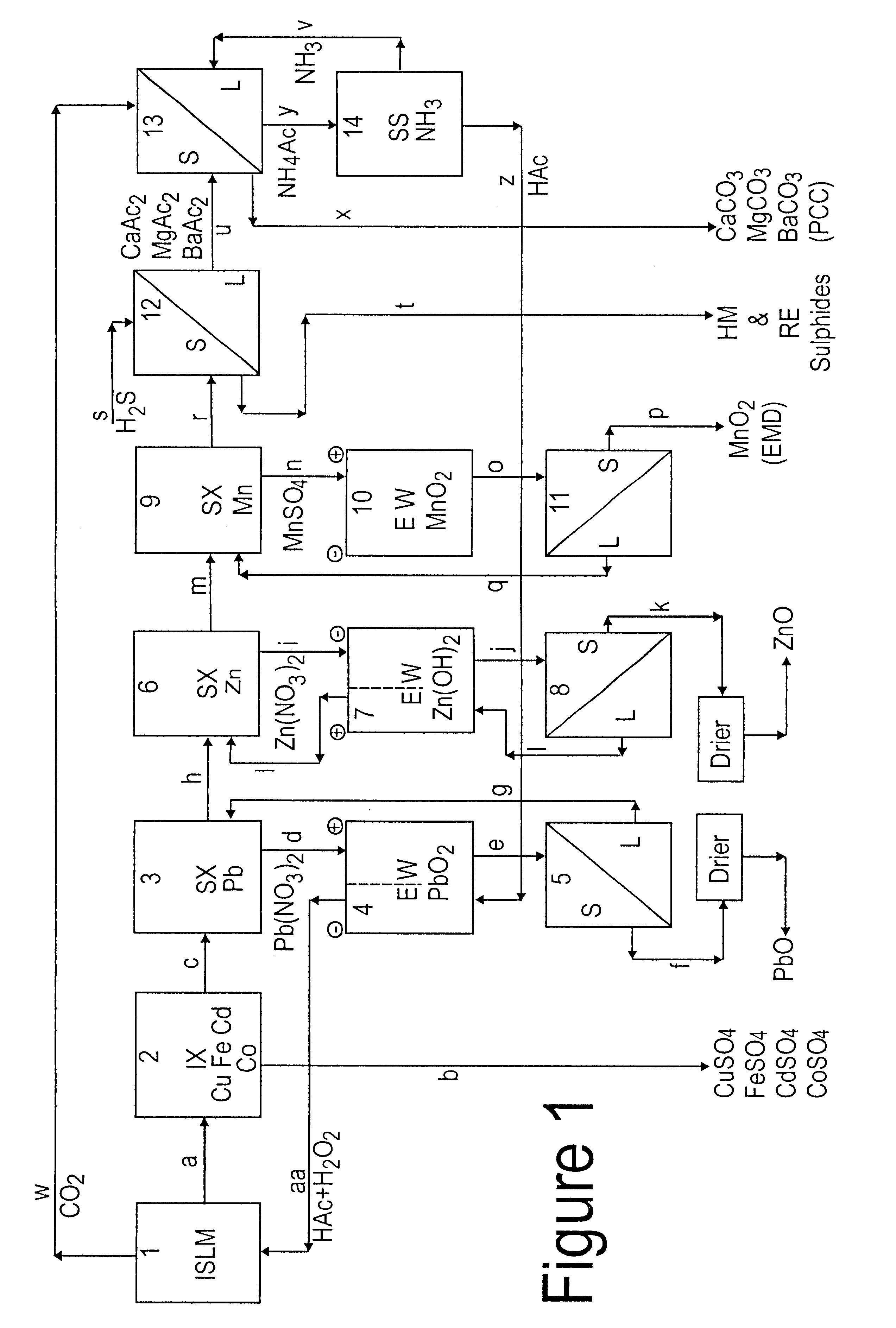
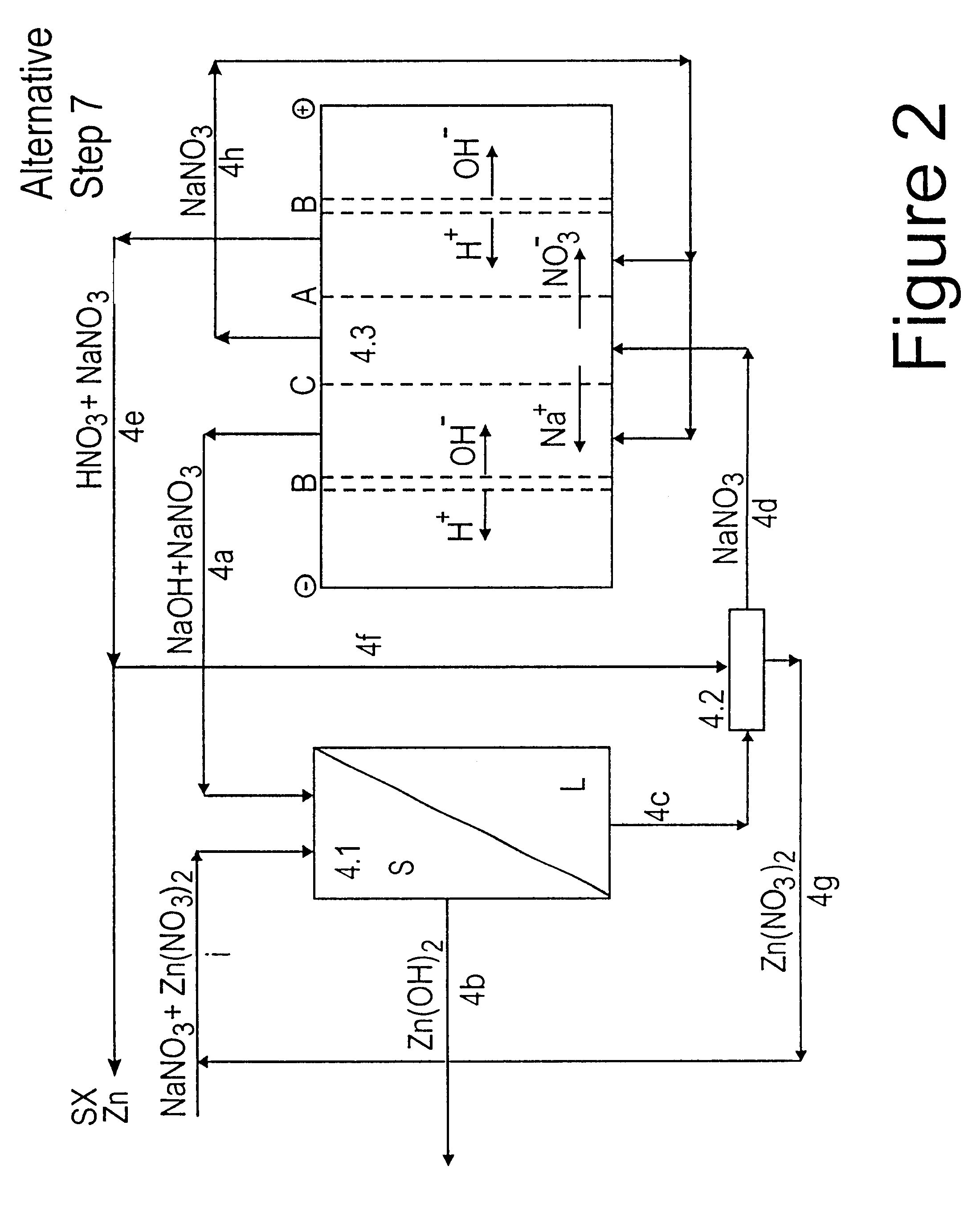
Lead, zinc and manganese recovery from aqueous solutions
InactiveUS6517701B1Low costShorten the timePhotography auxillary processesElectrolysis componentsIon exchangeManganese
Aqueous solutions containing lead, zinc and manganese are treated to recover these metals by sequential solvent extraction steps. Solvent extractants are selected to extract preferentially lead, then zinc and then manganese in that order. Any interfering metals are removed (as by ion exchange) before extraction. The loaded extractant phases are stripped with selected acids and lead, zinc and manganese each recovered from the strip solutions. Optionally calcium can be recovered when present. A preferred type of extractant (for lead especially) is substituted monothiophosphinic acids. A closed loop system is described which is advantageous with leachate from sulphide and carbonate ores.
Owner:CENTAUR MINING EXPLORATION
Method for effecting solvent extraction of metal ions using hydrocarbon soluble aminomethylene phosphonic acid compounds
Solvent extraction of one or more metal ions from an aqueous solution in the presence of hydrocarbon-soluble aminomethylenephosphonic acid derivatives.
Owner:BASF AG +1
Indium oxide-tin oxide powders and method for producing the same
InactiveUS6051166ADecrease productivityImprove sputtering efficiencyAluminium compoundsGallium/indium/thallium compoundsSaline waterIndium
A method for producing an indium oxide-tin oxide powder, which comprises supplying to react an aqueous solution of an indium salt, an aqueous solution of a tin salt and an aqueous alkaline solution into water at 40 DEG C. or more and less than 100 DEG C. so that the pH during the reaction is maintained within the range from 4 to 6, forming a precipitate, washing the formed precipitate after solid-liquid separation, and calcining the precipitate at 600 DEG C. or more and 1300 DEG C. or less.
Owner:SUMITOMO CHEM CO LTD
Methods of recovering rare earth elements
ActiveUS8216532B1Improve catalytic performanceNegative environmental impactMolecular sieve catalystsOther chemical processesMolecular sieveRare-earth element
Processes described include reacting a fresh or spent catalyst, or sorbent, with a solution containing an extracting agent (such as an acid or a base). Preferably, the catalyst contains both alumina and a molecular sieve (or a sorbent), and the reaction is performed under relatively mild conditions such that the majority of the base material does not dissolve into the solution. Thus, the catalyst can be re-used, and in certain instances the performance of the catalyst even improves, with or without re-incorporating certain of the metals back into the catalyst. Additionally, metals contained in the catalyst, such as Na, Mg, Al, P, S, Cl, K, Ca, V, Fe, Ni, Cu, Zn, Sr, Zn Sb, Ba, La, Ce, Pr, Nd, Pb, or their equivalent oxides, can be removed from the catalyst. Some of the metals that are removed are relatively valuable (such as the rare earth elements of La, Ce, Pr and Nd).
Owner:VIERHEILIG ALBERT A
Method for preparing inorganic pigments, resulting inorganic pigments, and apparatus therefor
PCT No. PCT / FR96 / 01202 Sec. 371 Date May 27, 1998 Sec. 102(e) Date May 27, 1998 PCT Filed Jul. 30, 1996 PCT Pub. No. WO97 / 06215 PCT Pub. Date Feb. 20, 1997A method for preparing inorganic pigments from steel mill dust, particularly electric steel mill dust, wherein (a) the dust is separated into a magnetic fraction and a non-magnetic fraction; (b) the non-magnetic fraction is subjected to a basic leaching reaction; (c) the resulting solid batch is rinsed until neutralized and then separated; (d) the resulting batch is calcined at 450-650 DEG C.; (e) the calcined batch is treated with sulfuric acid in the presence of a catalyst; (f) the inorganic pigments are recovered; and (g) the solutions from (c) and (e) are used to precipitate other pigments.
Owner:RECUPAC
Method for Collection of Valuable Metal from ITO Scrap
ActiveUS20100084279A1Easy to collectEfficient collectionAluminium compoundsTin compoundsElectrolysisIndium
Proposed is a method for collecting valuable metal from an ITO scrap in which a mixture of indium hydroxide and tin hydroxide or metastannic acid is collected by subjecting the ITO scrap to electrolysis in pH-adjusted electrolyte, and roasting this mixture as needed to collect the result as a mixture of indium oxide and tin oxide. This method enables the efficient collection of indium hydroxide and tin hydroxide or metastannic acid, or indium oxide and tin oxide from an ITO scrap of an indium-tin oxide (ITO) sputtering target or an ITO scrap such as ITO mill ends arisen during the manufacture of such ITO sputtering target.
Owner:JX NIPPON MINING& METALS CORP
Removal of lead from solid materials
InactiveUS20150050199A1Minimal costTin compoundsSolvent extractionMaterials scienceCathode Ray Tube Display
A leaching composition that substantially removes lead from solid materials and a method of using said composition. Preferably, the concentration of lead in the solid materials following processing is low enough that the solid materials can be reused and / or disposed of at minimal cost to the processor. Preferably, the solid materials comprise glass, such as cathode ray tube glass.
Owner:ENTEGRIS INC
GeSiSn-based compounds, templates, and semiconductor structures
InactiveUS8029905B2Polycrystalline material growthSemiconductor/solid-state device manufacturingSemiconductor structureActive layer
The present invention provides novel compounds of the formula Gei-x-ySixSny, wherein 0.01<y<0.11, and 0.26<x<0.35, and semiconductor structures comprising such compounds. The present invention also provides novel semiconductor structures comprising silicon substrates, an SiGe buffer layer, and a Group III-V or II-VI active layer. The present invention also provides novel semiconductor structures comprising silicon substrates, an SiGe buffer layer, an SiGeSn template layer, and an SiGe, Ge, Group III-V, or Group II-VI active layer.
Owner:ARIZONA STATE UNIVERSITY
Alkaline earth metal germanate nanomaterial and preparation method thereof and use thereof as cathode material of lithium ion battery
ActiveCN102502789ASimple processRaw materials are easy to getCell electrodesNanotechnologyAlkaline earth metalAluminium-ion battery
The invention discloses an alkaline earth metal germanate nanomaterial and a preparation method thereof and use of the nanomaterial as a cathode material of lithium ion batteries. The method comprises the following steps of: (1) mixing an aqueous solution of alkaline earth metal salt and germanium source compound GeO2 to obtain a liquid mixture; (2) allowing reaction of the liquid mixture obtained in the step (1) to take place in a polytetrafluoroethylene-lined high-pressure reaction kettle after heating, and cooling after the reaction is completed to obtain the alkaline earth metal germanate nanomaterial. The method is simple, has abundant and easily-available raw materials, is suitable for large-scale production and has a high level of practicability. The obtained alkaline earth metal germanate is a nanomaterial, has a high actual capacity, can be directly used as a cathode material of lithium ion batteries, solves the problem that the germanium-based material as lithium ion battery cathode material has a poor cycling property and takes a violent change of volume during charging / discharging process, and can be directly used as the cathode material of lithium ion batteries.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Stabilization method for lead projectile impact area
InactiveUS20030143031A1Increase surface areaLow water solubilityTin compoundsSolid waste disposalHealth riskEngineering
The invention pertains to a method for reducing the leaching of lead from lead projectile impact area acting to collect or stop such projectiles. The method includes contacting the lead projectile impact area with a seed of dry granular lead stabilizing agents. The seeding or coating of lead stabilizing agents provides a means to stabilize lead in the bullet / shot impact area while also allowing for future lead bullets, fragments, or lead shot fired into the soil to be stabilized upon contact with the lead stabilizing agent seeds or contact with rainwater leaching through the lead stabilizing agent seeds produced from the projectile coating, or excess seed from the lead shell projectiles. This method eliminates the need to remove or re-treat range soils and greatly reduces the environmental and health risks associated with the use of lead as projectiles in the open environment as well as at control trap ranges.
Owner:FORRESTER KEITH E
Method of preparing transition metal oxide nano-particles
ActiveCN101830496AEasy to manufactureEasy to handleNanostructure manufactureTantalum compoundsAlcoholMetallate
Provided is a method for preparing transition metal oxide nano-particles from a transition metal as a reactant. The method includes dissolving the transition metal into aqueous hydrogen peroxide to provide peroxi-metallate solution, and then adding a reactive solution containing an alcohol, water and an acid thereto to perform hydrothermal reaction. More particularly, the method for preparing transition metal oxide particles includes: dissolving transition metal powder as a reactant into aqueous hydrogen peroxide to provide a peroxi-metallate solution with a molar concentration of transition metal of 0.001-0.2 M; adding a reactive solution containing an alcohol, water and an acid to the peroxi-metallate solution to provide a mixed solution; and subjecting the mixed solution to hydrothermal reaction to provide transition metal oxide nano-particles.
Owner:THE IND & ACADEMIC COOPERATION & CHUNGNAM NAT UNIV
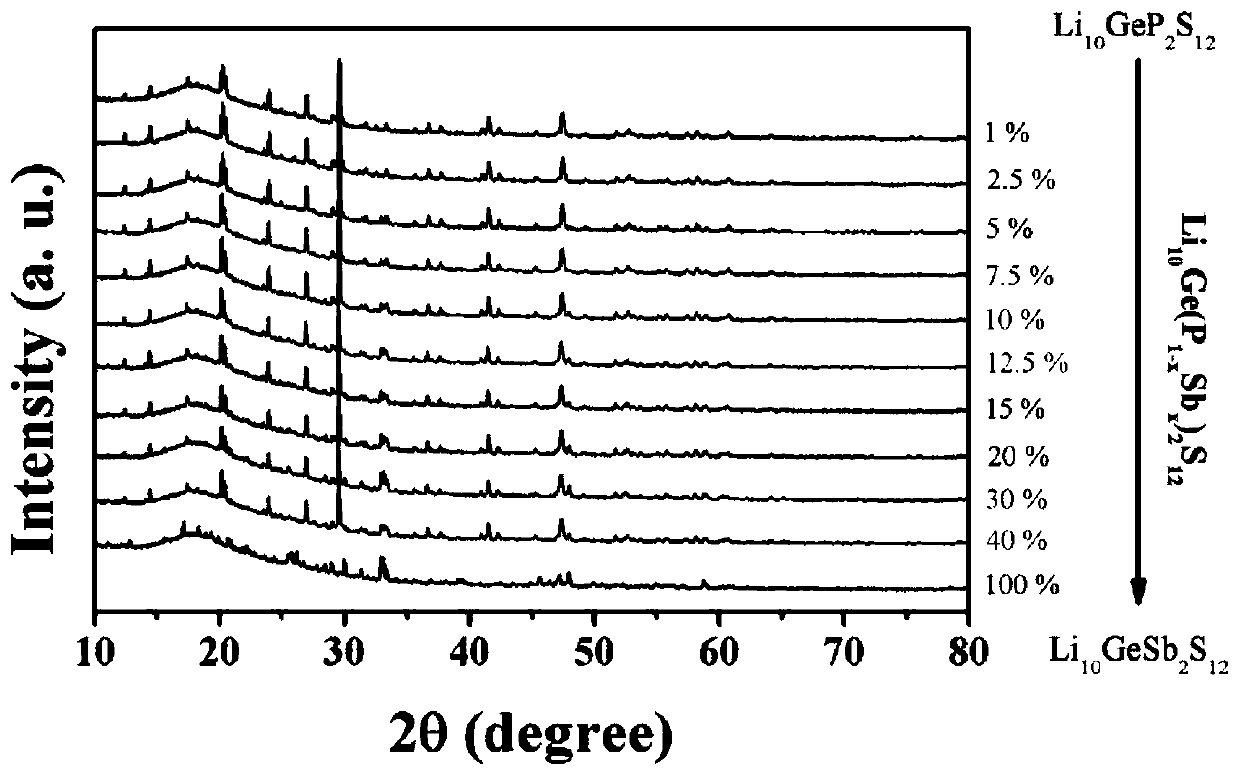
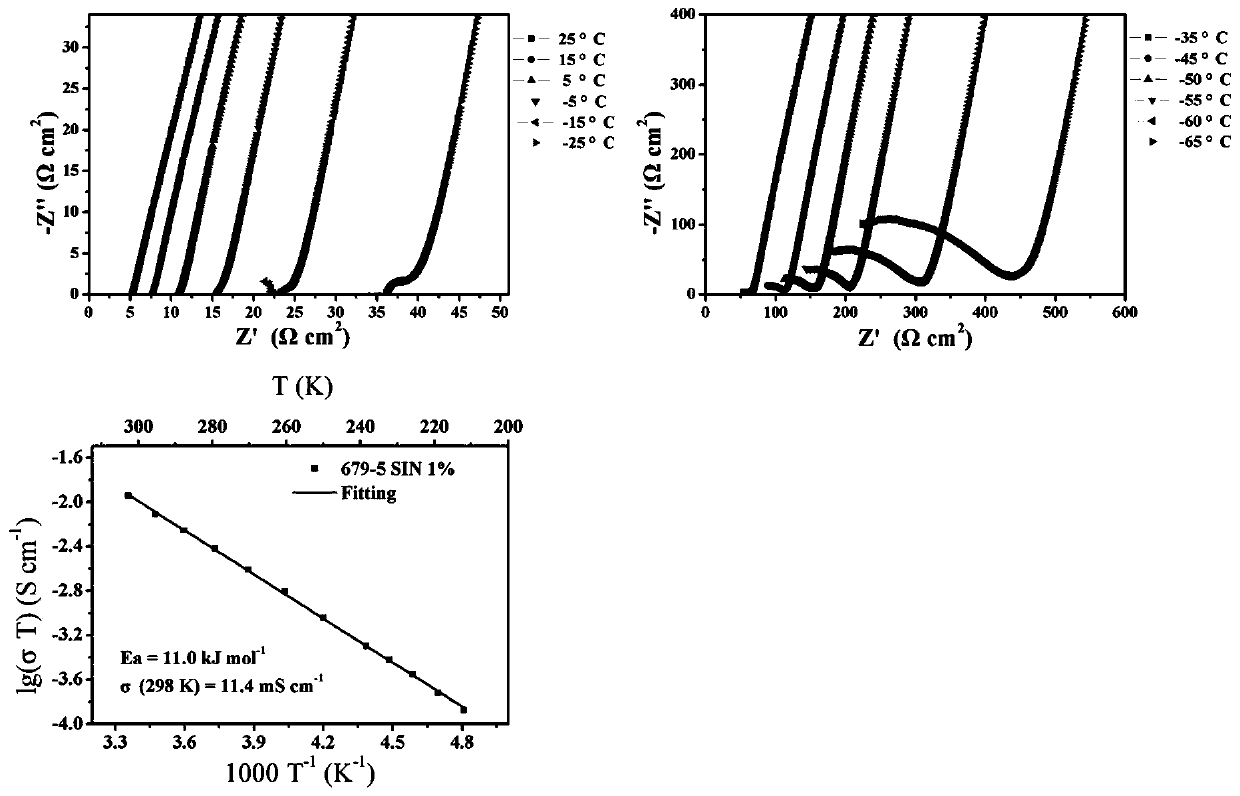
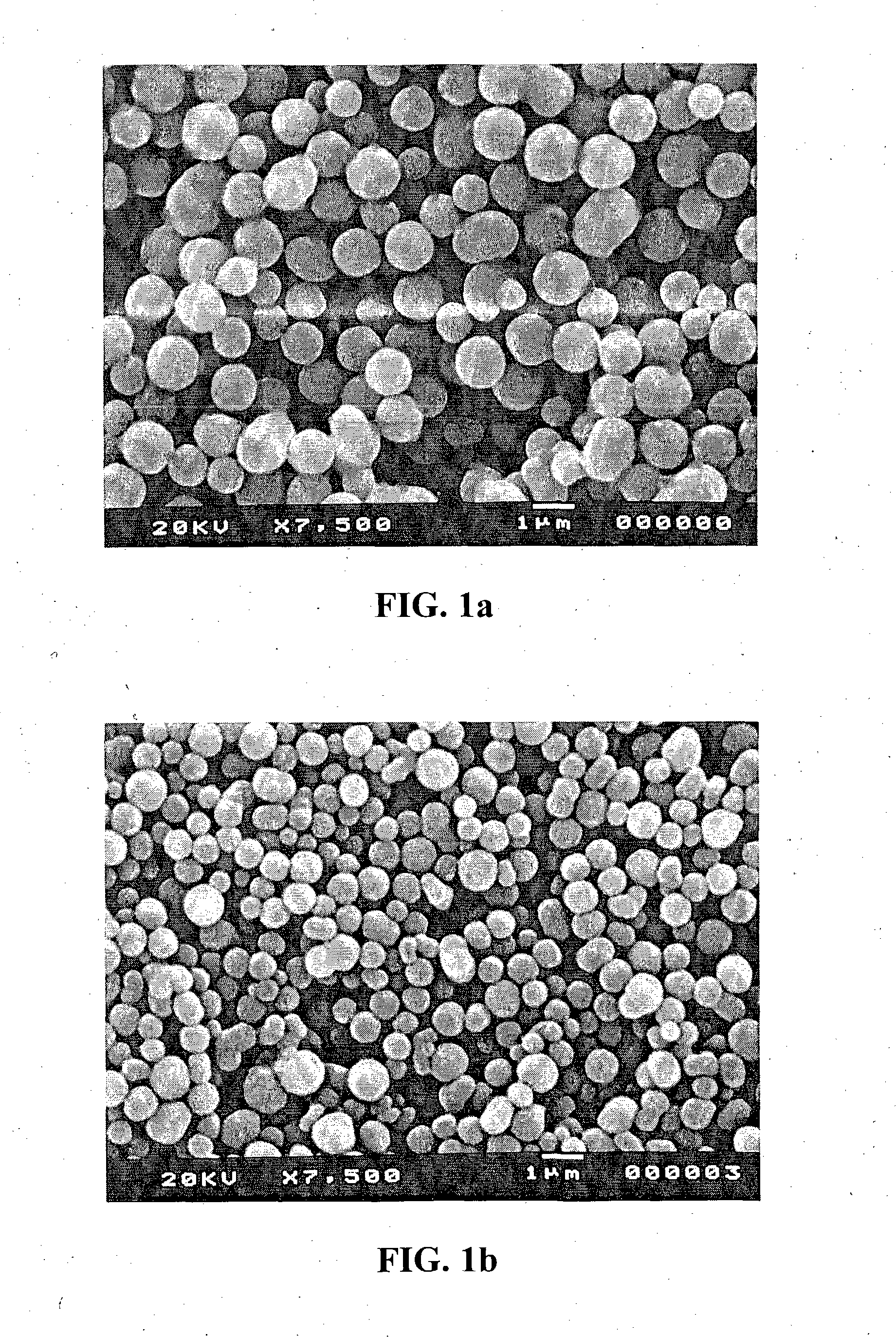
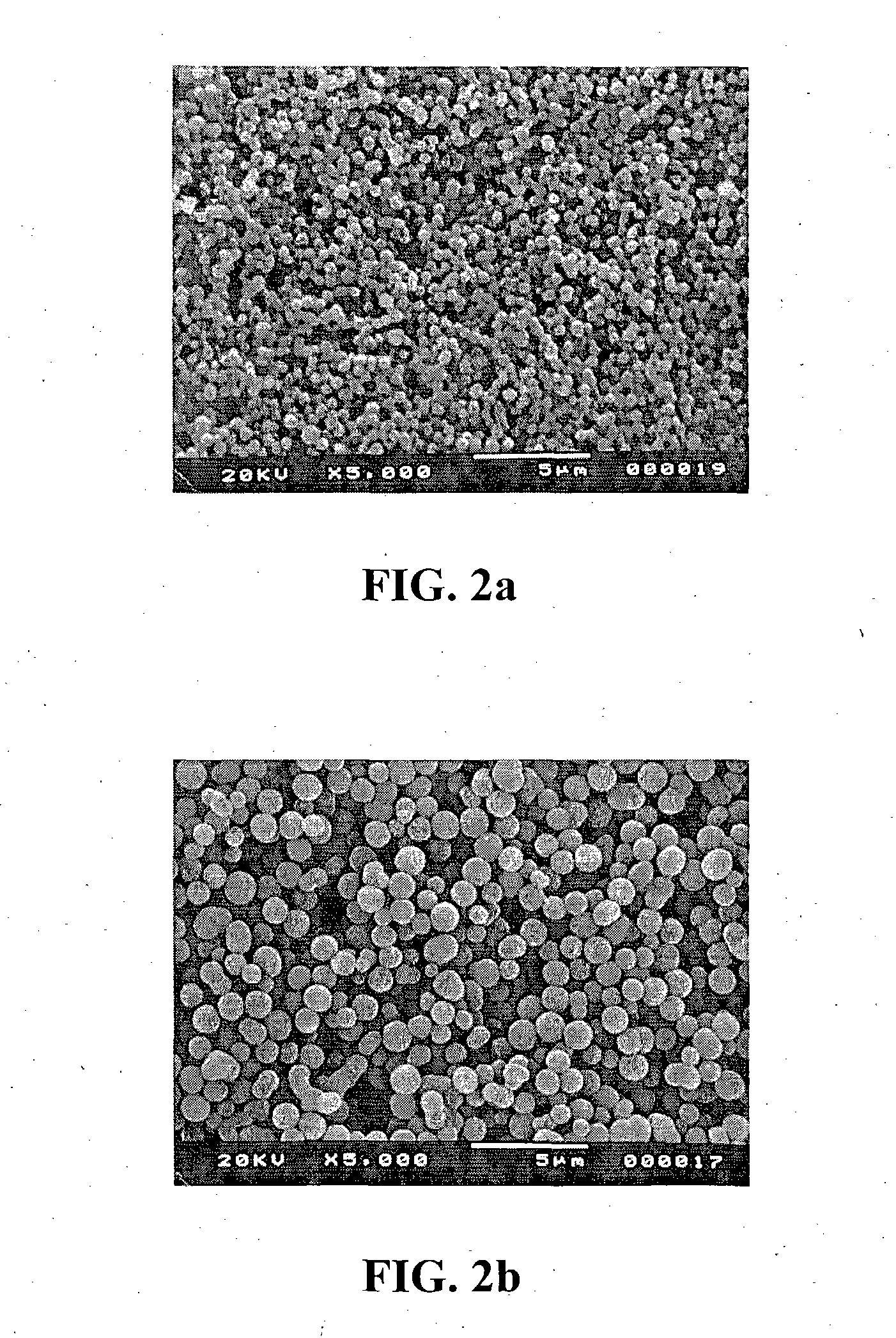
High-air-stability inorganic sulfide solid electrolyte and preparation method and application thereof
ActiveCN110085908AImprove air stabilitySimple production processSolid electrolytesTin compoundsAll solid stateElectrical battery
Owner:CHINA AUTOMOTIVE BATTERY RES INST CO LTD +1
Recovery of high purity PbO
ActiveUS7785561B1High purityTin compoundsPrimary cell maintainance/servicingLead oxideMetal particle
A process for producing high purity lead oxide from impure lead compounds particularly from waste lead battery paste which includes an oxidation-reduction step. The process results in a reduction of impure lead compounds to the +2 valence state and metal particle contaminants are oxidized to the +2 state.
Owner:RETRIEV TECH +1
Method for making fine and ultrafine spherical particles of zirconium titanate and other mixed metal oxide systems
Disclosed is a method for making amorphous spherical particles of zirconium titanate and crystalline spherical particles of zirconium titanate comprising the steps of mixing an aqueous solution of zirconium salt and an aqueous solution of titanium salt into a mixed solution having equal moles of zirconium and titanium and having a total salt concentration in the range from 0.01 M to about 0.5 M. A stearic dispersant and an organic solvent is added to the mixed salt solution, subjecting the zirconium salt and the titanium salt in the mixed solution to a coprecipitation reaction forming a solution containing amorphous spherical particles of zirconium titanate wherein the volume ratio of the organic solvent to aqueous part is in the range from 1 to 5. The solution of amorphous spherical particles is incubated in an oven at a temperature ≦100° C. for a period of time ≦24 hours converting the amorphous particles to fine or ultrafine crystalline spherical particles of zirconium titanate.
Owner:UT BATTELLE LLC
Method for Collection of Valuable Metal from ITO Scrap
ActiveUS20100316544A1Simple processEasy to collectAluminium compoundsPhotography auxillary processesElectrolysisIndium
Proposed is a method for collecting valuable metal from an ITO scrap including the steps of subjecting the ITO scrap to electrolysis in pH-adjusted electrolyte, and collecting indium or tin as oxides. Additionally proposed is a method for collecting valuable metal from an ITO scrap including the steps of subjecting the ITO scrap to electrolysis in an electrolytic bath partitioned with a diaphragm or an ion-exchange membrane to precipitate hydroxide of tin, thereafter extracting anolyte temporarily, and precipitating and collecting indium contained in the anolyte as hydroxide. With the methods for collecting valuable metal from an ITO scrap described above, indium or tin may be collected as oxides by roasting the precipitate containing indium or tin. Consequently, provided is a method for efficiently collecting indium from an ITO scrap of an indium-tin oxide (ITO) sputtering target or an ITO scrap such as ITO mill ends arisen during the manufacture of such ITO sputtering target.
Owner:JX NIPPON MINING& METALS CORP
Method for Collection of Valuable Metal from ITO Scrap
ActiveUS20100193372A1Efficient collectionEasy to collectAluminium compoundsPhotography auxillary processesIndiumElectrolysis
Proposed is a method for collecting valuable metal from an ITO scrap including the steps of subjecting the ITO scrap to electrolysis and collecting the result as indium-tin alloy. Additionally provided is a method for collecting valuable metal from an ITO scrap including the steps of providing an ITO electrolytic bath and an indium-tin alloy collecting bath, dissolving the ITO in the electrolytic bath, and thereafter collecting indium-tin alloy in the indium-tin alloy collecting bath. These methods enable the efficient collection of indium-tin alloy from an ITO scrap of an indium-tin oxide (ITO) sputtering target or an ITO scrap such as ITO mill ends arisen during the manufacture of such ITO sputtering target.
Owner:JX NIPPON MINING& METALS CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com