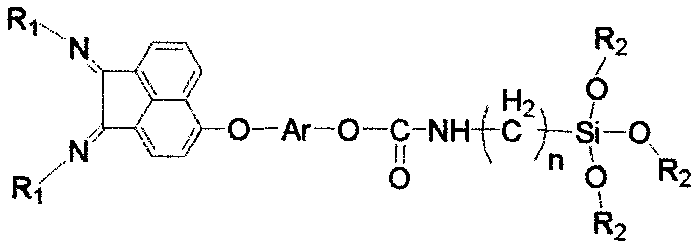
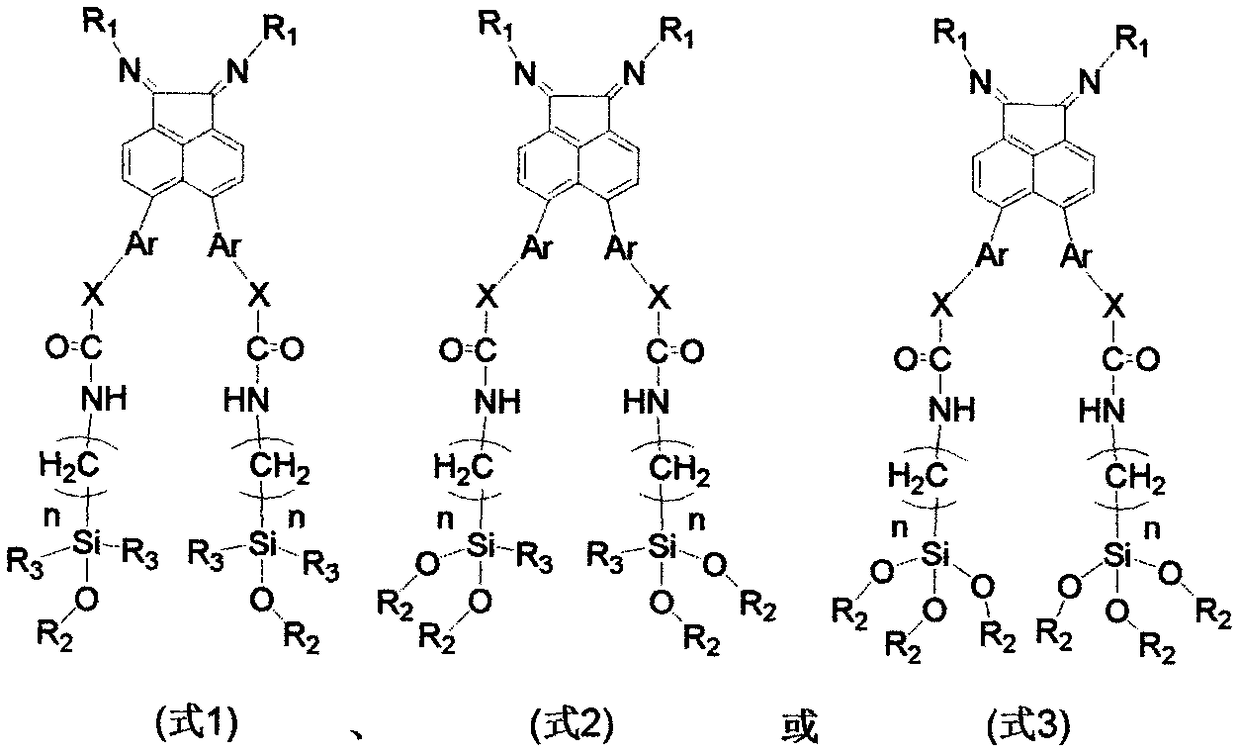
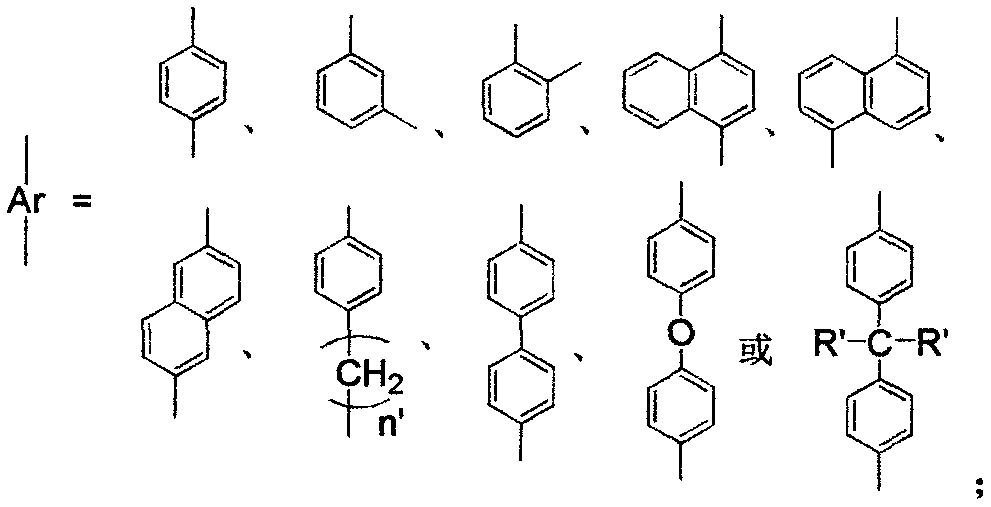
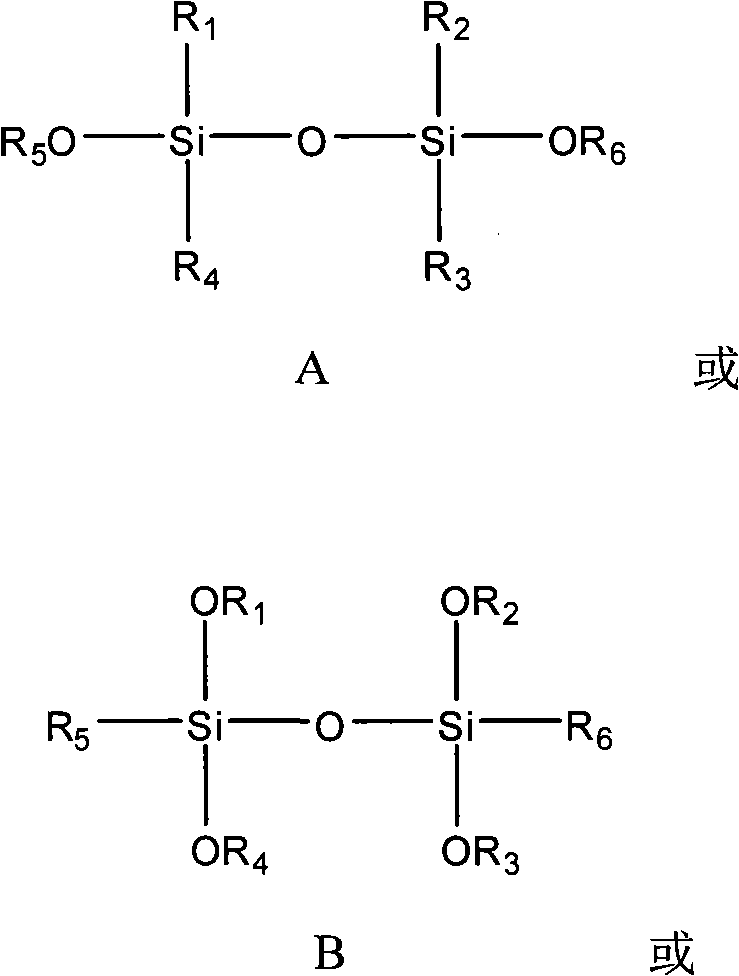
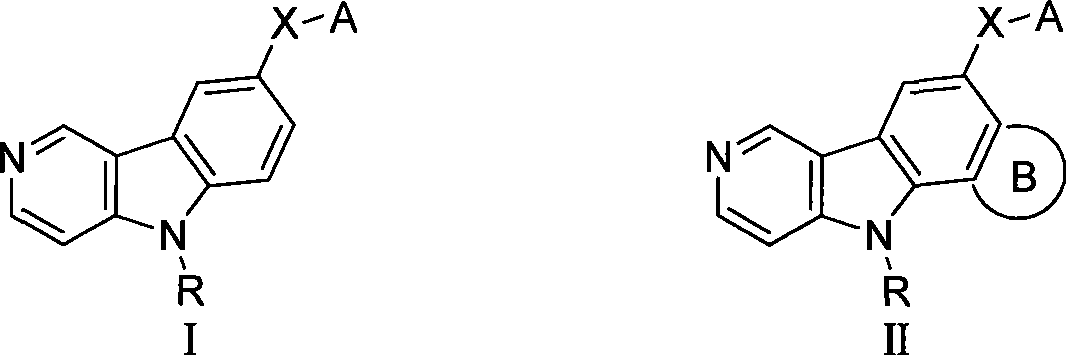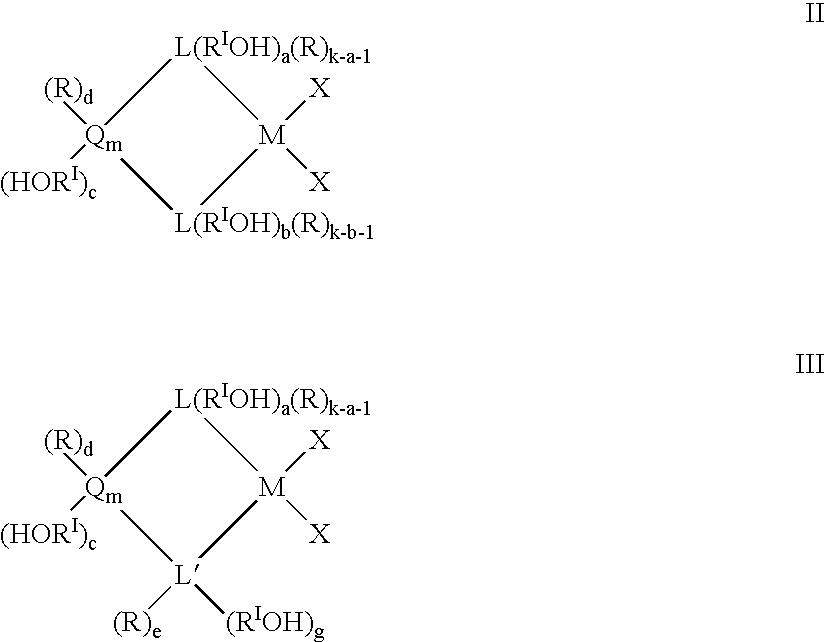Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
149 results about "Tubulin polymerization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tubulin heterodimers polymerize to form microtubules. Tubulin polymerizes to form structures called microtubules (MTs). When tubulin polymerizes it initially forms protofilaments, microtubules consist of 13 protofilaments and are 25 nm in diameter, each µm of microtubule length being composed of 1650 heterodimers.
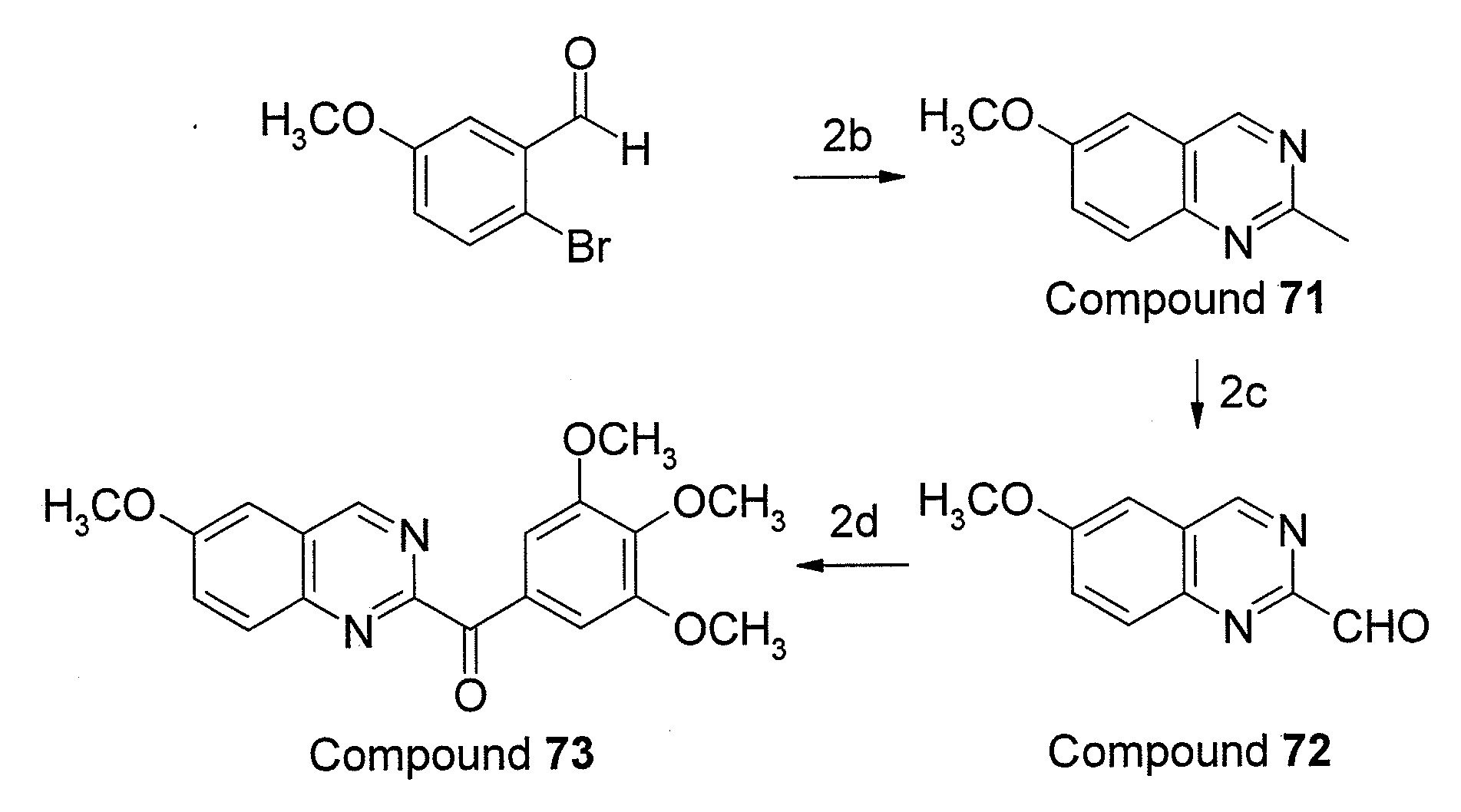
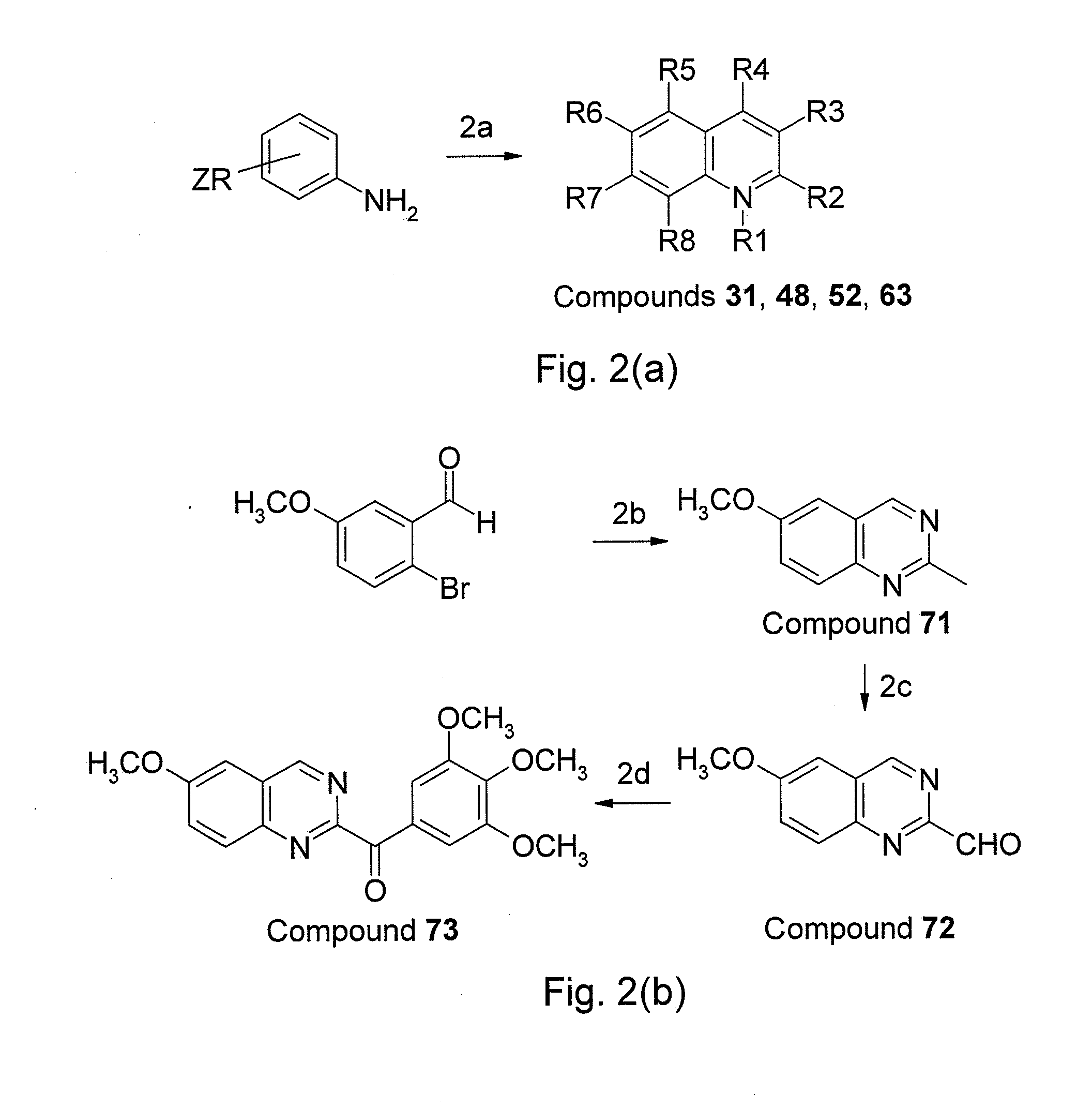
Aroylquinoline compounds
InactiveUS20110275643A1Improve bioavailabilityHigh activityBiocideOrganic chemistryTubulin polymerizationQuinolinium Compounds
A serious of nitro heterocyclic derivatives including a structure of formula (I) are provided. In formula (I), P, Q and R1 to R8 are defined in the specification. The derivatives disclosed in the present invention are characterized in inhibiting tubulin polymerization, and treating cancers and other tubulin polymerization-related disorders with a suitable pharmaceutical acceptable carrier.
Owner:TAIPEI MEDICAL UNIV +1
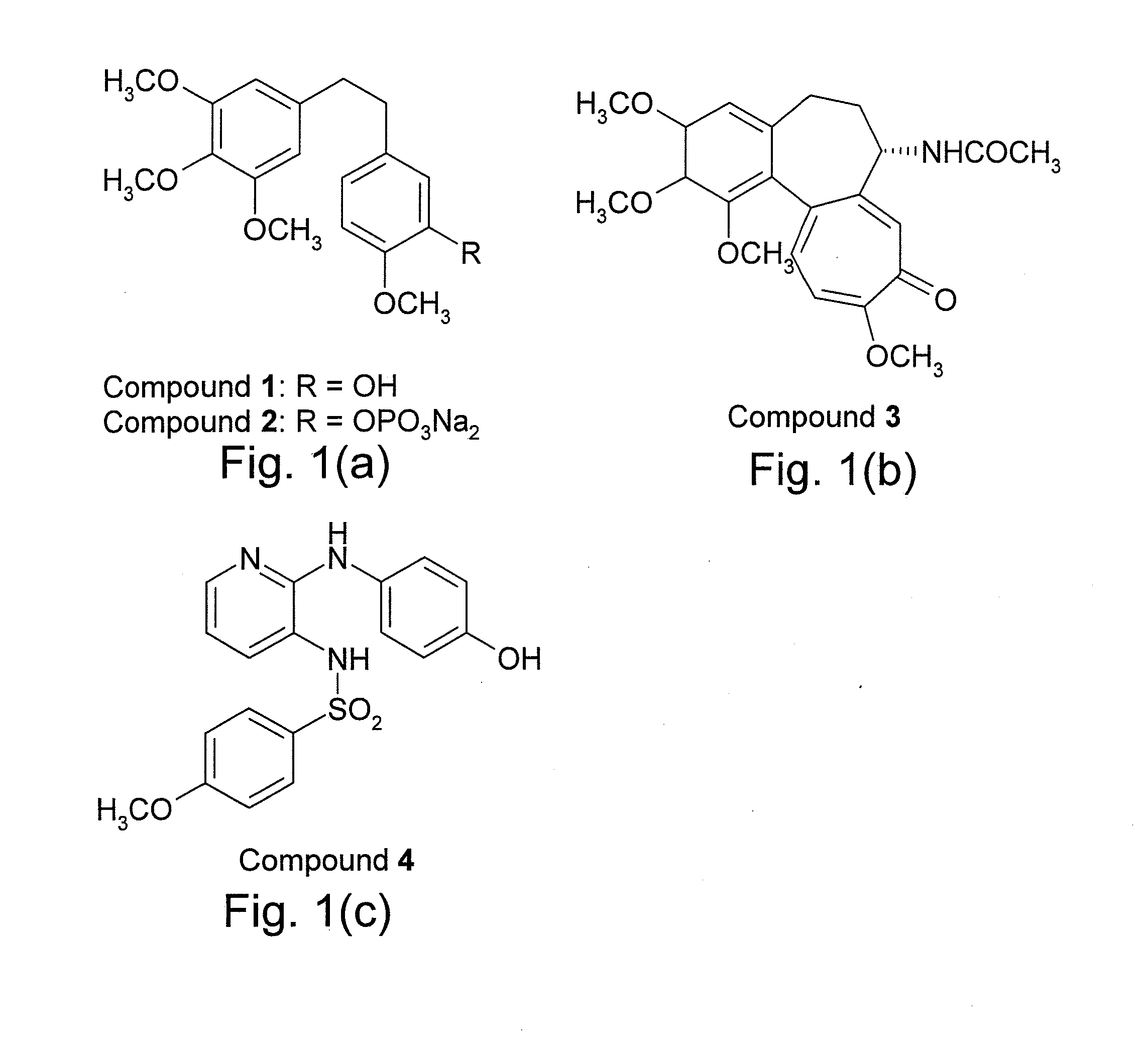
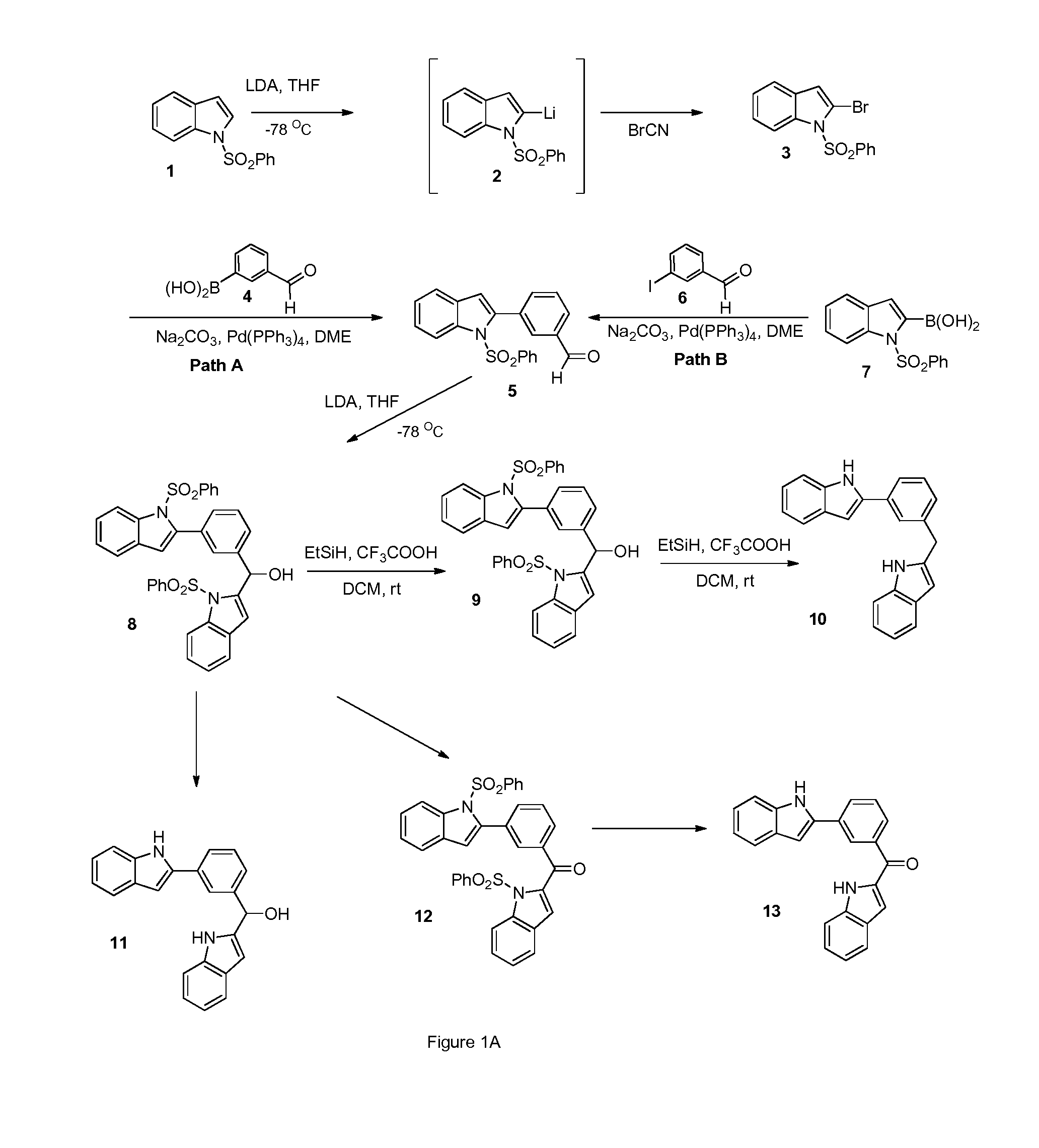
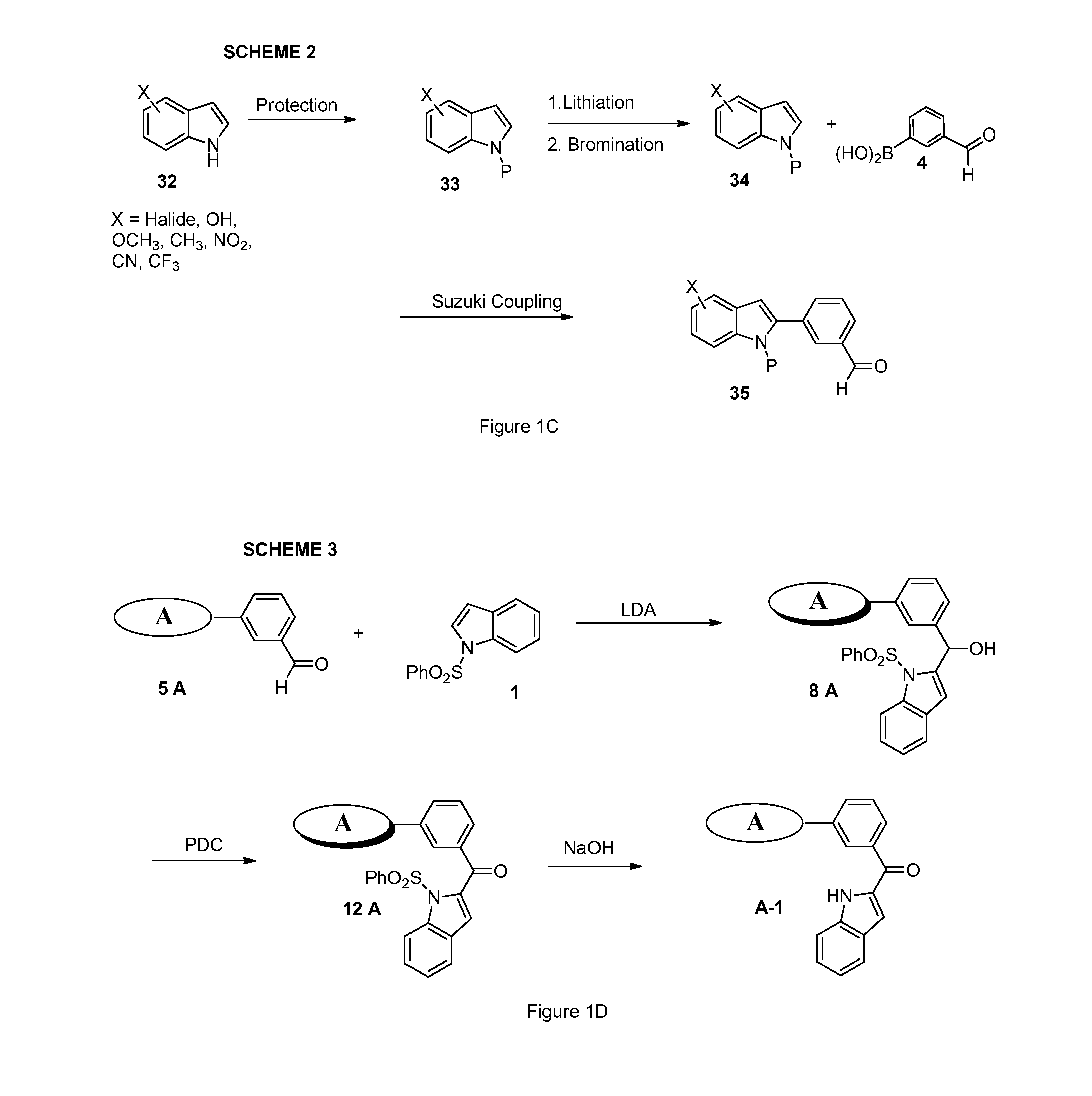
Indoles, derivatives and analogs thereof and uses therefor
InactiveUS20120022121A1Reduce severityReduce morbidityBiocideOrganic chemistryBladder cancerMelanoma
Indole derivatives and analogous compounds and pharmaceutical compositions comprising the same are provided. Also provided are methods of using these compounds to inhibit tubulin polymerization in a cell associated with a proliferative disease or to treat cancer, metastatic cancer, resistant cancer or multidrug resistant cancer, including inter-alia: prostate cancer, breast cancer, melanoma, colon cancer and bladder cancer.
Owner:DALTON JAMES T +5
Gel polymer electrolyte and lithium ion batteries employing the gel polymer electrolyte
The invention relates to a composition for preparing a gel polymer electrolyte, comprising: (1) a prepolymer; (2) a lithium salt; (3) an organic solvent; (4) a cross-linking agent; (5) an initiator; (6) optionally a monomer; and (7) optionally an additive; wherein the prepolymer comprises polyamides, polyimides and their combination. The invention also relates to a gel polymer electrolyte obtained by polymerization, especially in-situ polymerization of the composition and lithium-ion batteries employing the gelpolymer electrolyte, and a method of preparing the ge polymer electrolyte.
Owner:SHENZHEN CAPCHEM TECH CO LTD
Catalyst component used for olefin polymerization and preparation method thereof as well as catalyst used for olefin polymerization and application
The invention relates to a catalyst component used for olefin polymerization and a preparation method thereof as well as a catalyst used for olefin polymerization and application. The catalyst component comprises reaction products of the following components: (1) a solid component and (2) at least one titanium compound, wherein the solid component comprises a magnesium compound shown in Formula (1) (shown in the description) and epoxy alkanes compounds shown in Formula (2) (shown in the description); R1 is linear alkyls or branched alkyls with C1-C12; R2 is the same as or different from R3; R2 and R3 are hydrogen, or linear alkyls or branched alkyls with C1-C5 independently; hydrogen on alkyls is optionally replaced by halogen; X is halogen, m ranges from 0.1 to 1.9, n ranges from 0.1 to 1.9, and m plus n is equal to 2. By adopting the catalyst component in the process of olefin polymerization, relatively high polymerization activity and relatively high stereotactic capability can be acquired.
Owner:CHINA PETROLEUM & CHEM CORP +1
Antifungal phenylethylene
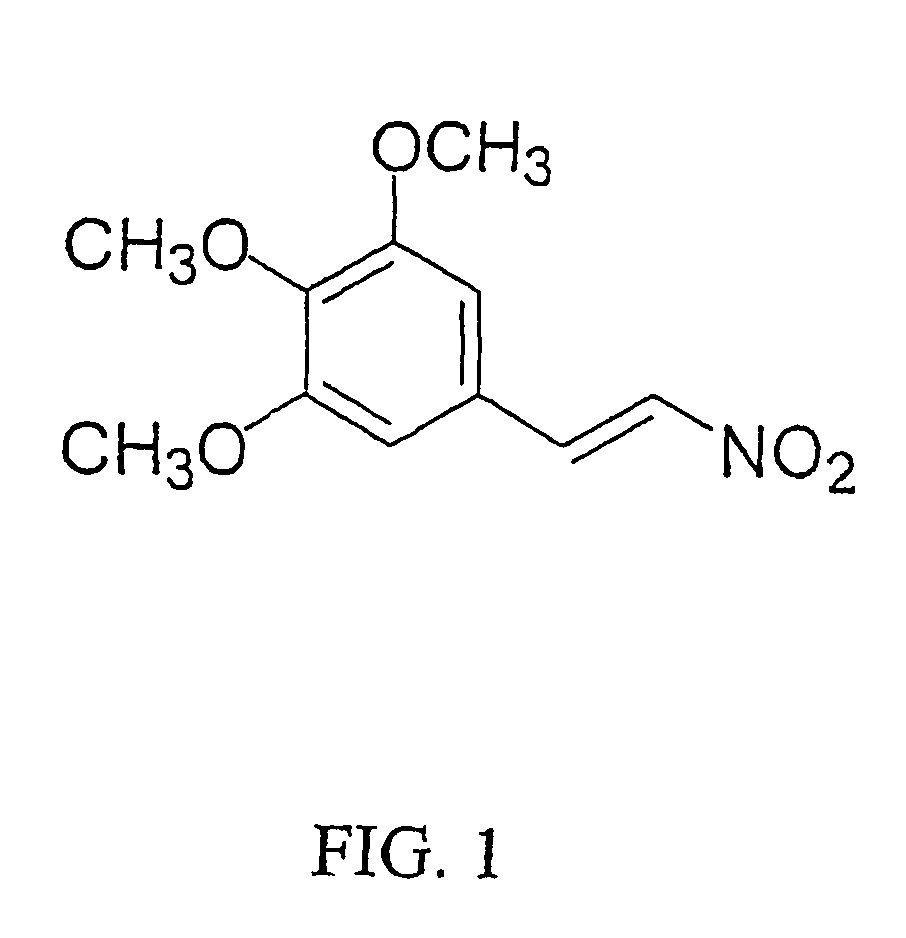
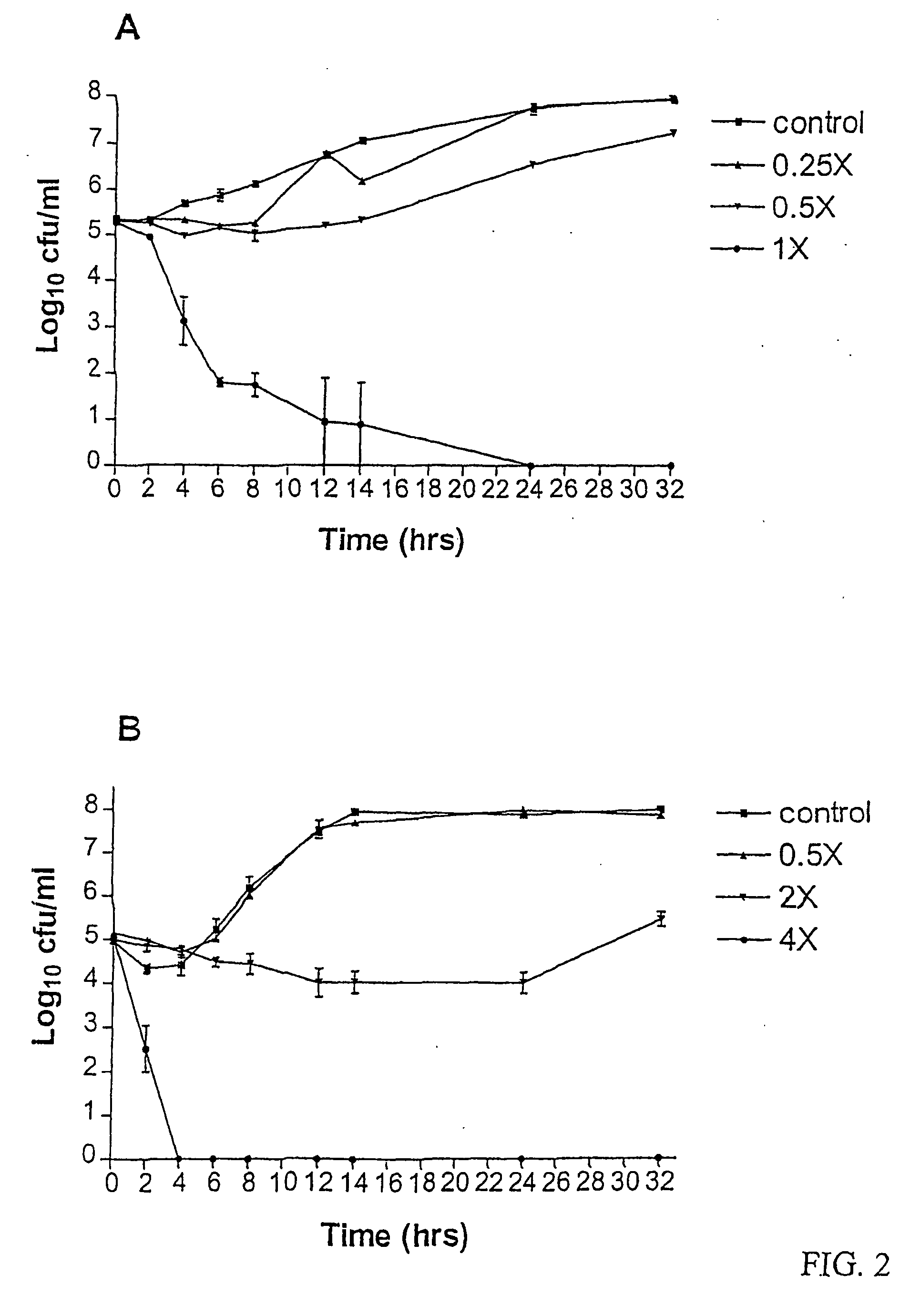
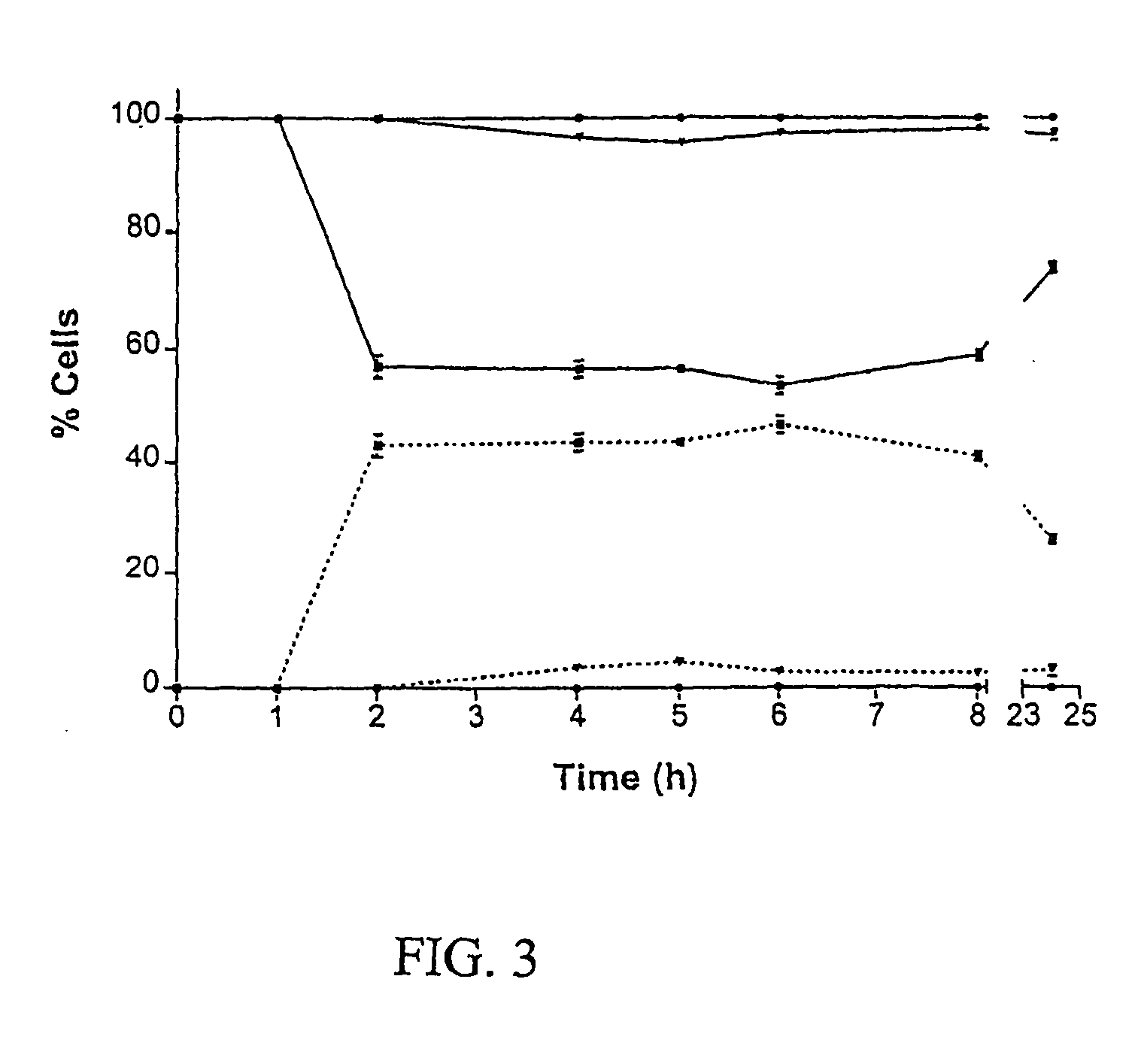
The antifungal and cancer cell growth inhibitory activities of 1-(3′,4′,5′-trimethoxyphenyl)-2-nitro-ethylene (TMPN) were examined. TMPN was fungicidal for the majority of 132 reference strains and clinical isolates tested, including those resistant to fluconazole, ketoconazole, amphotericin B or flucytosine. Minimum fungicidal concentration / minimum inhibitory concentration (MFC / MIC) ratios were ≦2 for 96% of Cryptococcus neoformans clinical isolates and 71% of Candida albicans clinical isolates. TMPN was fungicidal for a variety of other basidiomycetes, endomycetes and hyphomycetes, and its activity was unaffected by alterations in media pH. TMPN was slightly cytotoxic for murine and human cancer cell lines (GI50=1-4 μg / ml), and weakly inhibited mammalian tubulin polymerization (IC50=0.60 μg / ml). TMPN may also be used as a biochemical probe for tubulin and fungal dimorphism studies.
Owner:ARIZONA STATE UNIVERSITY
Catalyst component for olefin polymerization and catalyst thereof
Owner:CHINA PETROLEUM & CHEM CORP +1
Indoles, Derivatives, and Analogs Thereof and Uses Therefor
InactiveUS20090142832A1Growth inhibitionTreat cancerOrganic chemistryTissue cultureTubulin polymerizationStereochemistry
Indole derivatives and analog compounds and pharmaceutical compositions comprising the same are provided. Also provided are methods of using these compounds to inhibit tubulin polymerization in a cell associated with a proliferative disease or to treat a cancer.
Owner:UNIV OF TENNESSEE RES FOUND +1
Perylene diimide photoelectric functional materials and preparation method thereof
InactiveCN102070771ATest thermal stabilityImprove thermal stabilityOrganic chemistryFinal product manufactureOrganic solar cellPerylene
The invention discloses perylene diimide-based photoelectric functional materials and a preparation method thereof. A series of polymers with different contents are prepared through Suzuki polymerization, Yamamoto polymerization, Stille polymerization or Sonogashira polymerization. The materials have good thermal, photic and chemical stability and good application prospects in the fields such as organic solar cells and the like. The structural formula of the perylene diimide-based polymers is shown in the specification, wherein a is a number between 1 and 30; b is a number between 0 and 30; R is C1-C10 alkyl or alkoxy; A1 is any one of the following structures shown in the specification, wherein n is a number between 1 and 10; and R is C1-C10 alkyl.
Owner:NANJING UNIV OF POSTS & TELECOMM
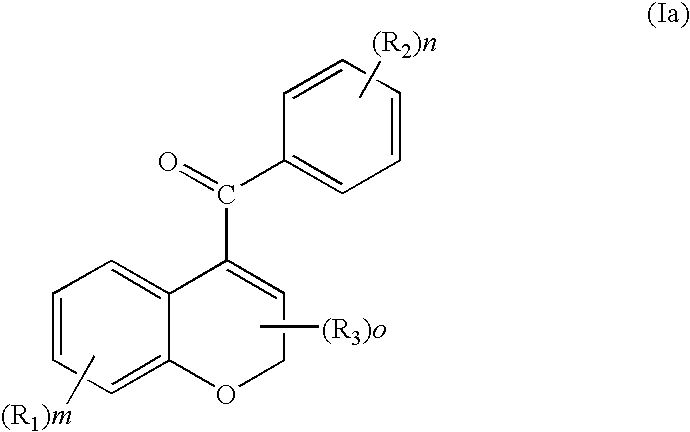
Chromene-containing compounds with anti-tubulin and vascular targeting activity
Chrome compounds have been discovered which demonstrate impressive cytotoxicity as well as a remarkable ability to inhibit tubulin polymerization. Such compounds as well as related derivatives are excellent clinical candidates for the treatment of cancer in humans. In addition, certain of these ligands, as pro-drugs, may well prove to be tumor selective vascular targeting chemotherapeutic agents or to have vascular targeting activity resulting in the selective prevention and / or destruction of nonmalignant proliferating vasculature.
Owner:MATEON THERAPEUTICS INC +1
Catalyst component, catalyst and method for olefin polymerization
The invention provides a catalyst component, a catalyst and a method for olefin polymerization. An internal donor a and an internal donor b are used as internal donors, so that when the catalyst component and the catalyst are used for olefin polymerization, the catalyst component and the catalyst have high stereo selectivity, high activity and high hydrogen response, and the prepared olefin polymer has wide molecular weight distribution, wherein the internal donor a is a compound shown as a formula (I); and in the formula (I), R1 and R3 are same or different, and refer to a straight-chain or branched-chain alkyl with the carbon atom number of 1-10, naphthenic base with the carbon atom number of 3-10 or substituted or non-substituted aryl with the carbon atom number of 6-20; R2 refers to straight-chain or branched-chain alkylene with the carbon atom number of 1-6 and substituted or non-substituted aryl with the carbon atom number of 6-20; and n is an integer from 2 to 10.
Owner:CHINA PETROLEUM & CHEM CORP +1
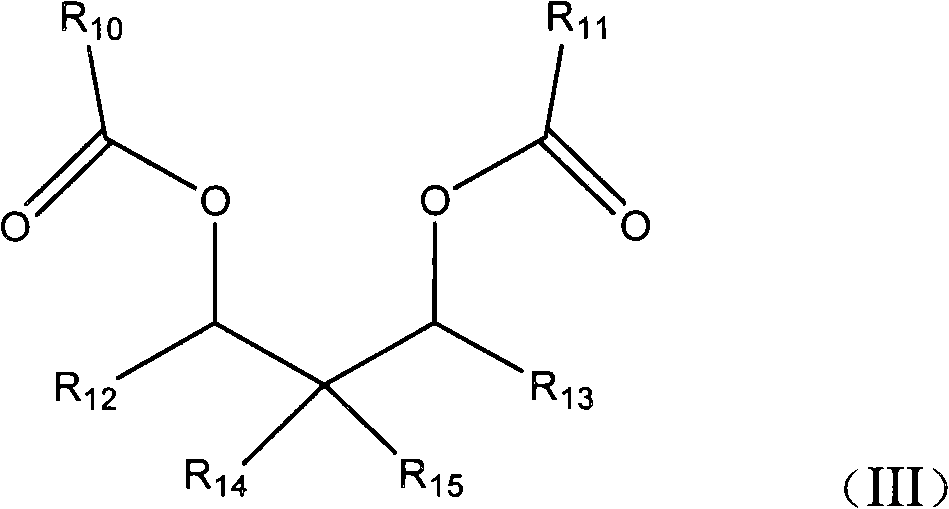
Compounds for the treatment of proliferative disorders
InactiveUS20070238699A1Inhibit tubulin polymerizationBiocideAnimal repellantsDiseasePharmaceutical medicine
The invention relates to compounds of structural formula (I): or a pharmaceutically acceptable salt, solvate, clathrate, and prodrug thereof, wherein Ra, Rb, and R2 are defined herein. These compounds inhibit tubulin polymerization and / or target vasculature and are useful for treating proliferative disorders, such as cancer.
Owner:SYNTA PHARMA CORP
Chromene-containing compounds with anti-tubulin and vascular targeting activity
InactiveUS20050245489A1Substantial necrosisReduced tumor blood flowBiocideOrganic chemistryCytotoxicityCancer research
Trimethoxyphenyl substituted indole ligands have been discovered which demonstrate impressive cytotoxicity as well as a remarkable ability to inhibit tubulin polymerization. Such compounds as well as related derivatives are excellent clinical candidates for the treatment of cancer in humans. In addition, certain of these ligands, as pro-drugs, may well prove to be tumor selective vascular targeting chemotherapeutic agents or to have vascular targeting activity resulting in the selective prevention and / or destruction of nonmalignant proliferating vasculature.
Owner:OXIGENE +1
Combretastatin analogs with tubulin binding activity
Analogs of combretastatin have been discovered which demonstrate impressive cytotoxicity as well as a remarkable ability to inhibit tubulin polymerization. Such compounds are excellent clinical candidates for the treatment of cancer in humans. In addition, certain of these ligands, as pro-drugs, may well prove to be tumor selective vascular targeting chemotherapeutic agents or to have vascular targeting activity resulting in the selective prevention and / or destruction of nonmalignant proliferating vasculature.
Owner:BAYLOR UNIVERSITY
Compounds for the treatment of proliferative disorders
InactiveUS20060217389A1Useful in treatmentBiocideSenses disorderStructural formulaTubulin polymerization
The invention relates to compounds of structural formula (I): or a pharmaceutically acceptable salt, solvate, clathrate, and prodrug thereof, wherein Ra, Rb, and R2 are defined herein. These compounds inhibit tubulin polymerization and / or target vasculature and are useful for treating proliferative disorders, such as cancer.
Owner:SYNTA PHARMA CORP
Phenoxy ester coordinated transition metal organic complex, olefin polymerization catalytic system comprising same and application of catalytic system to olefin polymerization
ActiveCN103554173AEasy to controlHigh catalytic activityTitanium organic compoundsOrganic compoundCoordination complex
The invention discloses a novel phenoxy ester coordinated transition metal organic complex, an olefin polymerization catalytic system comprising the same and an application of the catalytic system. The catalytic system comprises (A) a transition metal organic complex with a general formula (I) and (B) main group metal organic compounds, organic aluminoxane and ionic compounds which can be ionized. The transition metal organic complex is shown in the general formula (I), wherein in the formula, M is one or more of transition metal elements in an IV, V or VIII subgroup in the periodic table of elements, m is an integer from 1 to 3, n is a number satisfying the M valence state, X is halogen, alkoxy and the like, R1 is C1-20 alkyl or C6-20 aryl or substituted aryl, and R2-R5 are the same or different and are selected from hydrogen atoms, halogen atoms, alkyl, silyl and the like. The catalytic system has the advantages of high catalytic activity, good thermal stability, strong copolymerization capability and the like, can catalyze olefin polymerization and copolymerization, and can be used in various polymerization methods.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Mixed decyl mercaptans compositions and use thereof as chain transfer agents
A chain transfer agent composition comprises at least one branched C10 mercaptan selected from 5-methyl-1-mercapto-nonane, 3-propyl-1-mercapto-heptane, 4-ethyl-1-mercapto-octane, 2-butyl-1-mercapto-hexane, 5-methyl-2-mercapto-nonane, 3-propyl-2-mercapto-heptane, 4-ethyl-2-mercapto-octane, 5-methyl-5-mercapto-nonane, or combinations thereof. The chain transfer agent composition can be a component of an emulsion polymerization mixture and can be used in a process for emulsion polymerization for the production of polymers, for example, via free-radical polymerization.
Owner:CHEVRON PHILLIPS CHEMICAL CO LP
Novel tubulin inhibitors and methods of using the same
Compounds represented by the formula (I) or pharmaceutically acceptable salts thereof:R2—Y—Z-Q-A-R1 Formula (I)wherein R1, R2, Y, Z, Q, and A are as defined. These compounds are inhibitors of tubulin polymerization by binding at colchicines binding site and are useful in the treatment of tumors or mitotic diseases such as cancers, gout, and other conditions associated with abnormal cell proliferation.
Owner:DEV CENT FOR BIOTECHNOLOGY
Inhibitors of Tubulin Polymerization
The invention relates to the inhibition of tubulin polymerization. The invention provides compounds and methods for inhibiting tubulin polymerization. The invention also provides compositions and methods for treating cell proliferative diseases and conditions.
Owner:RIGEL PHARMA
Application of quinazoline compounds
InactiveCN101926802AInhibits mitosisInhibit abnormal proliferationOrganic active ingredientsNervous disorderMitotic ProcessQuinazoline
The invention relates to the technical field of drugs, in particular to application of quinazoline compounds. The invention discloses application of the quinazoline compounds shown in the general formula I or pharmaceutically acceptable salt, prodrugs or isomers thereof in preparing mitotic inhibitors. The quinazoline compounds shown in the general formula I or pharmaceutically acceptable salt, prodrugs or isomers have the effect of inhibiting tubulin polymerization and can effectively inhibit mitosis.
Owner:XIANGBEI WELMAN PHARMA CO LTD +1
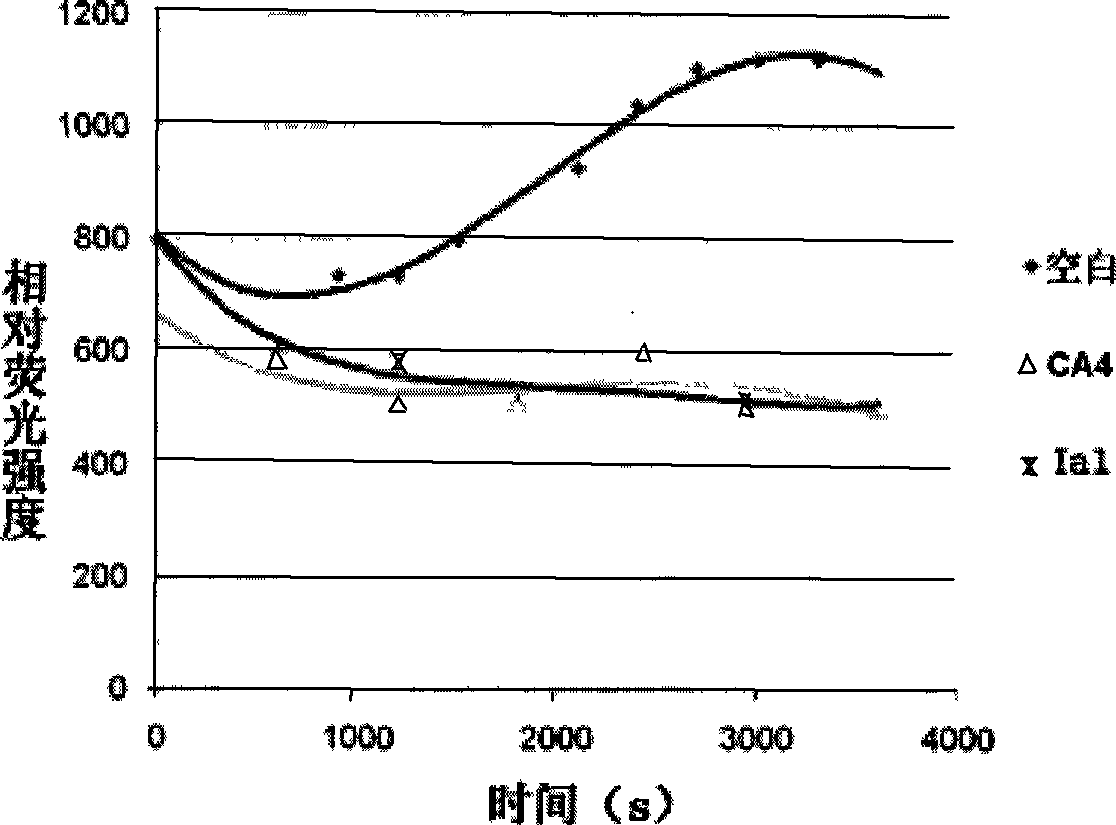
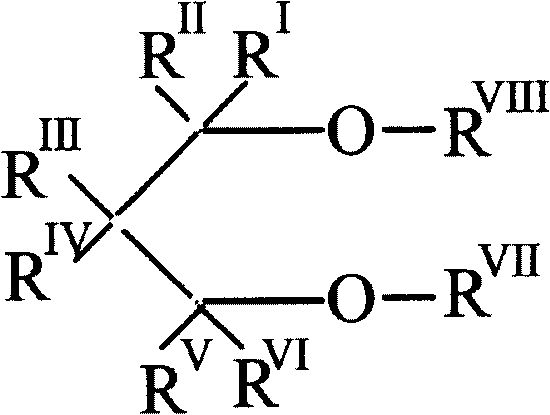
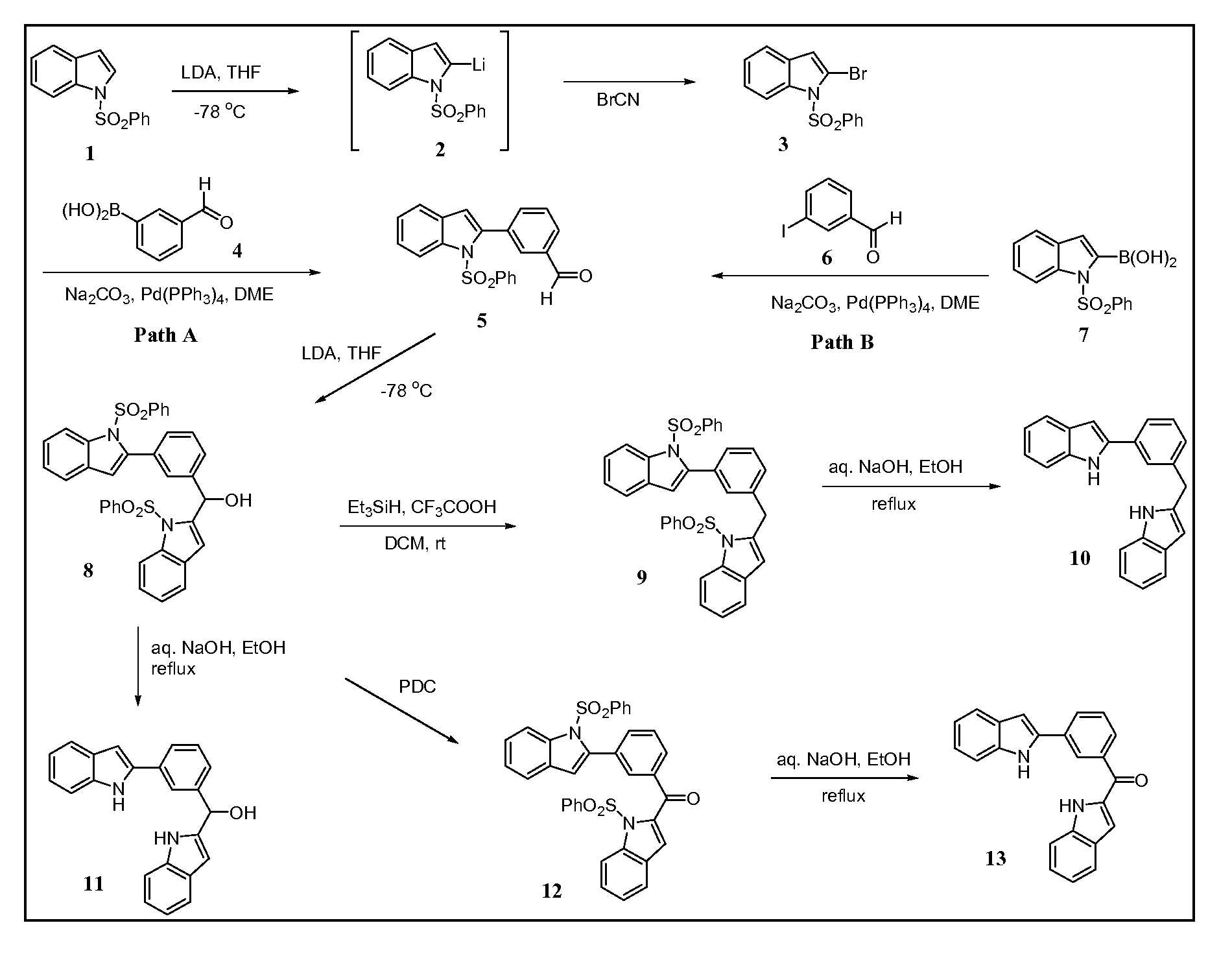
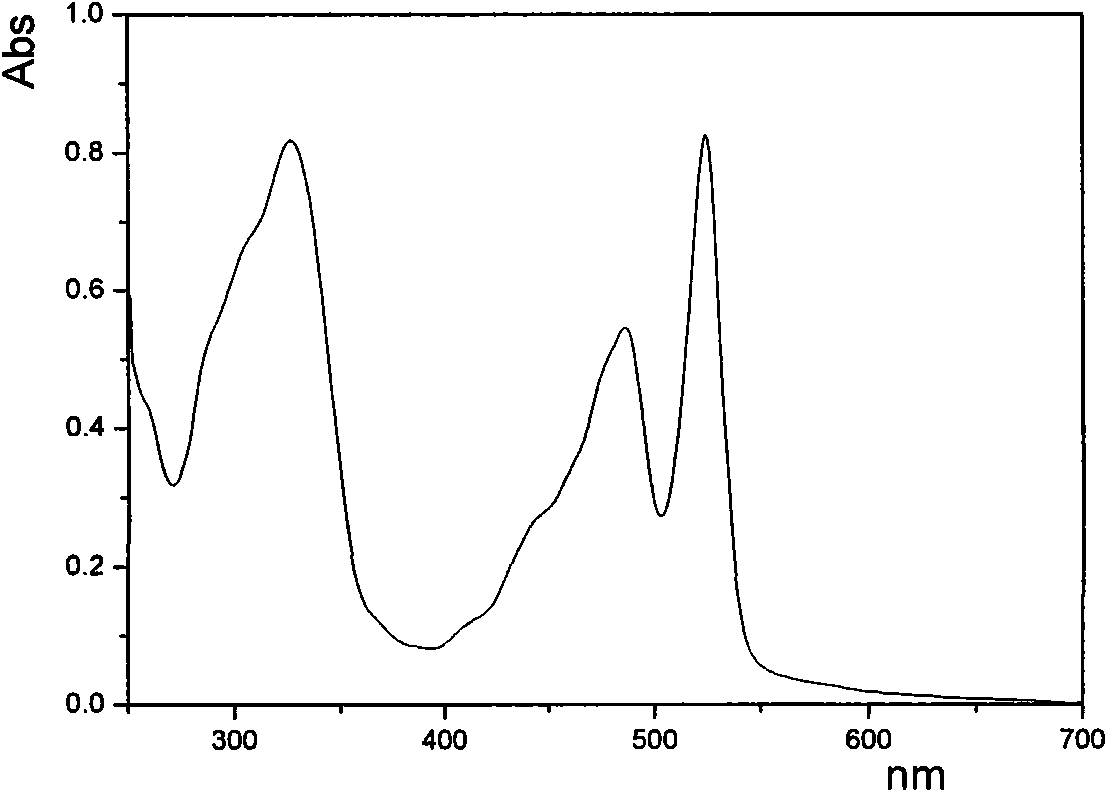
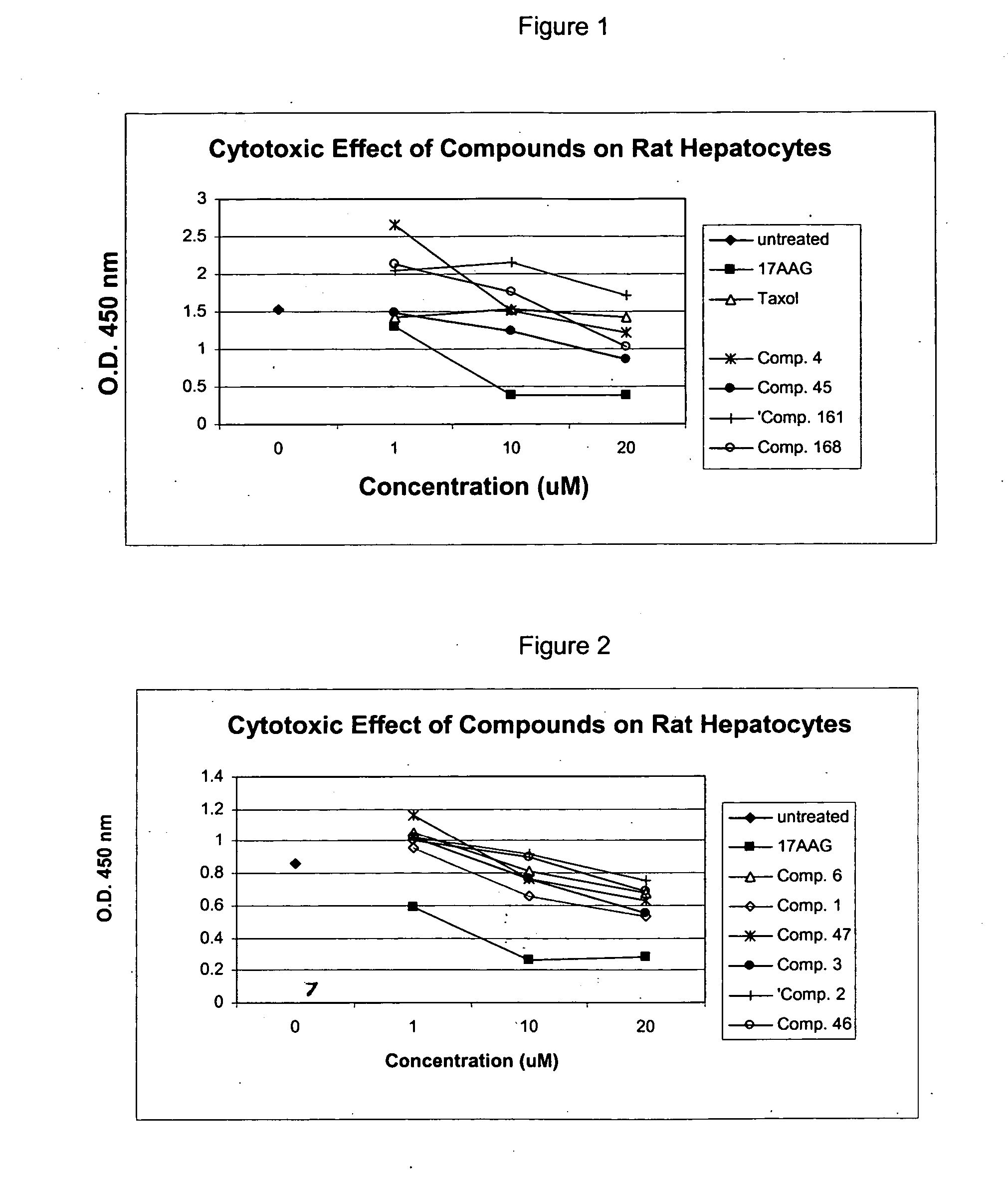
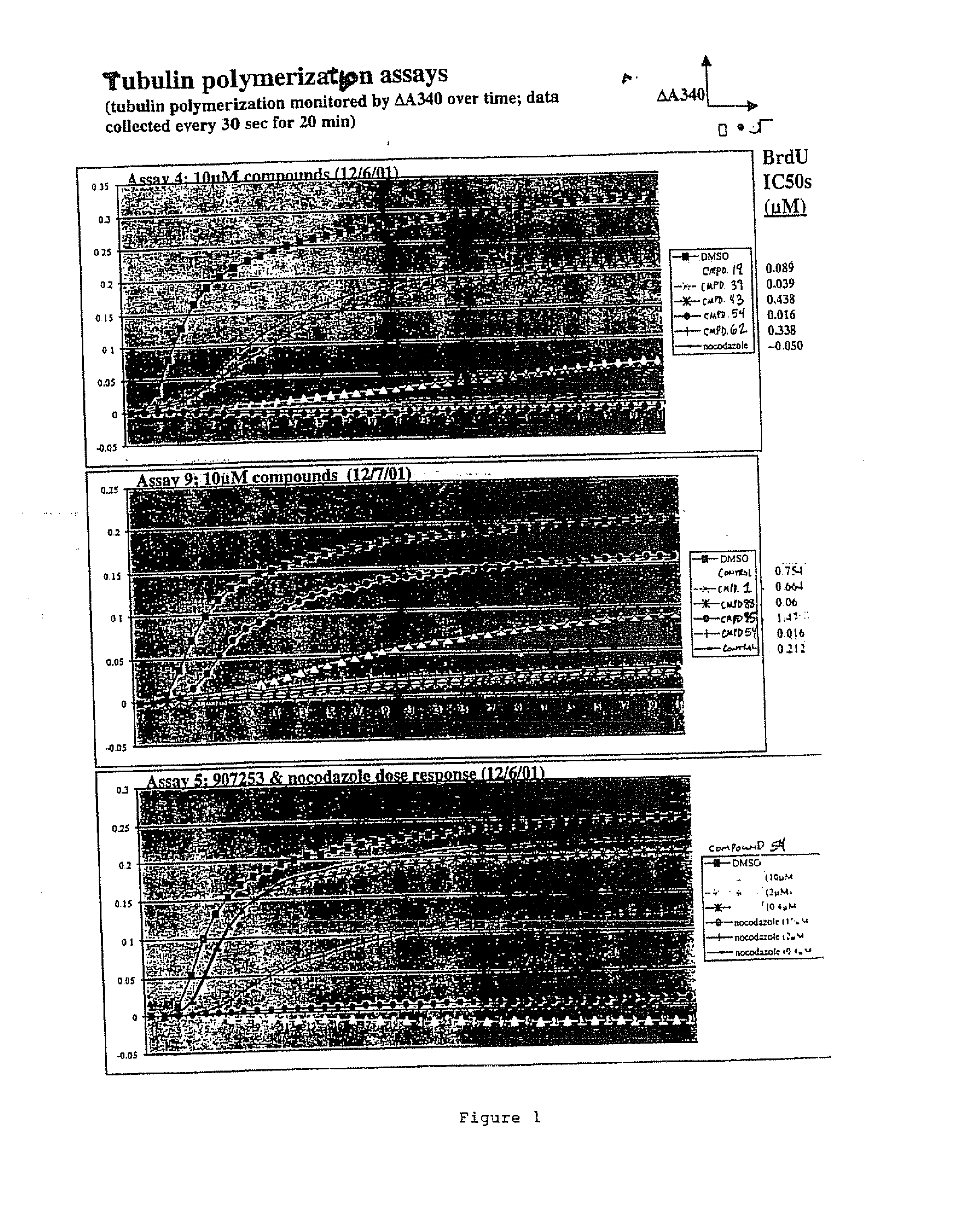
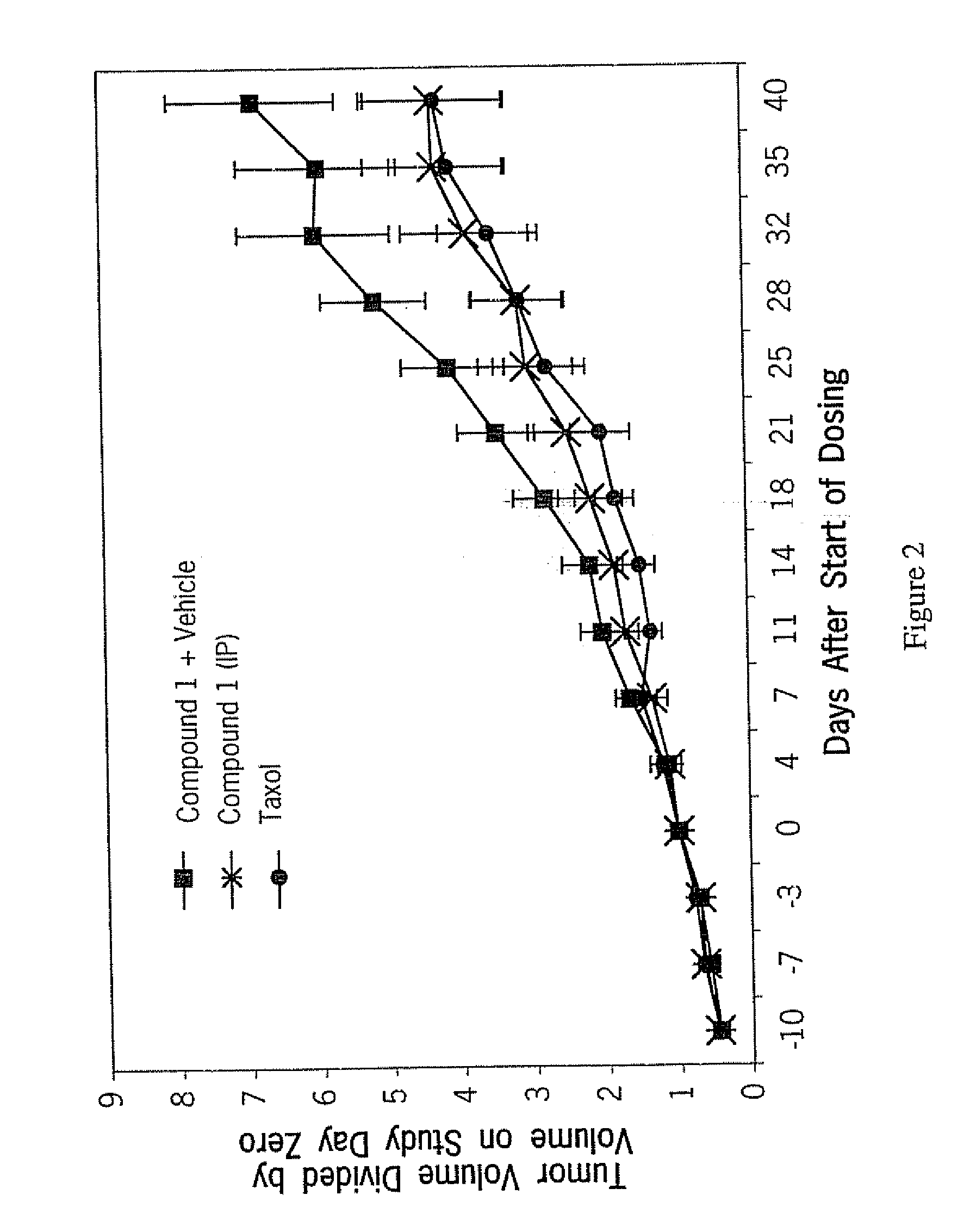
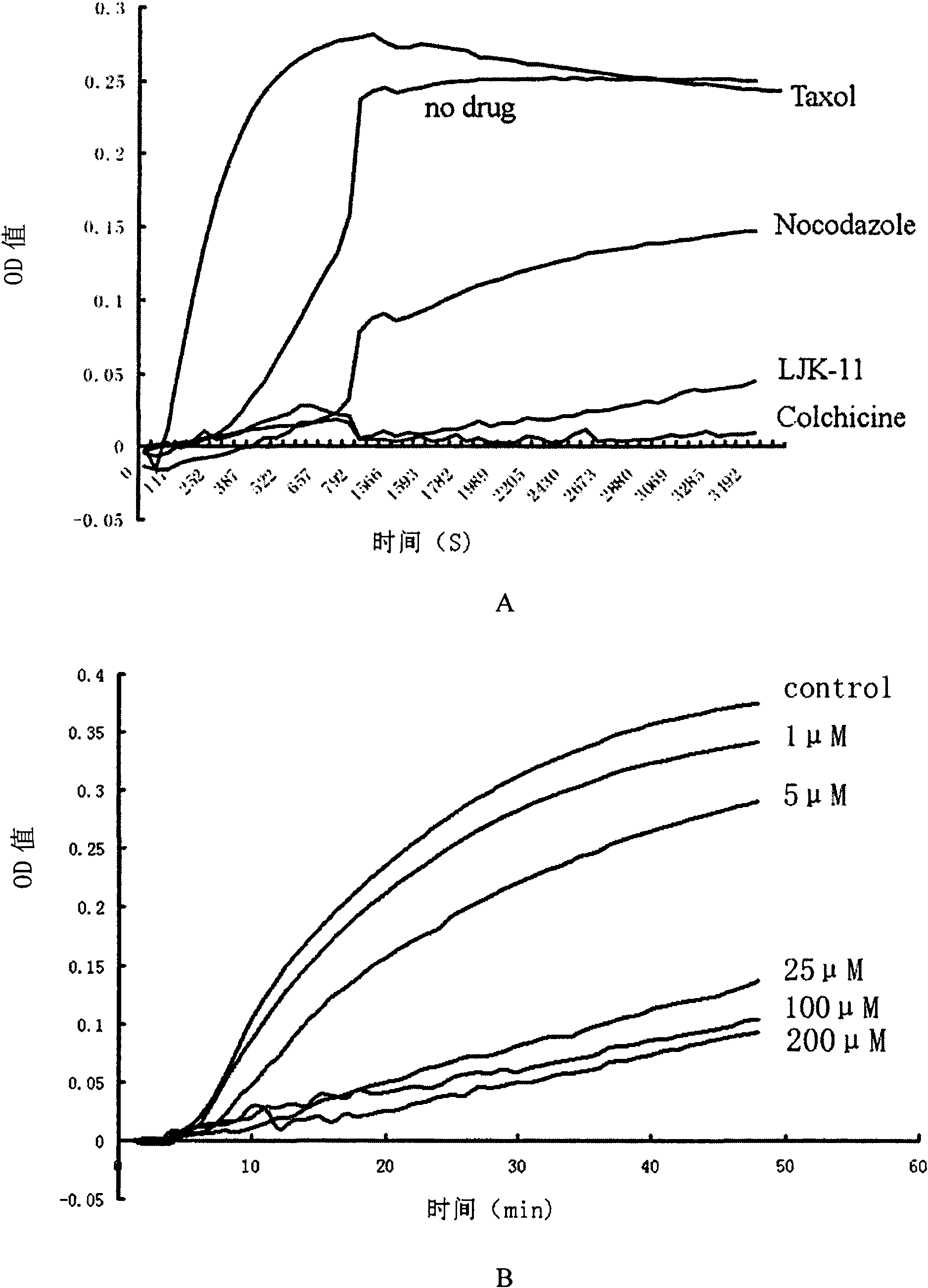
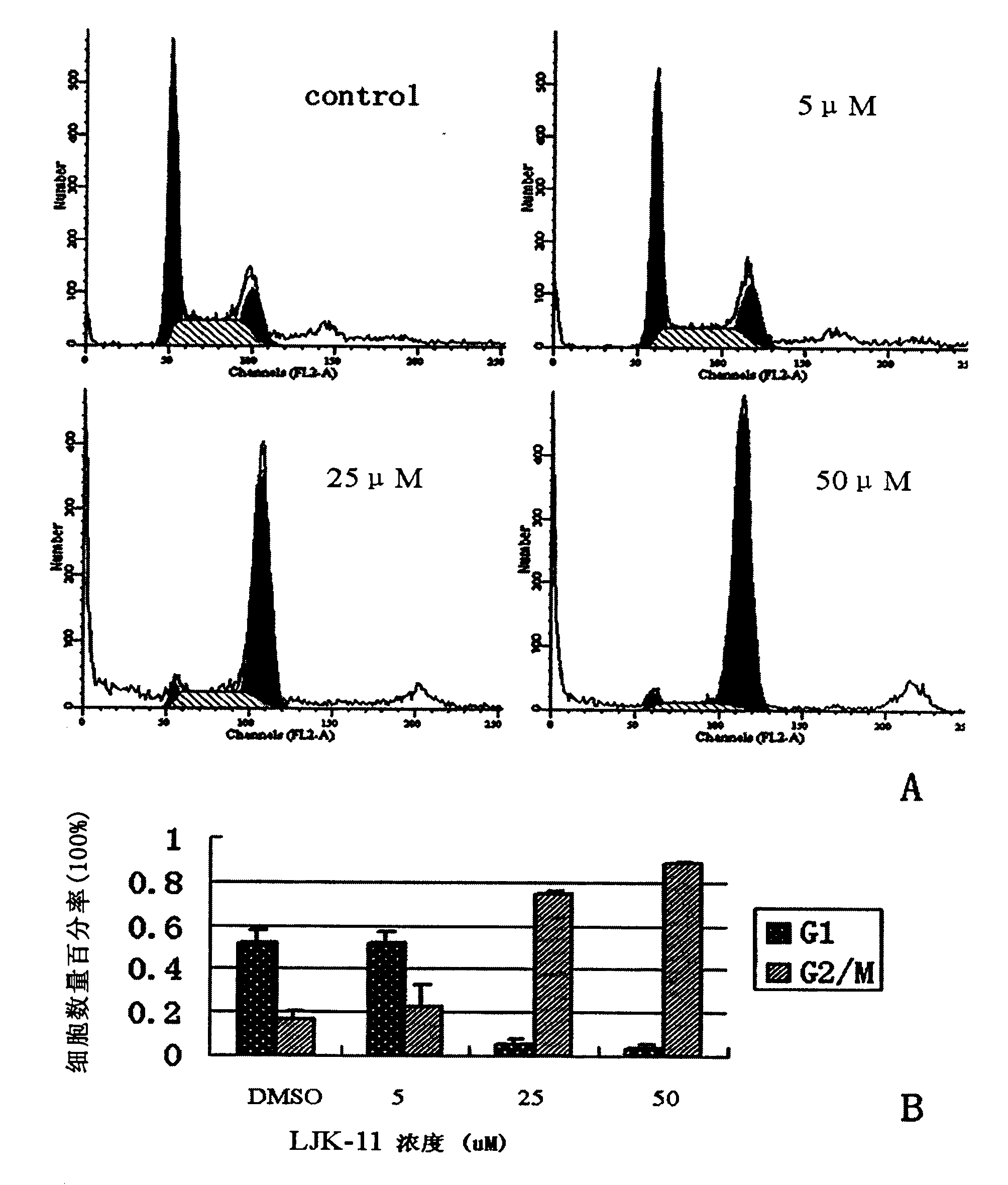
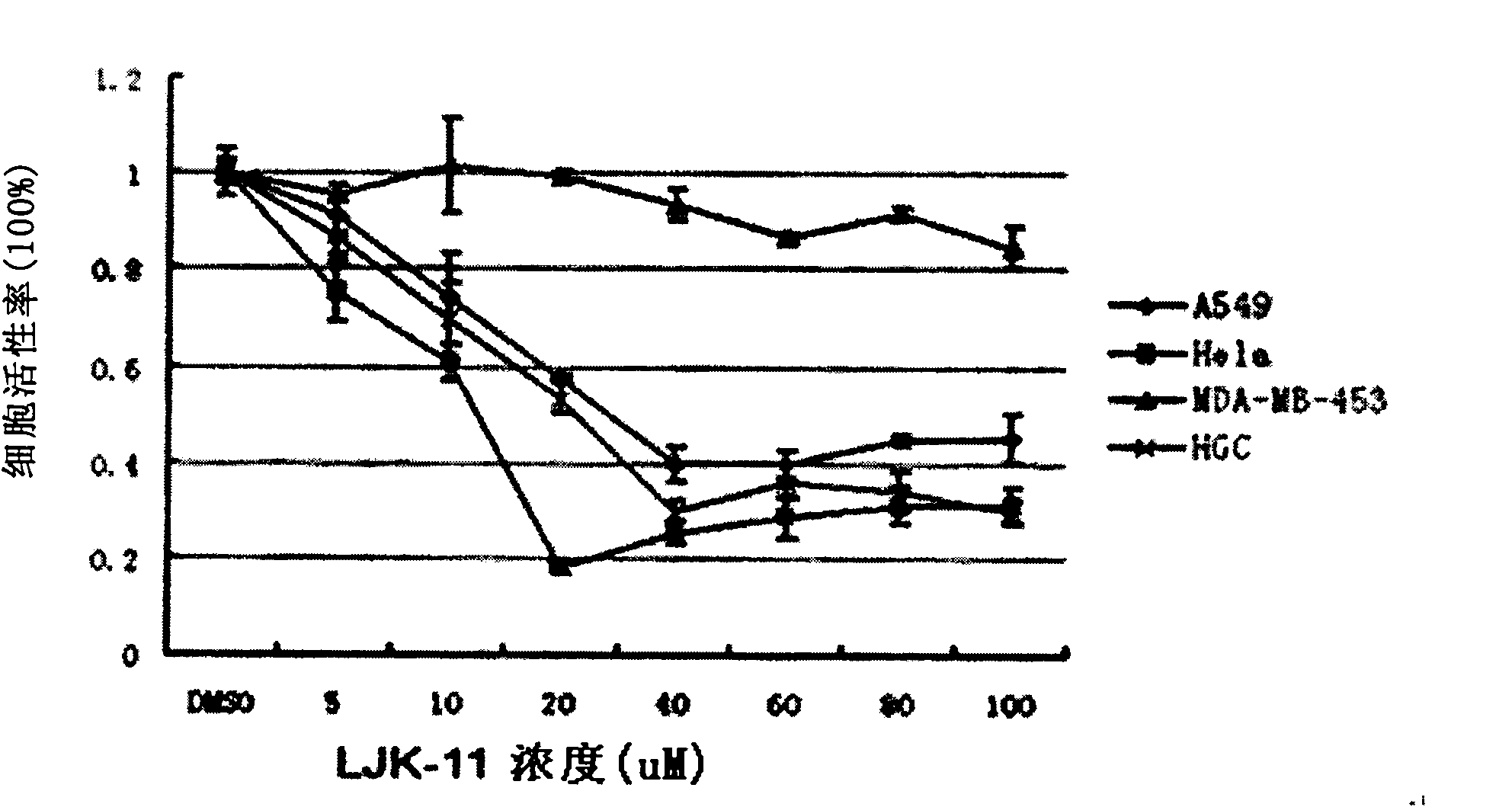
COMPOSITION FOR PREVENTION OR TREATMENT OF CANCER COMPRISING N-METHYLENENAPHTHO[2,1-b]FURAN-2-CARBOHYDRAZIDE DERIVATIVES AS AN ACTIVE INGREDIENT
The present invention relates to ethyl(2-methyl-3{(E)-[(naphtho[2,1-b]furan-2-ylcarbonyl)hydrazono]methyl}-1H-indole-1-yl)acetate, the novel mitosis inhibitor. The said ethyl(2-methyl-3{(E)-[(naphtho[2,1-b]furan-2-ylcarbonyl)hydrazono]methyl}-1H-indole-1-yl)acetate not only induces apoptosis by inhibiting tubulin polymerization in the course of mitosis but also displays an excellent anticancer effect in the multi-drug resistant cancer cells, so that it can be effectively used for the treatment of cell proliferative disease including various cancers.
Owner:KOREA RES INST OF BIOSCI & BIOTECH
Gamma-carbolines derivates as well as preparation method and application thereof
InactiveCN101423517AWide variety of sourcesReasonable designOrganic active ingredientsOrganic chemistryPositive controlSide chain
The invention provides a gamma-carboline derivative, which is obtained by using gamma-carboline with antineoplastic activity as a lead compound, and introducing different lateral chains, aromatic rings, aromatic heterocycles or heterocycles to the sixth position of the gamma-carboline. Pharmacological active screening test shows that the compound has in-vitro antihyperplasia function on tumor cells; partial compound has obvious anti-tumor activity; the value of IC50 reaches mu M grade to show cytotoxicity similar to positive control polyenic taxusol; and significant microtubulin polymerization retardation activity and DNA intercalation function are presented in primary activity mechanism research; therefore, the compound is expected to be a new antineoplastic reagent with double functions of micromolecular microtubulin and DNA. The gamma-carboline derivative has the advantages of reasonable design, wide source of raw materials, easy preparation, mild reaction condition, simple operation and practicality. The structural formula of the compound is shown as the above.
Owner:ZHEJIANG UNIV
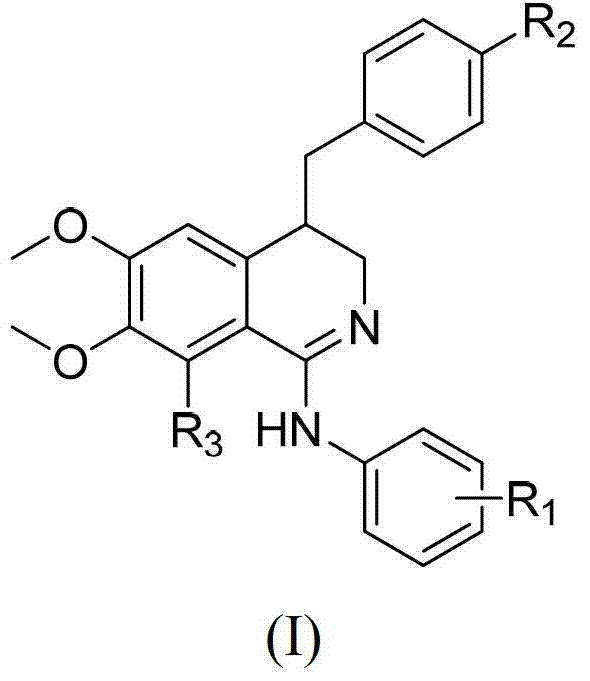
3, 4-dihydroisoquinoline antitumor compounds as well as preparation method and application thereof
The invention discloses 3, 4-dihydroisoquinoline antitumor compounds, a preparation method of the compounds, and an application of the compounds in preparation of antitumor medicines. The compounds have a structural formula (I) as shown in the specification. The compounds disclosed by the invention are structurally novel antitumor compounds which have the double mechanisms of inhibiting kinase VEGFR-2 (Vascular Endothelial Growth Factor Receptor-2) and promoting tubulin polymerization, and have good antitumor activity.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Two-alkoxyl-silicon-containing alpha-diimine compound and application of load type metal complex thereof in olefin polymerization
InactiveCN108530562AImprove stabilityNot easy to fall offGroup 4/14 element organic compoundsReaction temperatureHigh activity
The invention discloses a two-alkoxyl-silicon-containing alpha-diimine compound and the application of a load type metal complex thereof in olefin polymerization. The alpha-diimine compound structurecontains two active groups, namely alkoxyl silicon, so that the alpha-diimine compound can be loaded on the surface of a carrier in a covalent grafting manner; moreover, the two active groups greatlyincrease the firmness of the alpha-diimine compound loaded on the carrier. The loaded alpha-diimine compound is matched with the metal complex to obtain an alpha-diimine compound-loaded metal complexwhich is applied to the olefin polymerization. During the olefin polymerization, the loaded metal complex used as a catalyst is high in thermal stability and can still keep relatively high activity ata reaction temperature of 100 DEG C; the catalyst is hard to fall off, and the phenomenon of kettle sticking during the polymerization is obviously improved.
Owner:TIANJIN POLYTECHNIC UNIV
Use of compound with structure similia combretastatin A4 in preparation medicine for inhibiting tubulin polymerization or treating tumor
InactiveCN101073561AEther/acetal active ingredientsAntineoplastic agentsWilms' tumorOrganic chemistry
The invention is concerned with the compositions that the structure is similar with Combretastatin A4 and using for depression tubulin polymerization or anti-tumor drug.
Owner:SUN YAT SEN UNIV
Catalyst component used for vinyl polymerization and catalyst
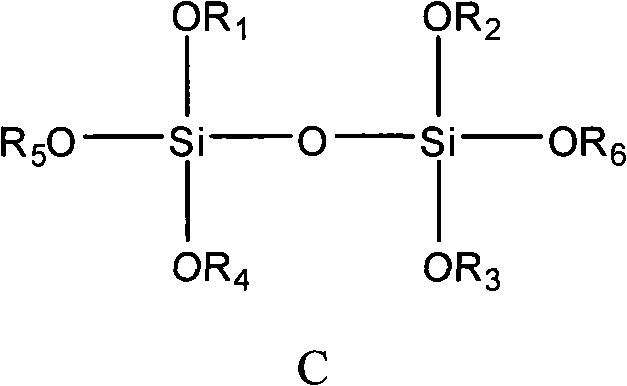
The invention provides a catalyst component used for vinyl polymerization. The catalyst component comprises the following components: a magnesium composite, a titanium compound and an organosilicon compound, wherein the magnesium composite is a product prepared by dissolving alkoxy magnesium in a solvent system containing an organic epoxy compound, boron trihalide and at least one kind of organicalcohol; the titanium compound has the general formula of Ti(OR)aXb, wherein R refers to aliphatic hydrocarbon group or aryl group with 1 to 14 carbon atoms, X refers to halogen, a is 0, 1 or 2, b isan integer from 1 to 4, and a+b=3 or 4; and the organosilicon compound has at least one structural formula of a structural formula a, a structural formula b and a structural formula C.
Owner:CHINA PETROLEUM & CHEM CORP +1
Nitrogen substitutive 3-oxygen substitutive-6-tetrahydroquinoxaline substitutive structure compound and preparation method and medical application thereof
Disclosed are nitrogen substitutive 3-oxygen substitutive-6-tetrahydroquinoxaline substitutive structure compound and preparation method and medical application thereof. The general structure of the compound is as shown in the formula (I). The compound has tubulin polymerization inhibition activities, and is good in tumor disease resistance and proliferative disease apart from tumors.
Owner:HENAN UNIVERSITY
Taccalonolide microtubule stabilizing agents
InactiveUS6878699B1Promote efficient proliferationTransport becomes poorOrganic active ingredientsSteroidsGlycophorinMDA-MB-435
In accordance with the present invention, there have been identified extracts from a tropical plant that initiate paclitaxel-like microtubule bundling. Bioassay-directed purification yields the steroid taccalonolide A. Taccalonolide, like paclitaxel, initiates the formation of abnormal interphaes and mitotic microtubules. Cells treated with taccalonolide exhibit thick bundles of microtubules that appear to nucleate independent of the microtubule organizing center. Abnormal mitotic spindles consisting of multi-polar spindles are initiated by taccalonolide and resemble abnormal mitotic spindles found in the presence of paclitaxel. Like paclitaxel, taccalonolide causes the breakdown of the nucleus into micronuclei. Taccalonolide causes G2 / M arrest, Bc1-2 phosphorylation and initiates an apoptotic cascade that includes the activation of caspase 3. Taccalonolide is an effective inhibitor of proliferation against both SK-OV-3 and MDA-MB-435 cell with IC50 values of 2.3 μM and 2.1 μM respectively. In contrast to paclitaxel, taccalonolide is effective against the multidrug resistant SKVLB-1 cellline and thus appears to be a poor substrate for P-glycoprotein-mediated transport. Although taccalonolide is almost equipotent with paclitaxel in its effects on cellular microtubules, it is much less potent than paclitaxel in its ability to initiate the polymerization of purified tubulin and microtubule protein. Taccalonolide A is the first microtubule stabilizing agent to be discovered from a plant since identification of the mechanism of action of paclitaxel and it is the first natural product steroid identified to have these cellular effects.
Owner:UNIV OF HAWAII
Catalyst component for ethylene polymerization, and preparation method thereof, and catalyst for ethylene polymerization
ActiveCN109694423AParticle size controllableNarrow particle size distributionEpoxyOrganophosphorous compounds
The present invention relates to the technical field of catalysts for ethylene polymerization, and provides a catalyst component for ethylene polymerization, and a preparation method thereof, and a catalyst for ethylene polymerization. The catalyst component comprises the reaction product of the following components: (1) a magnesium compound, (2) an organophosphorus compound, (3) an alcohol compound, (4) an organic epoxy compound, (5) an oxygen-containing organotitanium compound, and (6) a halogenated aromatic ester compound, wherein the halogenated aromatic ester compound has a general formula of R<1>C6H4COO(CH2)nCHmX3-m, R<1> is selected from hydrogen, halogen, C1-C20 alkyl, C1-C20 substituted alkyl, C3-C20 cycloalkyl and C6-C20 aromatic group, X is halogen, n and m are integers, n is more than or equal to 0 and is less than or equal to 10, and m is more than or equal to 0 and is less than 3. According to the present invention, the obtained catalyst component has characteristics of increased particle size and significantly-narrowed particle size distribution, and the obtained polymer has high bulk density and good hydrogen sensitivity.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation and use of heterogeneous catalyst components for olefins polymerization
InactiveUS20030144135A1Effective controlGuaranteed effective sizeOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationLanthanideHomogeneous catalysis
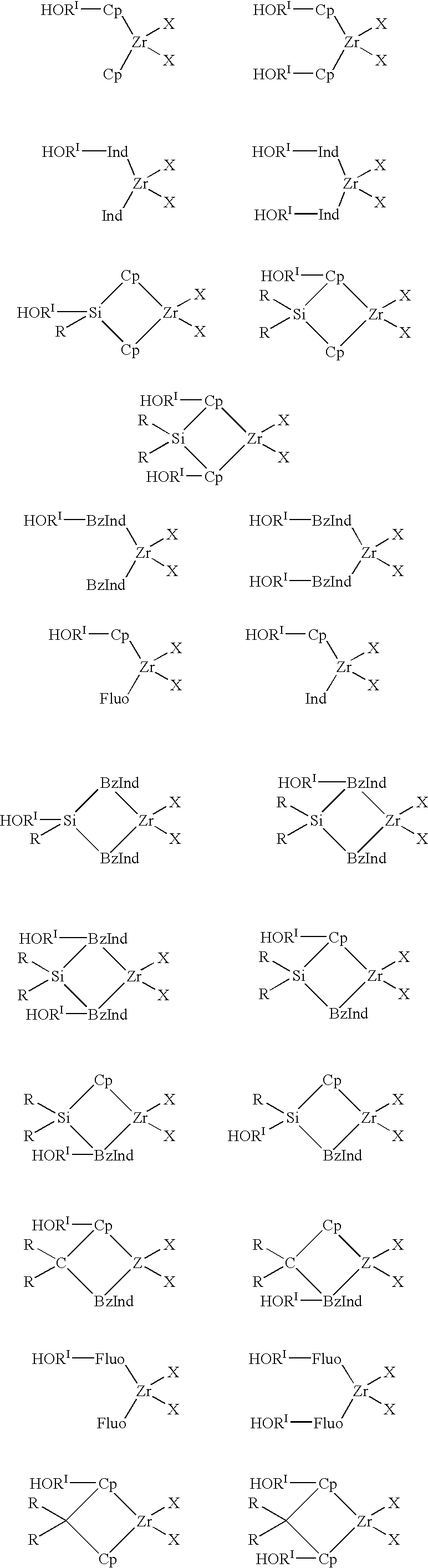
Heterogeneous catalytic component obtainable by reacting a porous inorganic support with a metallocene compound characterized in that the metallocene compound is defined by the following general formulas: (LRk)z[LRk-f(RIOH)f]xMXy I wherein: L, equal to or different from each other, is selected from the group comprising: cyclopentadienyl, indenyl, tetrahydroindenyl, fluorenyl, octahydrofluorenyl or benzoindenyl; each R is independently selected from hydrogen, C1-C20 alkyl, C3-C20 cycloalkyl, C6-C20 aryl, C3-C20 alkenyl, C7-C20 arylalkyl, C7-C20 alkylaryl, C8-C20 arylalkenyl, linear or branched, optionally substituted by 1 to 10 halogen atoms, or a group SiRII3; each RI equal to or different from each other is a divalent aliphatic or aromatic hydrocarbon group containing from 1 to 20 carbon atoms, optionally containing from 1 to 5 heteroatoms of groups 14 to 16 of the periodic table of the elements and boron; each Q is independently selected from B, C, Si, Ge, Sn; M is a metal of group 3, 4 or 10 of the Periodic Table, Lanthanide or Actinide; each X is independently selected from: hydrogen, chlorine, bromine, ORII, NRII2, C1-C20 alkyl or C6-C20 aryl; each RII is independently selected from C1-C20 alkyl, C3-C20 cycloalkyl, C6-C20 aryl, C3-C20 alkenyl, C7-C20 arylalkyl, C7-C20 arylalkenyl or alkylaryl, linear or branched; RII is methyl, ethyl, isopropyl; L' is N or O; when L is cyclopentadienyl k is equal to 5, when L is indenyl k is equal to 7, when L is fluorenyl or benzoindenyl k is equal to 9, when L is tetrahydroindenyl k is equal to 11 and when L is octahydrofluorenyl, k is equal to 17; z is equal to 0, 1 or 2; x is equal to 1, 2 or 3; y is equal to 1, 2 or 3; x+y+z is equal to the valence of M; m is an integer which can assume the values 1, 2, 3 or 4; a and b are integers whose value ranges from 0 to k-1; f is an integer whose value ranges from 1 to k; g is 0 or 1; c and e are equal to 0 or 1; a+b+c is
Owner:REPSOL QUIMICA
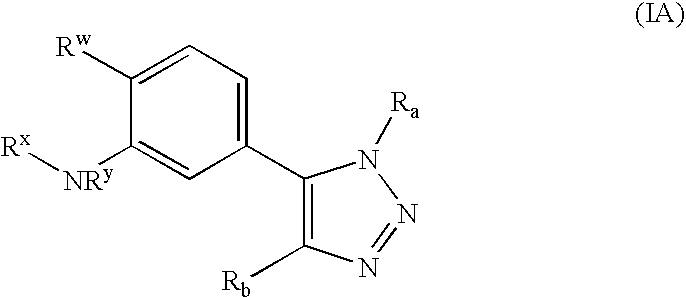
Cinnamoyl sulfonamide compound preparation and applications of cinnamoyl sulfonamide compounds in anti-tumor treatment drugs
InactiveCN102838515ASulfonic acid amide preparationAmide active ingredientsMelanomaStructural formula
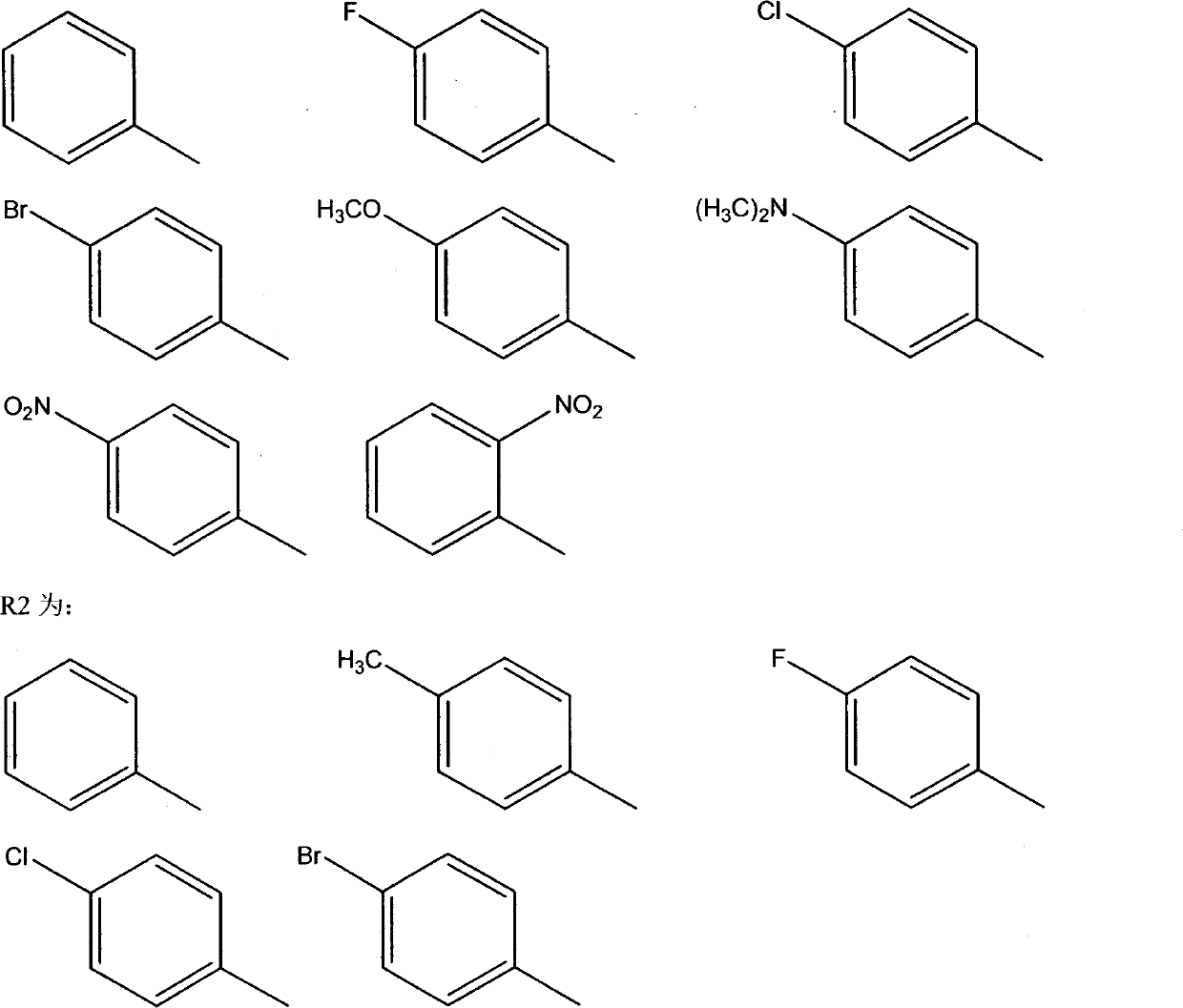
The invention relates to a class of cinnamoyl sulfonamide derivatives, wherein the derivatives are characterized in that the derivatives have the following general formula, and R1 in the structural formula is represented in the picture. The cinnamic acid derivatives of the present invention have effects of tubulin polymerization resistance and mice melanoma cell B16-F10 proliferation inhibition, such that the cinnamic acid derivatives of the present invention can be adopted as potential anti-tumor drugs. The present invention further discloses a production method for the derivatives.
Owner:NANJING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
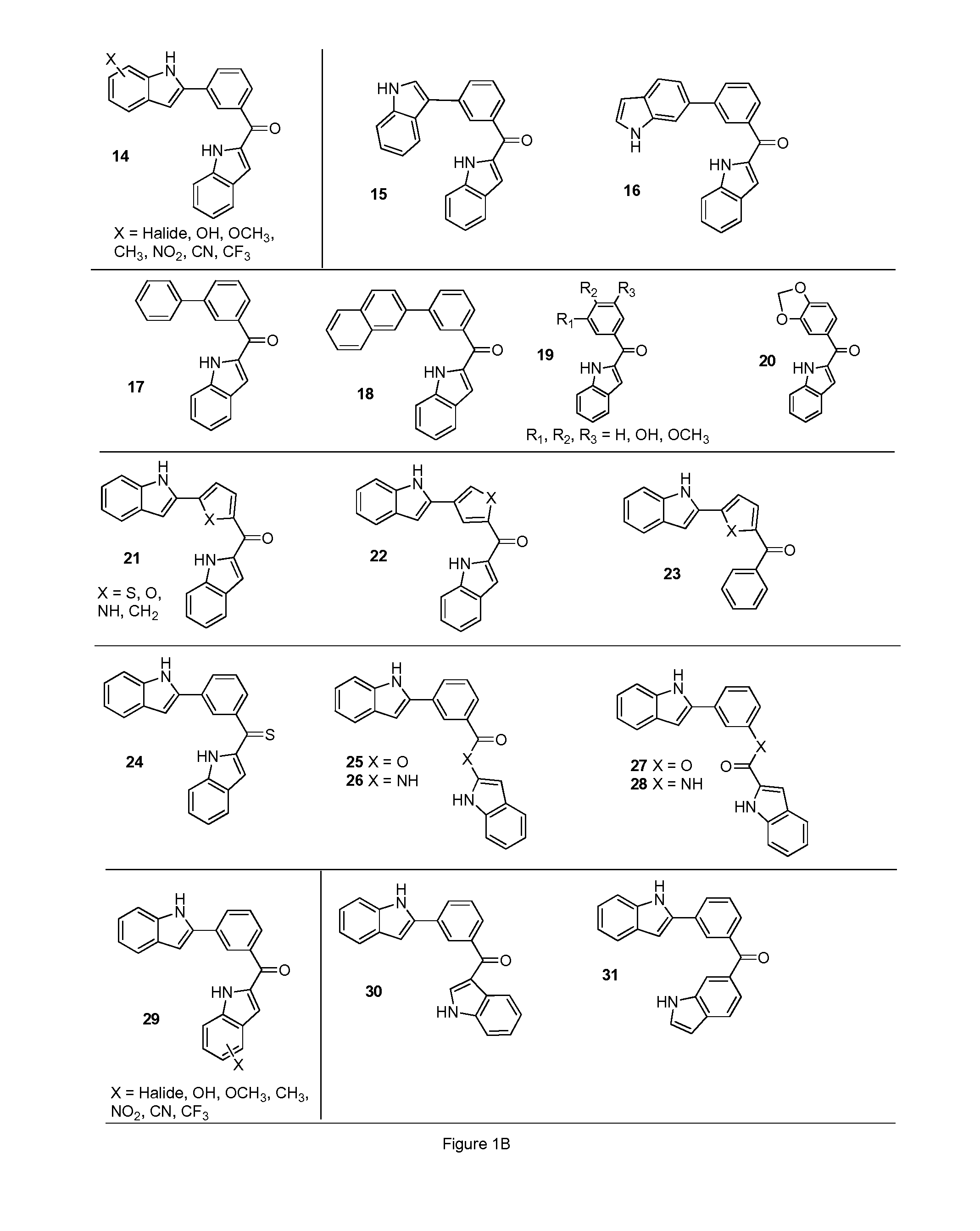

























![COMPOSITION FOR PREVENTION OR TREATMENT OF CANCER COMPRISING N-METHYLENENAPHTHO[2,1-b]FURAN-2-CARBOHYDRAZIDE DERIVATIVES AS AN ACTIVE INGREDIENT COMPOSITION FOR PREVENTION OR TREATMENT OF CANCER COMPRISING N-METHYLENENAPHTHO[2,1-b]FURAN-2-CARBOHYDRAZIDE DERIVATIVES AS AN ACTIVE INGREDIENT](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2c8378f1-20a2-4e09-ae60-e4f0b1b26579/US20150182502A1-20150702-D00001.PNG)
![COMPOSITION FOR PREVENTION OR TREATMENT OF CANCER COMPRISING N-METHYLENENAPHTHO[2,1-b]FURAN-2-CARBOHYDRAZIDE DERIVATIVES AS AN ACTIVE INGREDIENT COMPOSITION FOR PREVENTION OR TREATMENT OF CANCER COMPRISING N-METHYLENENAPHTHO[2,1-b]FURAN-2-CARBOHYDRAZIDE DERIVATIVES AS AN ACTIVE INGREDIENT](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2c8378f1-20a2-4e09-ae60-e4f0b1b26579/US20150182502A1-20150702-D00002.PNG)
![COMPOSITION FOR PREVENTION OR TREATMENT OF CANCER COMPRISING N-METHYLENENAPHTHO[2,1-b]FURAN-2-CARBOHYDRAZIDE DERIVATIVES AS AN ACTIVE INGREDIENT COMPOSITION FOR PREVENTION OR TREATMENT OF CANCER COMPRISING N-METHYLENENAPHTHO[2,1-b]FURAN-2-CARBOHYDRAZIDE DERIVATIVES AS AN ACTIVE INGREDIENT](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2c8378f1-20a2-4e09-ae60-e4f0b1b26579/US20150182502A1-20150702-D00003.PNG)