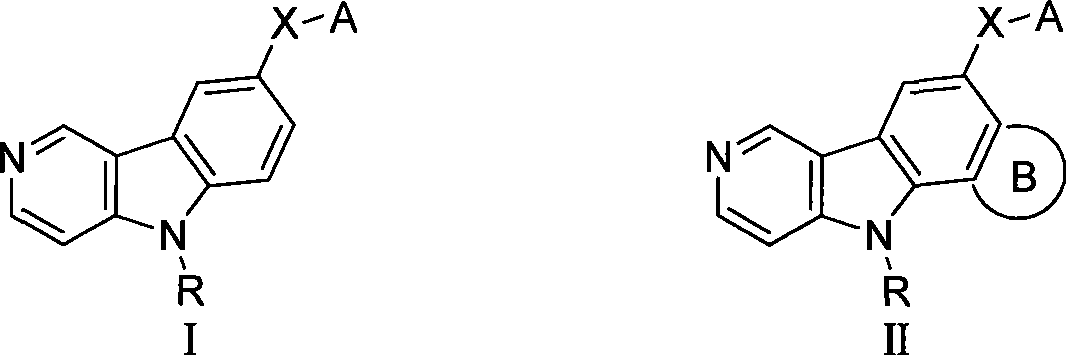Gamma-carbolines derivates as well as preparation method and application thereof
A carboline and product technology, applied in the field of synthesis of organic compounds, can solve the problems of complex structure, limited sources, and difficult synthesis, and achieve the effects of mild reaction conditions, reasonable design, and wide sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
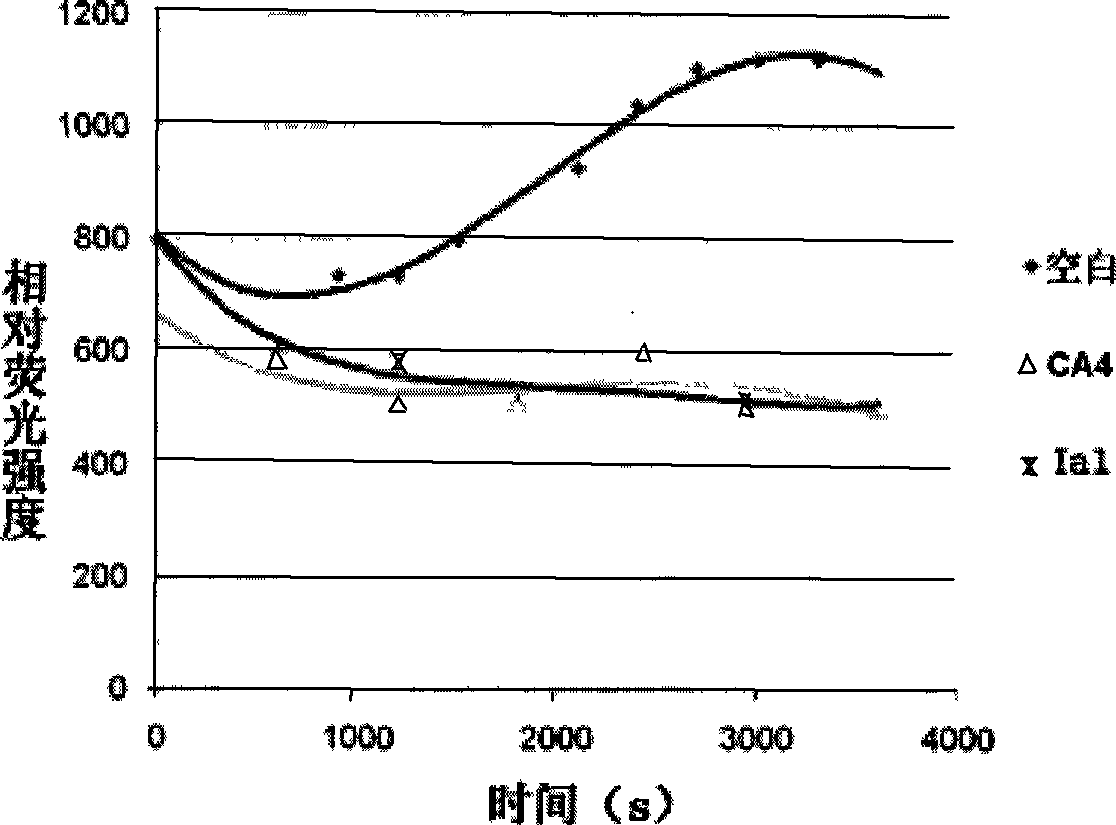
[0037] The preparation of embodiment 1,9-ethyl-6-(4-bromobenzenesulfonamido)-γ-carboline (compound Ia1)
[0038]Dissolve homemade 6-amino-9-ethylγ-carboline (53mg, 0.25mmol) in DMF (1mL), add homemade 4-bromobenzenesulfonyl chloride (Su Yanxi et al., "Chemical World" , 2001, 12, 657-658) (64 mg, 0.25 mmol) and pyridine (81 μL, 1 mmol), and stirring was continued for 0.5 hours. The reaction solution was poured into water, extracted with ethyl acetate (5 mL×3), washed with saturated sodium chloride solution (5 mL×2), and dried over anhydrous sodium sulfate. After filtration, the filtrate was recovered under reduced pressure to obtain a yellow solid, which was separated by silica gel column chromatography (eluent: petroleum ether: ethyl acetate: ethanol = 10:10:1) to obtain a white solid (compound Ia1), with a yield of 56%. Melting point: 154-156°C.
[0039] 1 H-NMR (δ, DMSO-d 6 ): 9.24(s, 1H), 8.57(d, 1H, J=6.4Hz), 8.04(s, 1H), 7.86(d, 1H, J=6.4Hz), 7.62(d, 2H, J=8.8Hz ), 7...
Embodiment 2
[0040] The preparation of embodiment 2,9-ethyl-6-(4-fluorobenzenesulfonamido)-γ-carboline (compound Ia2)
[0041] The operation process is the same as in Example 1, except that 4-bromobenzenesulfonyl chloride is replaced with 4-fluorobenzenesulfonyl chloride. A white solid (compound Ia2) was obtained in a yield of 52%. Melting point: 178-180°C.
[0042] 1 H-NMR (δ, DMSO-d 6 ): 10.18(brs, 1H), 9.28(s, 1H), 8.47(d, 1H, J=5.2Hz), 7.95(s, 1H), 7.78-7.76(m, 2H), 7.63-7.57(m, 2H), 7.37(t, 2H, J=8.8Hz), 7.18(d, 1H, J=8.4Hz), 4.41(q, 2H, J=6.8Hz), 1.29(t, 3H, J=6.8Hz) .
Embodiment 3
[0043] The preparation of embodiment 3,9-ethyl-6-(3-nitrobenzenesulfonamido)-γ-carboline (compound Ia3)
[0044] The operation process is the same as in Example 1, except that 4-bromobenzenesulfonyl chloride is replaced by self-made 3-nitrobenzenesulfonyl chloride (Ma Ying et al., CNZL200610052268.5, 2006). A white solid (compound Ia3) was obtained in a yield of 44%. Melting point: 159-161°C.
[0045] 1 H-NMR (δ, DMSO-d 6 ): 10.46 (brs, 1H), 9.33 (s, 1H), 8.49 (d, 2H, J=1.2Hz), 8.44 (dd, 1H, J=1.2, 8, 8Hz), 8.08 (d, 1H, J =8.8Hz), 8.00(d, 1H, J=2.0Hz), 7.82(t, 1H, J=8.8Hz), 7.65(d, 1H, J=6.0Hz), 7.62(d, 1H, J=8.8 Hz), 7.21(dd, 1H, J=8.8, 2, 0Hz), 4.43(q, 2H, J=7.2Hz), 1.29(t, 3H, J=7.2Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com