Inhibitors of Tubulin Polymerization
a technology of tubulin and polymerization, which is applied in the field of inhibition of tubulin polymerization, can solve problems such as failure of mitosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Inhibition of Tubulin Polymerization
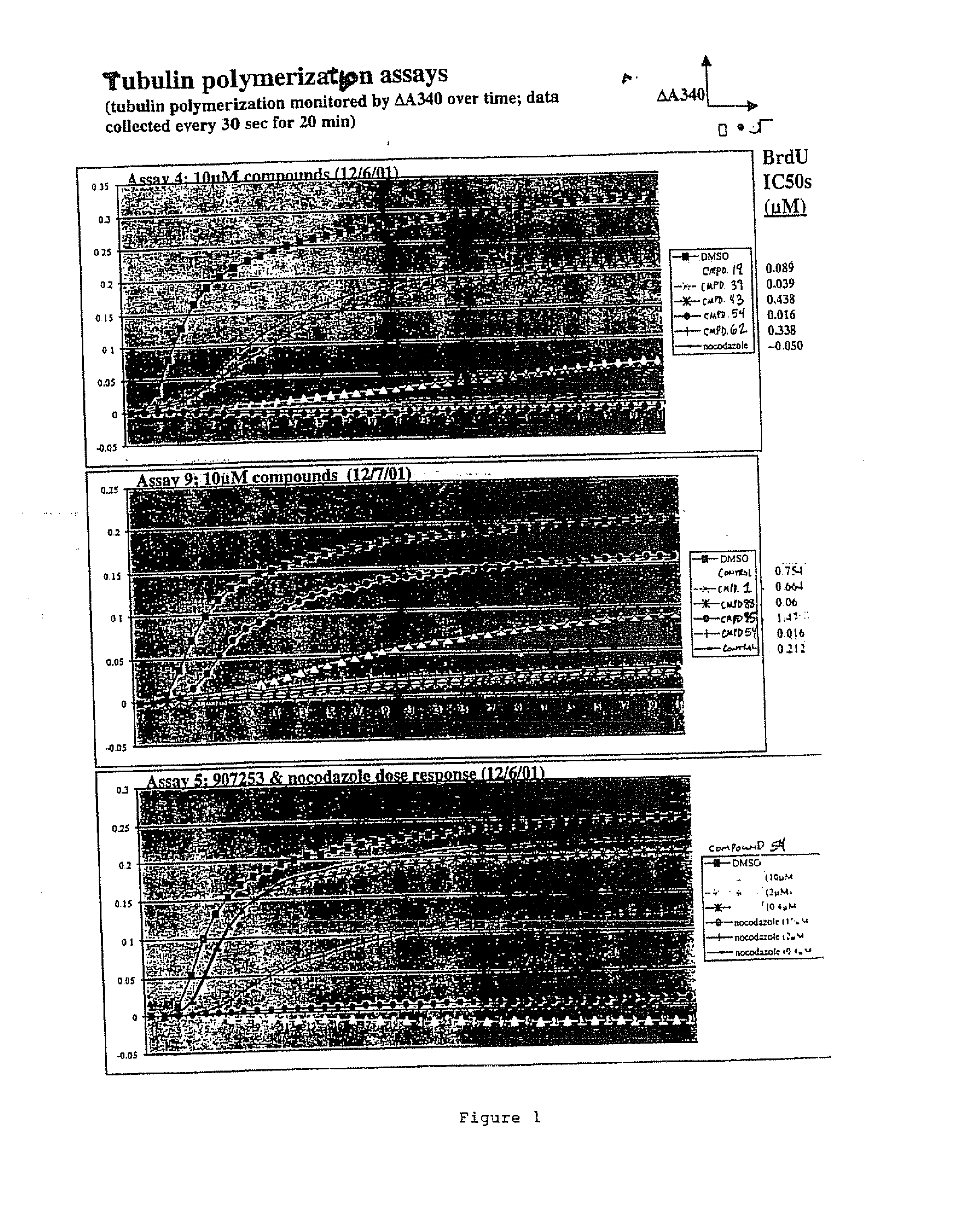
[0087]FIG. 1 illustrates the inhibition of tubulin polymerization activity conferred by exemplary compounds of the invention.
[0088] The assay measures tubulin polymerization as an increase in turbidity (A340) over time. Assays for tubulin polymerization were performed using a kit from Cytoskeleton (Denver, Colo.) and as described by Tahir, S, K., et al, (2001) Cancer Res., 61: 5480-5, incorporated herein by reference, and as described below.
[0089] To determine the ability of the compounds to inhibit microtubule polymerization in vitro, the following protocol was followed: (i) 70 μl ice-cold MAP-rich tubulin protein from bovine brain (1.5 mg / ml final concentration) was added to polymerization buffer (80 mM PIPES pH6,8, 1 mM EGTA, 1 mM MgCl2, 1 mM GTP) in a quartz microcuvette; (ii) 0.7 μl of DMSO or 100× stock of control (nocodazole) or test compound (thienopyrimidine) in DMSO were added to the cuvette; (iii) the contents were mixed by pipetting...
example 2
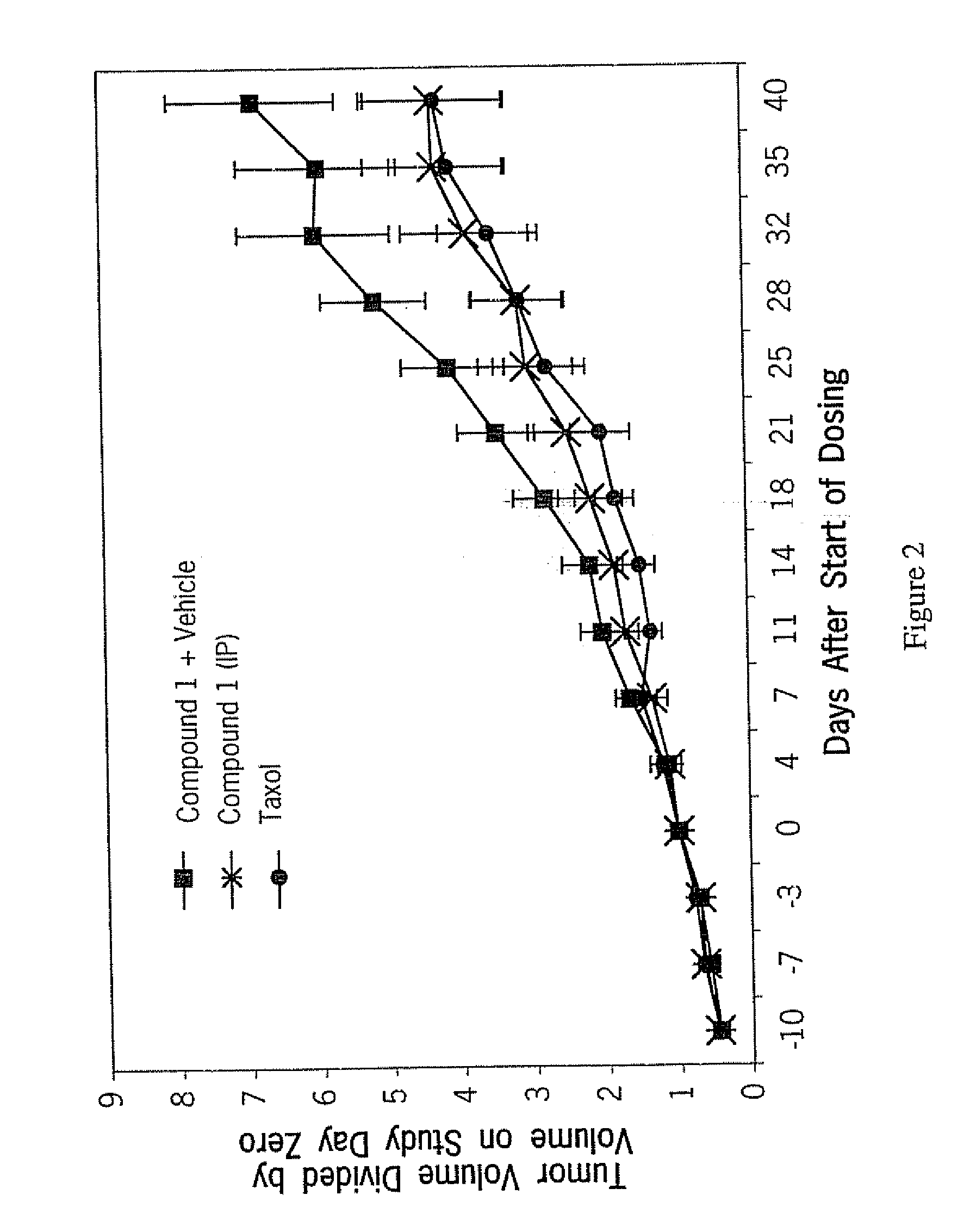
Antitumor Activity of Compound 1 Against Human Lung and Colon Tumors in Nude Mouse Xenografts
[0090] A nude mouse / human carcinoma model was used to examine the effect of tubulin inhibitors on tumor growth and animal survival. Groups of immune deficient (nude) mice were implanted subcutaneously with suspensions of the human tumor lines A549 (lung) and SW480 (colon). When palpable tumors appeared and were of a preselected volume (approximately 40-150 mm3), animals were subdivided into various treatment groups such that the variation in mean tumor volume of each group was within 10%. The animals were then treated daily with either control vehicle or Compound 1 administered by intraperitonel injection. Twice weekly, the volume of the tumors in each animal in each group was measured to gather information on tumor growth (volume) as a function of time. Systemic toxicity was assessed by measuring individual animal body weights, and toxicity was evaluated by examination of differences in bo...
example 3
Bromodeoxyuridine (BrdU) Proliferation Assay
[0111] In order to determine the antiproliferative activity of microtubule inhibitors, A549 cells were plated in 96 well plates at 2000 cells / well 24 hours prior to the addition of a compound of the invention in 80 ul growth media (F12K, 10% FBS, Pen / Strep). Prior to addition to cells, compounds were solubilized in 100% DMSO to 5 mM and serially diluted in 6 semi-log steps in 100% DMSO. The diluted compounds were added to cells by two 15.8-fold dilution steps, the first being a dilution into a 23% ethanol / H20 mixture and the second being a dilution into growth media. Finally, these dilutions were followed by a 1:1 dilution onto cells. The resulting final solvent concentration was 0.2% DMSO / 0,75% ethanol. Compounds were tested at final concentrations of 10, 3, 1, 0.3, 0.1 and 0.03 uM. Forty-eight hours after the addition of compound, the effects on cellular proliferation were measured using a cell proliferation BrdU ELISA (Roche Molecular ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com