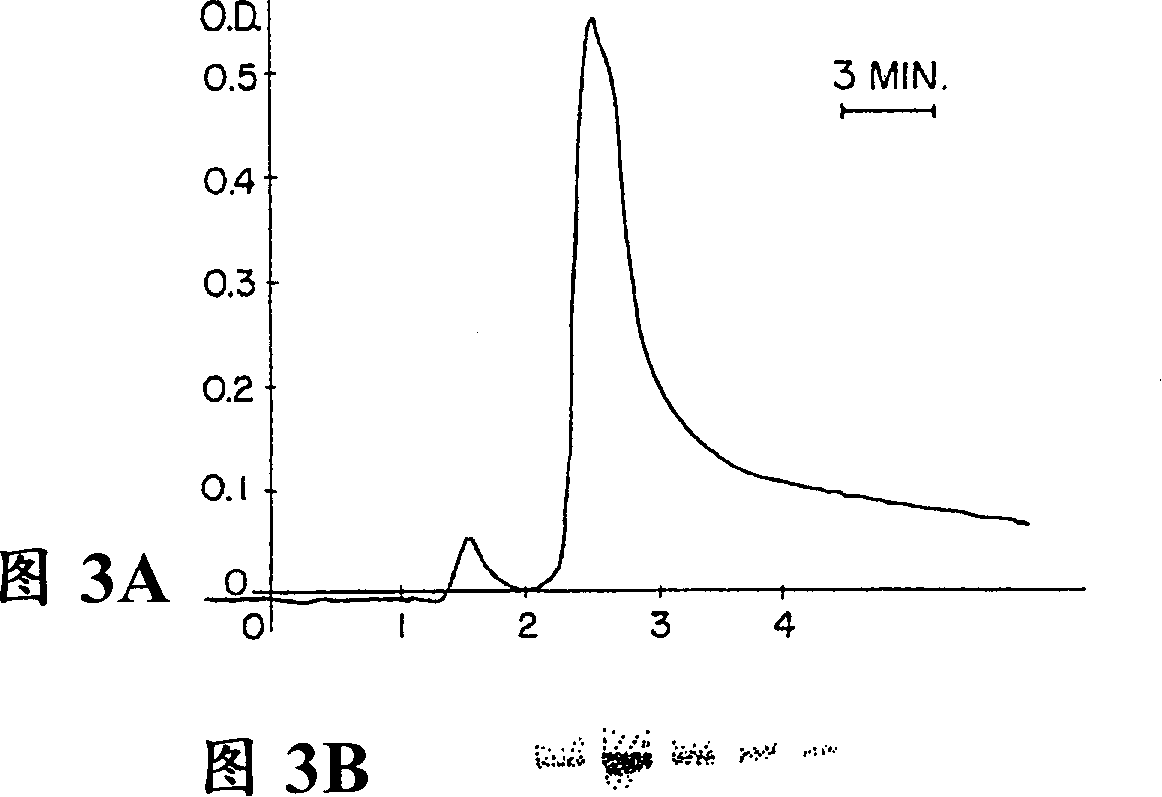
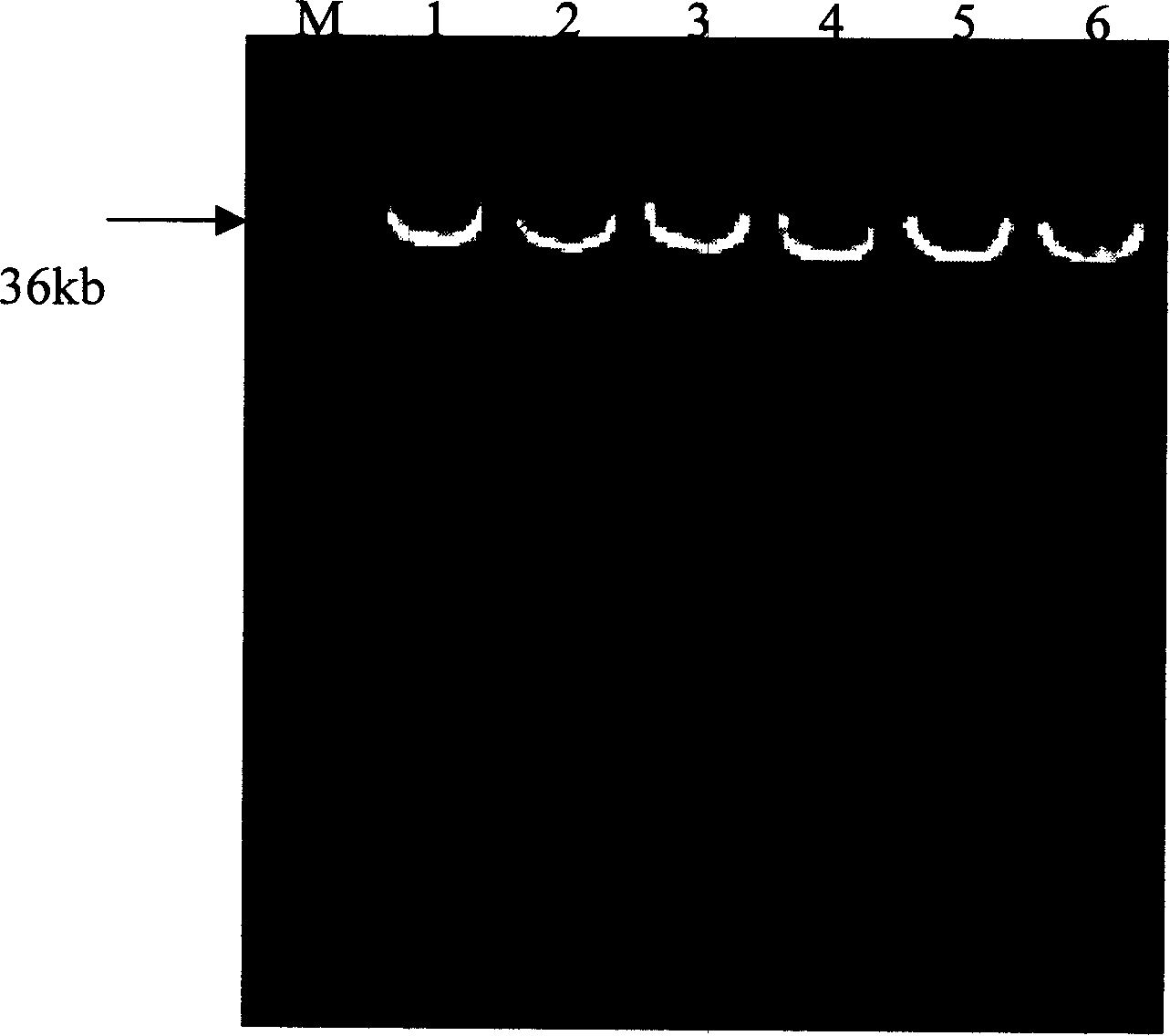
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Transgelin Gene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
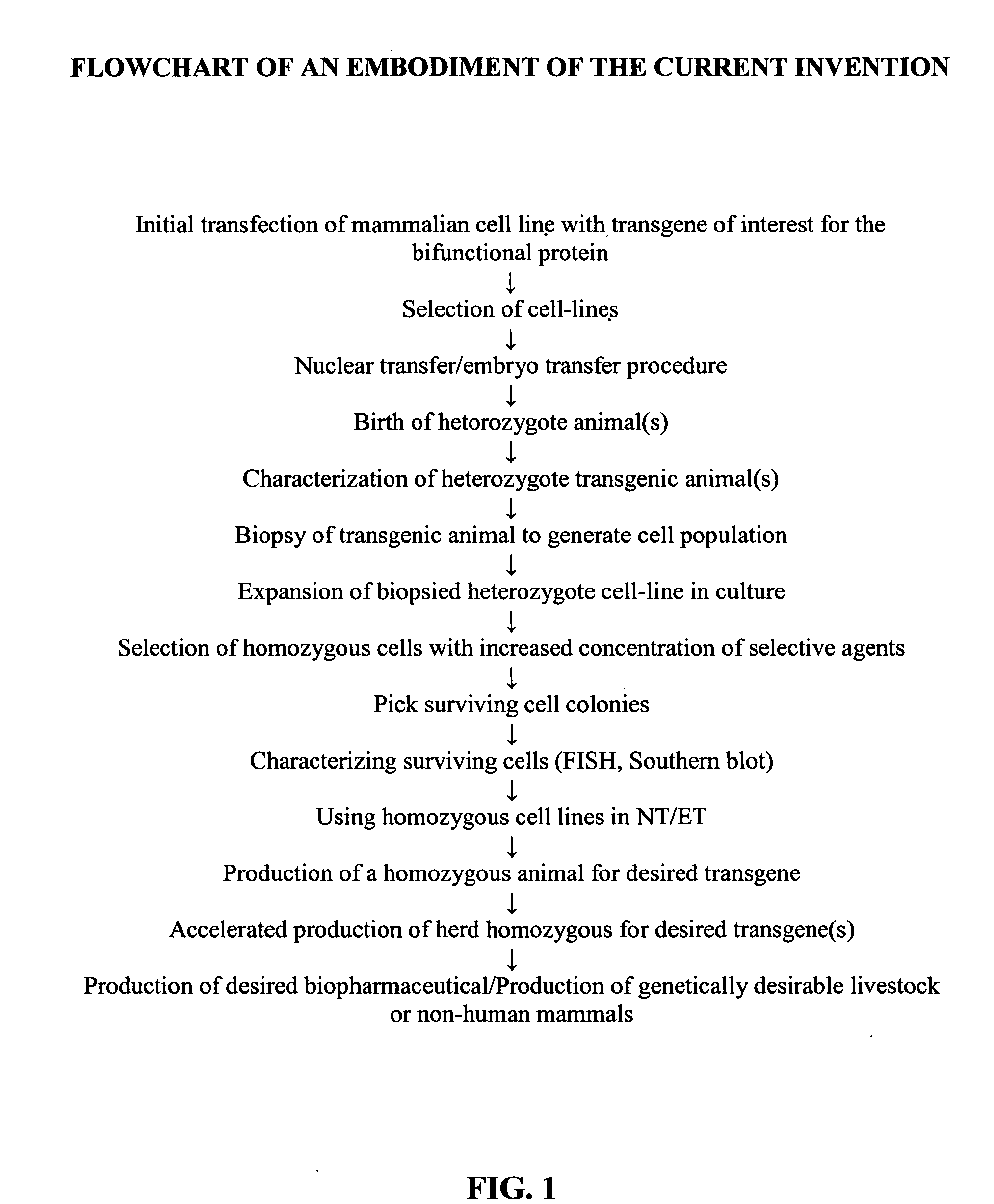
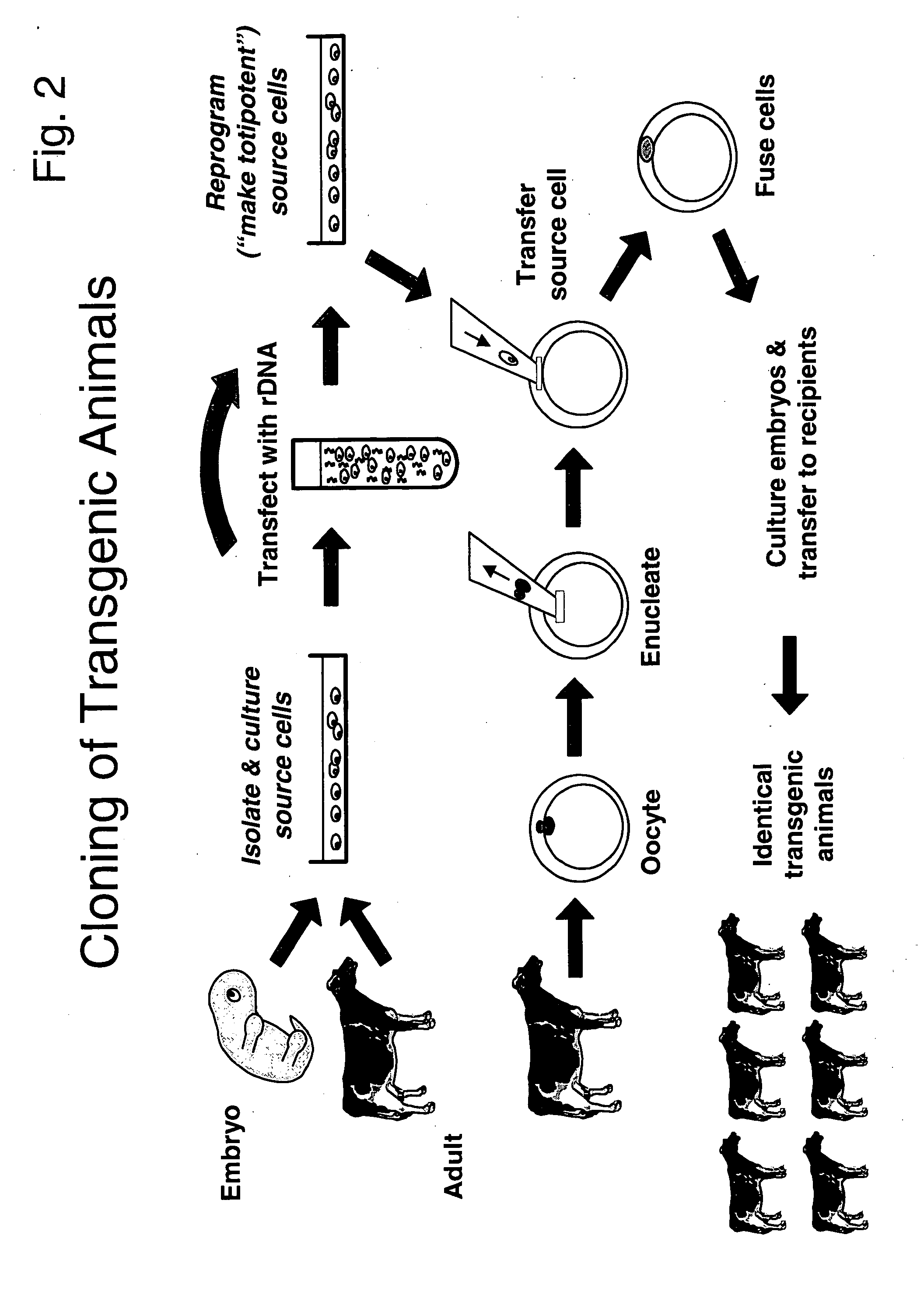
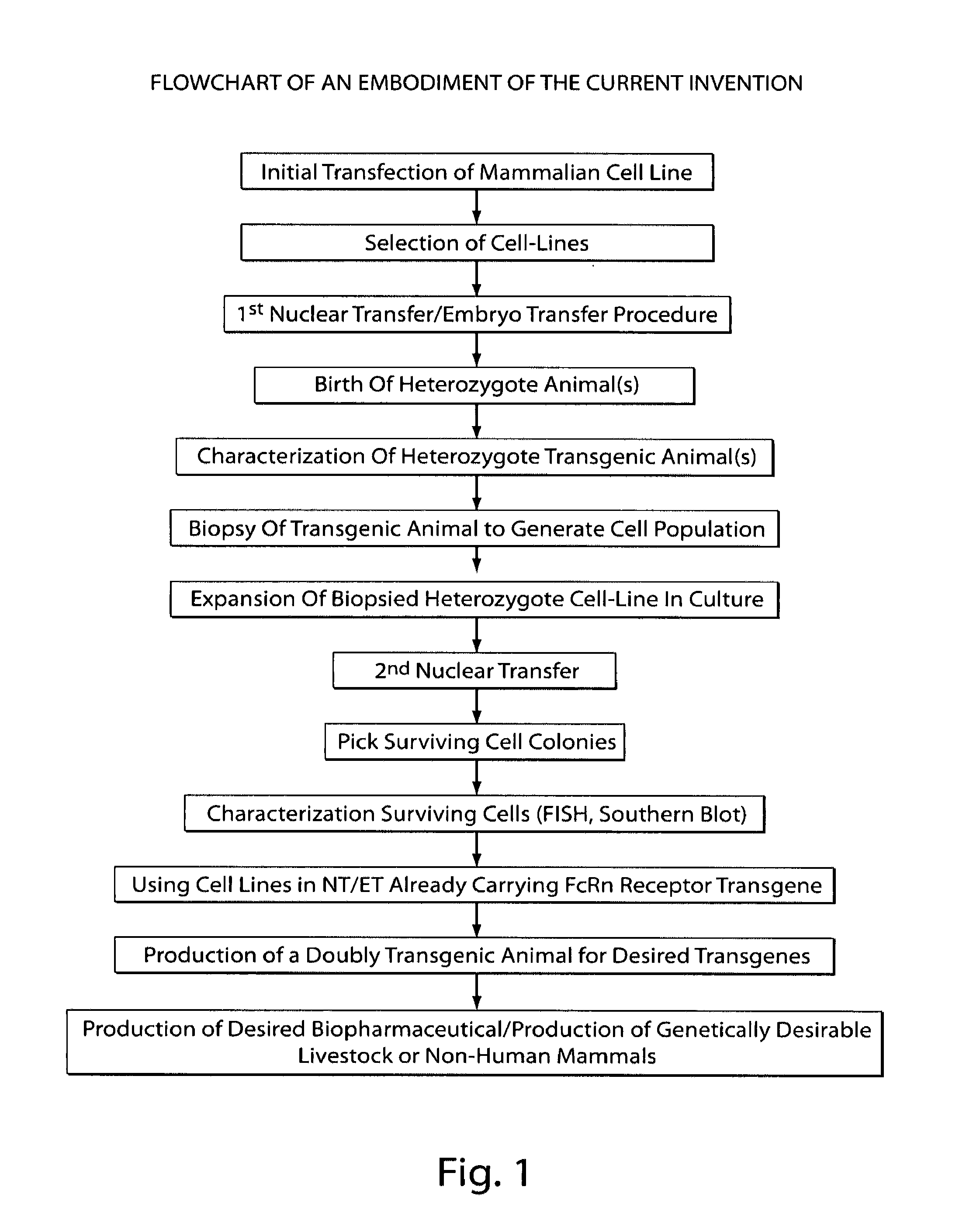
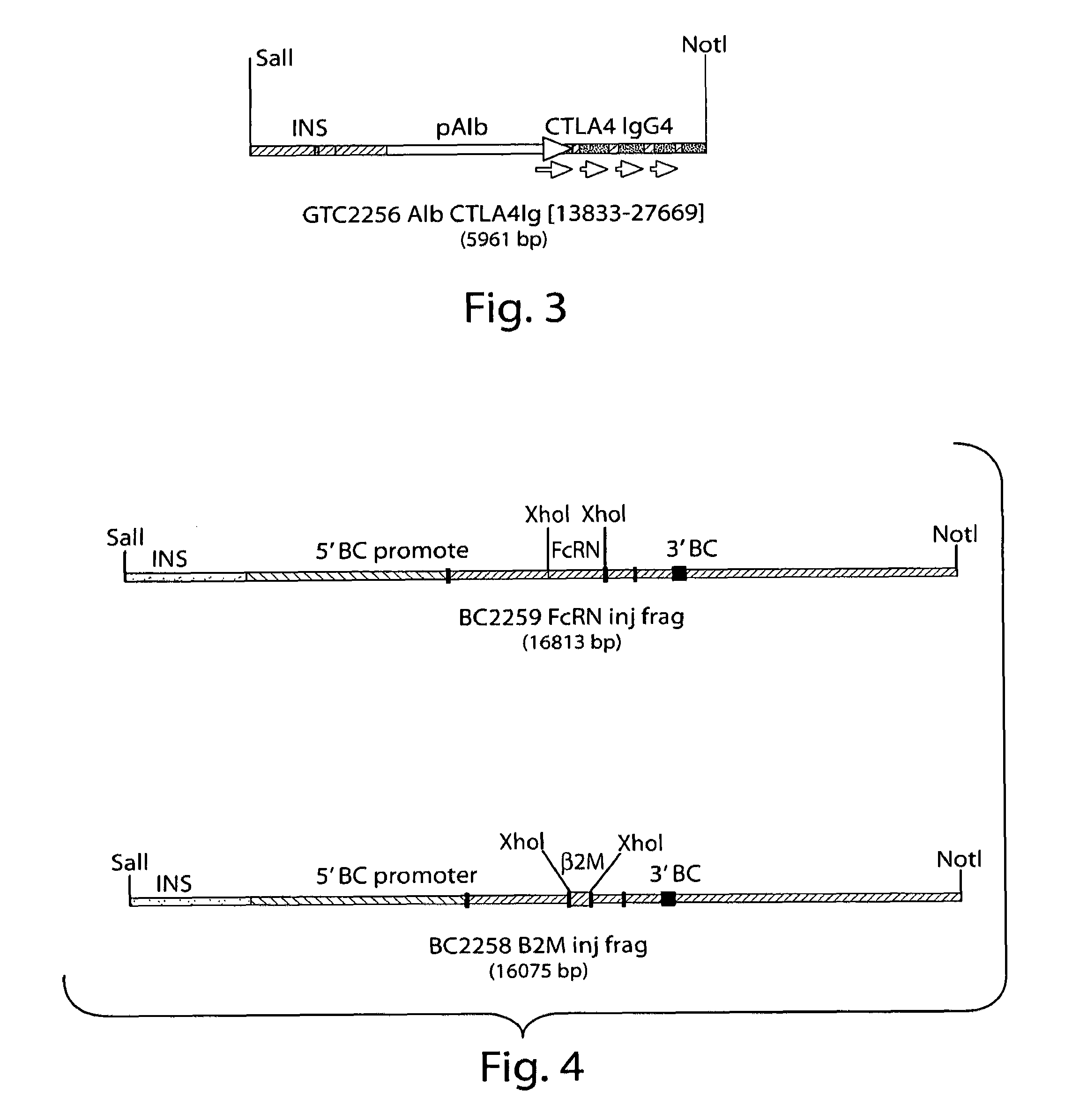
Method for the production of transgenic proteins useful in the treatment of obesity and diabetes
InactiveUS20050186608A1Short timeEfficient productionBiocidePeptide/protein ingredientsBiotechnologyDiabetes mellitus
Owner:GTC BIOTHERAPEUTICS INC
Microfluidic protein chip for detecting genetically modified crops and kit of microfluidic protein chip
InactiveCN102759628ADecreased probe activityHigh sensitivityBiological testingTransgelin GenePhosphate
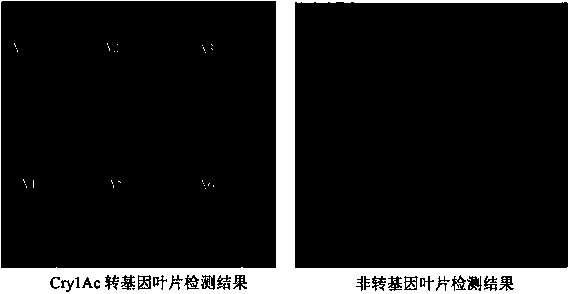
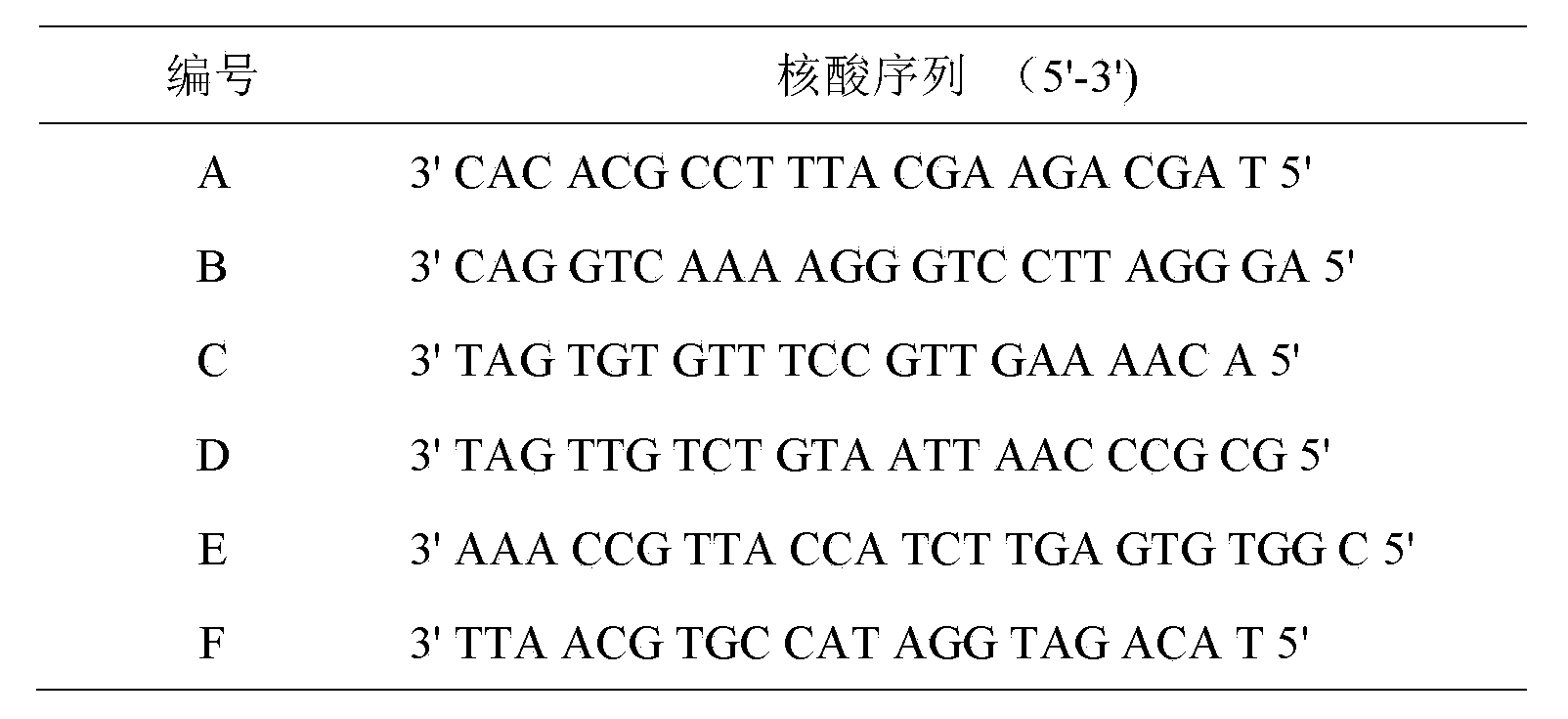
The invention discloses a microfluidic protein chip for high-through detecting genetically modified crops. The microfluidic protein chip comprises a nucleic acid chip which is synthesized on the basis of mu ParafloTM microfluidic protein chip and contains six nucleotide sequences and 6 types of antibody-oligonucleotide composite probes, thereby simultaneously detecting 6 transgenetic proteins of trans-bacillus thuringiensis Bt insecticidal crystal protein (Cry1Ac), trans-bacillus thuringiensis Bt insecticidal crystal protein (Cry1c), trans-bacillus thuringiensis Bt insecticidal crystal protein (Cry1F), trans-bacillus thuringiensis Bt insecticidal crystal protein (Cry1Ab), soybean trypsin (SBTI) and neomycin phosphate transferase II gene (NPTII). The invention further provides a transgenic assay kit which comprises a mu ParafloTM microfluidic deoxyribose nucleic acid (DNA) chip which contains six types of probes, six types of antibody-oligonucleotide composite probes, six types of detection antibodies and the like.
Owner:ZHEJIANG UNIV
Immunochromatography test paper assembled by taking chitin/chitosan fiber membrane as colloid label pad and preparation method thereof
The invention provides immunochromatography test paper assembled by taking a chitin / chitosan fiber membrane as a colloid label pad and a preparation method thereof. The test paper has the advantages of uniform adsorption of negative-charge colloid particles, hydrophilic steady flow, neutral biomacromolecules, stable test, high sensitivity, long useful life and the like. In the colloid label pad, pure chitin fiber and deacylated chitosan fiber are mixed in any ratio, and a fiber membrane with thickness of 0.2-4mm is prepared by a pulping method, spinning method or solution method; the test paper which can load multiple kinds of colloid particle markers and are assembled with different detection membranes can be widely applied to the fields such as physical health, disease detection, drug and doping detection and transgenic protein detection.
Owner:RES INST OF ZHEJIANG UNIV TAIZHOU
Colloidal gold immunodetection kit for detecting transgenic protein Cry1Ab/Ac and use thereof
InactiveCN107271688AEasy to operateShort detection timeBiological testingTransgelin GeneVisual inspection
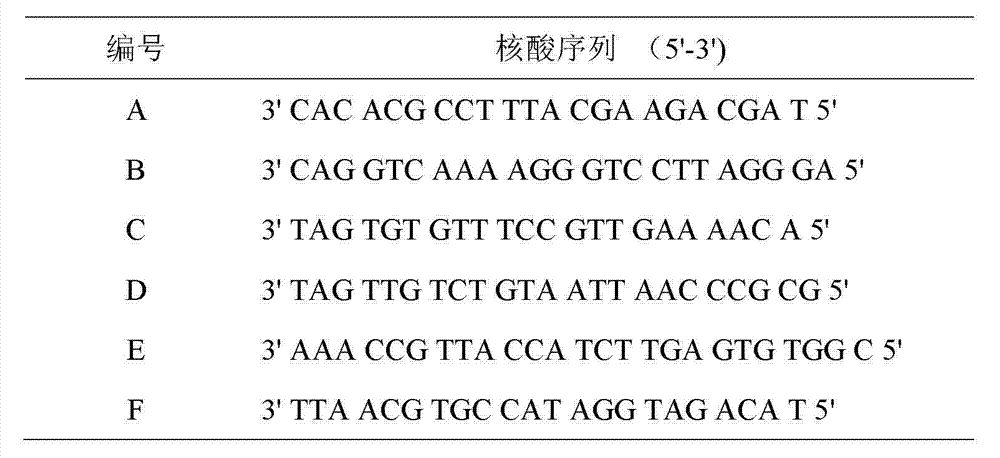
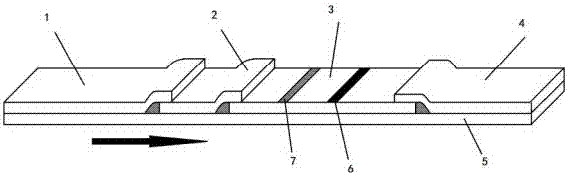
The invention discloses a colloidal gold immunodetection kit for detecting a transgenic protein Cry1Ab / Ac. The colloidal gold immunodetection kit comprises a pretreatment PCR tube and a test paper. The test paper is composed of a sample pad, a label pad, a nitrocellulose membrane and an absorbent pad, and the sample pad, the label pad, the nitrocellulose membrane and the absorbent pad are orderly lap-connected to and bonded to a base plate. The label pad is coated with a colloidal gold-labeled anti-digoxin antibody. The nitrocellulose membrane comprises a detection zone coated with an anti-FITC antibody and a control zone coated with a secondary antibody. The pretreatment PCR tube comprises a digoxin-labeled sequence, a FITC-labeled sequence, a Taq enzyme, dNTP and buffer. The digoxin-labeled sequence is GCTCCTACAAATGCCATCATTGC and the FITC-labeled sequence is GATAGTGGGATTGTGCGTCATCCC. The colloidal gold immunodetection card can realize fast and on-site sensitive detection of the PCR amplification product, is easy to operate, realizes visual inspection of the PCR amplification result and can finish detection in 8-10min.
Owner:WUXI FUYANG BIOTECH
Transgenic protein g10-epsps quantitative detection method and used kit
InactiveCN105866414AQuantitative detection method is accurateQuantitative detection method is reliableBiological material analysisTransgelin GeneTest sample
The invention discloses a g10-epsps quantitative detection kit including the following components: I, an antibody pre-coated ELISA plate; II, a sample diluent which is a phosphate buffer solution; III, a washing liquid which is a phosphate buffer solution containing Tween-20; IV, a reaction stop liquid which is a H2SO4 solution; V, an enzyme labeled antibody which is a mouse anti g10-epsps monoclonal antibody labeled by horseradish peroxidase; VI, a color developing agent; and VII, a g10-epsps standard substance. The invention also discloses a method for quantitative detection of a transgenic protein g10-epsps in a plant by using the kit, wherein the method includes the following steps: extracting a protein from a to-be-tested sample, to obtain a protein extract liquid; using the g10-epsps quantitative detection kit for detecting the protein extract liquid; and making a concentration-absorbance standard curve by using g10-epsps protein standard substances, and calculating the g10-epsps protein concentration in the to-be-tested sample.
Owner:ZHEJIANG UNIV +1
Plant bioreactors
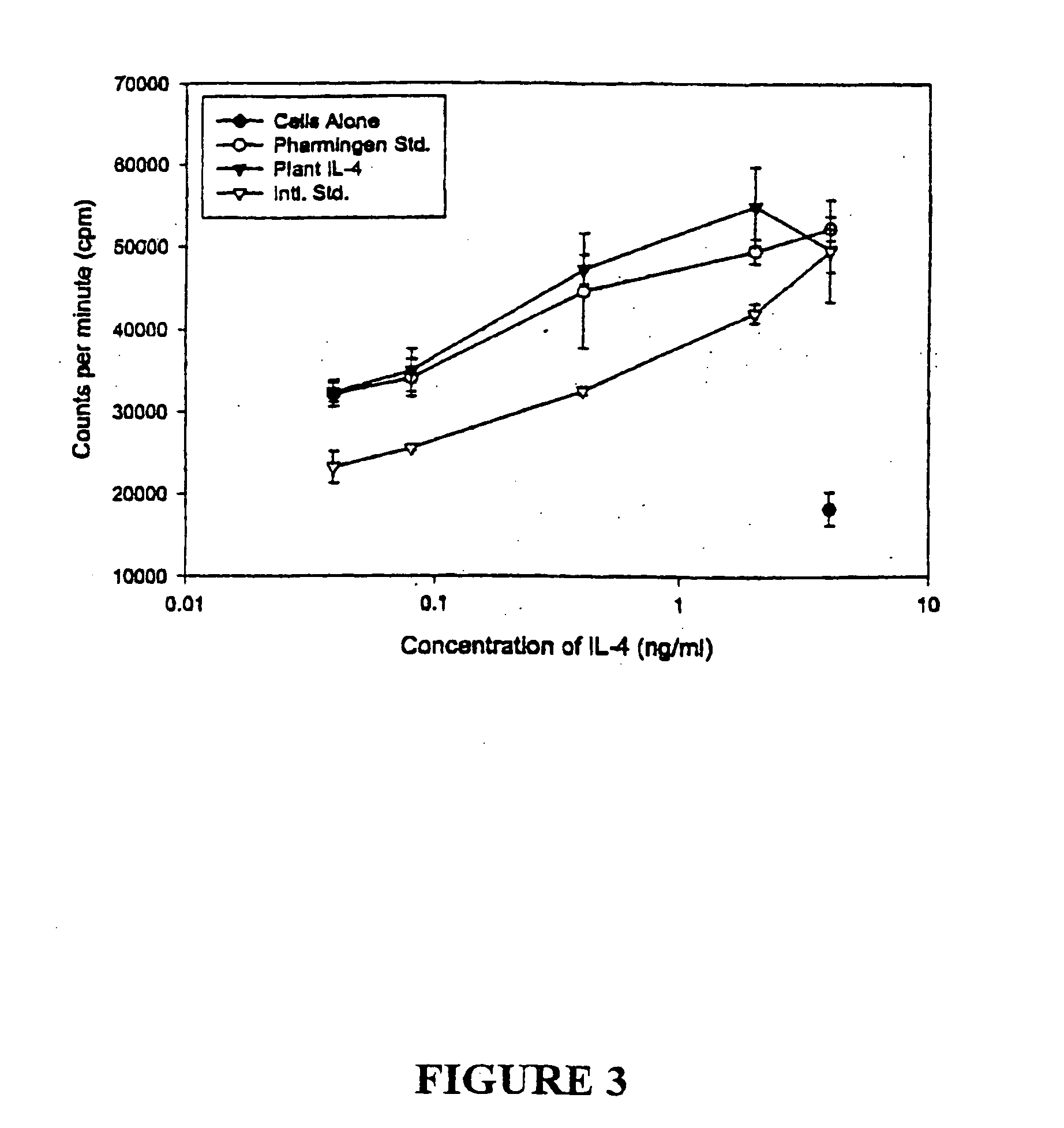
A plant expressing a cytokine and an autoantigen is disclosed. An example of a cytokine is a contra-inflammatory cytokine, for example IL-4 and IL-10, and an example of autoantigen is GAD. A novel method for the production of transgenic proteins of interest suitable for oral administration is also disclosed. Further, non-food crop plants expressing one or more proteins of interest are disclosed, as is a method involving the preparation of a protein of interest suitable for oral administration within a non-food crop plant comprising, transforming the non-food crop plant with a suitable vector containing a gene of interest and appropriate regulatory regions to ensure expression of the gene of interest within the non-food crop plant, such that the non-food crop plant is characterized as being non-toxic, non-addictive, palatable, and requiring minimal or no processing prior to oral administration. An example of a non-food crop plant is low alkaloid tobacco. Proteins of interest may include pharmaceutically active proteins such as growth regulators, insulin, interferons, interleukins, growth hormone, erythropoietin, G-CSF, GM-CSF, hPG-CSF, M-CSF, Factor VIII, Factor IX, tPA, antibodies, antigens and combinations and / or derivatives thereof.
Owner:AGRI & AGRI FOOD +2
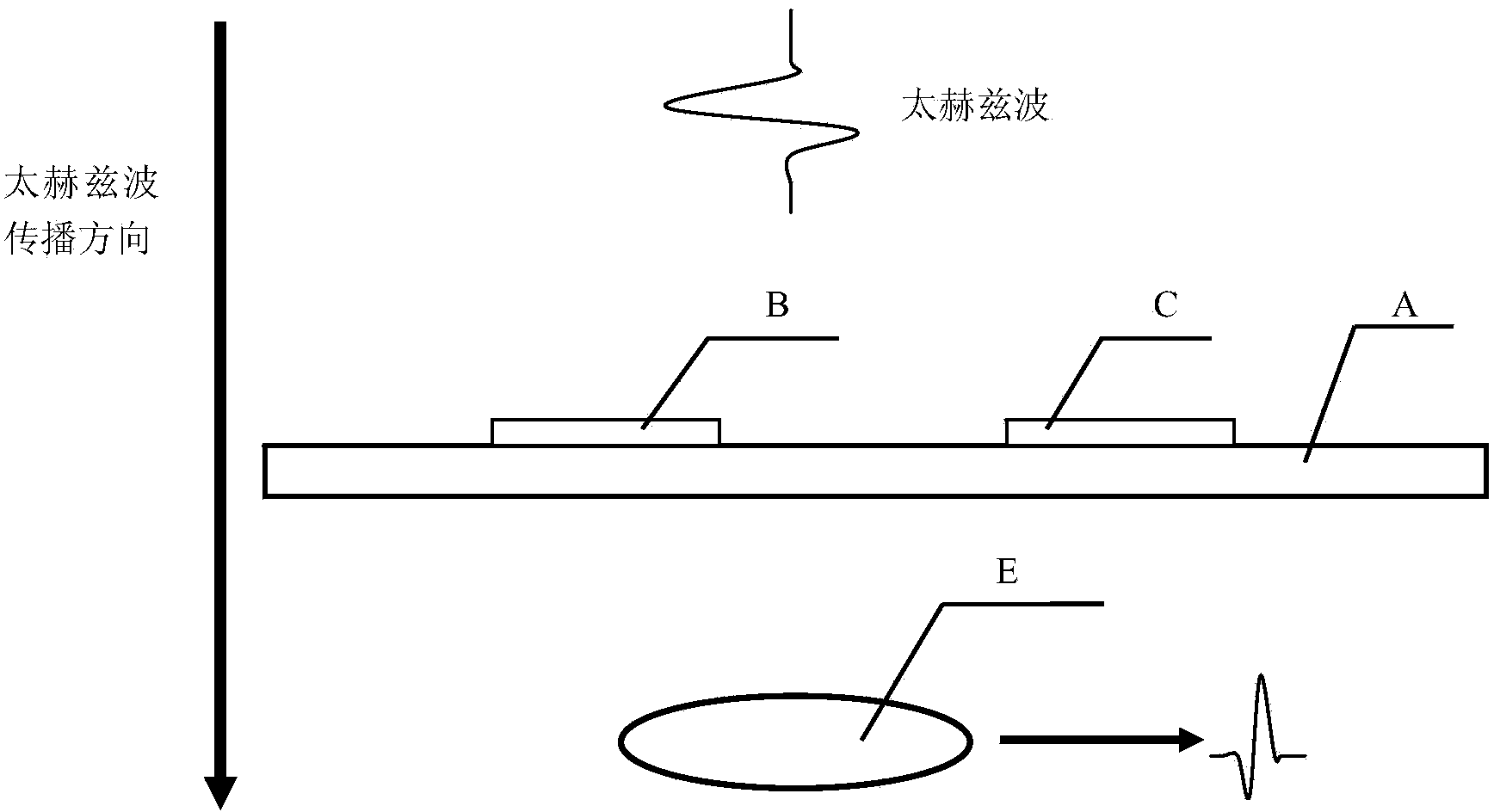
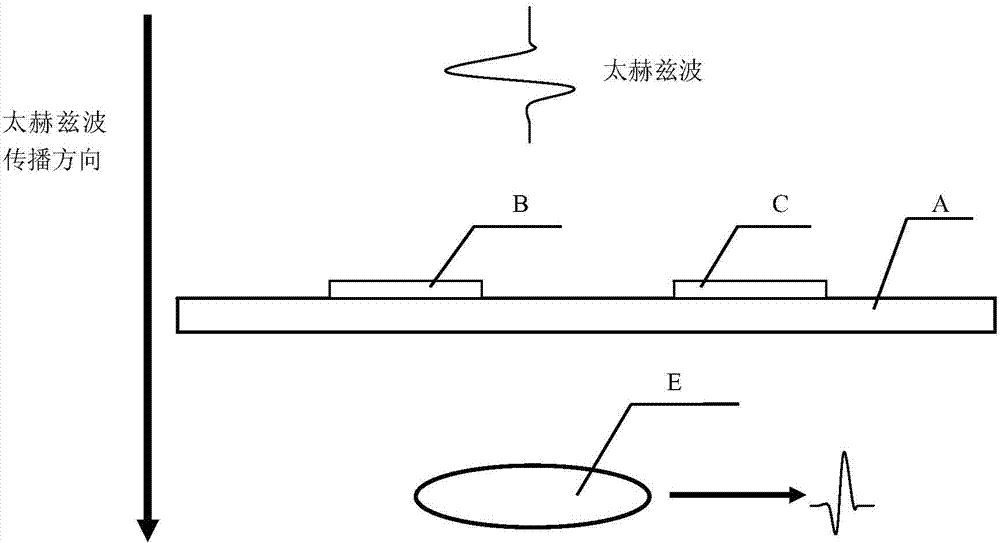
Transgenic protein detection method combining antibody modification and terahertz spectrum
ActiveCN103528987AImprove accuracyAvoid the Fluorescence StepMaterial analysis by optical meansTime domainTransgelin Gene
The invention relates to a transgenic protein detection method combining antibody modification and terahertz spectrum. The invention aims to provide the method with the characteristics of high accuracy and rapid and convenient detection. According to a technical scheme involved in the invention, the transgenic protein detection method combining antibody modification and terahertz spectrum comprises the steps of: (1) quartz slide cleaning; (2) quartz slide surface modification; (3) quartz slide surface activation; (4) antibody immobilization; (5) quartz slide surface redundant site close; (6) transgenic protein immobilization; (7) acquisition of the terahertz time-domain spectrums of a to-be-measured point on a quartz slide and a reference point; and (8) comparison of the terahertz time-domain spectrums of the to-be-measured point on the quartz slide and the reference point.
Owner:ZHEJIANG UNIV
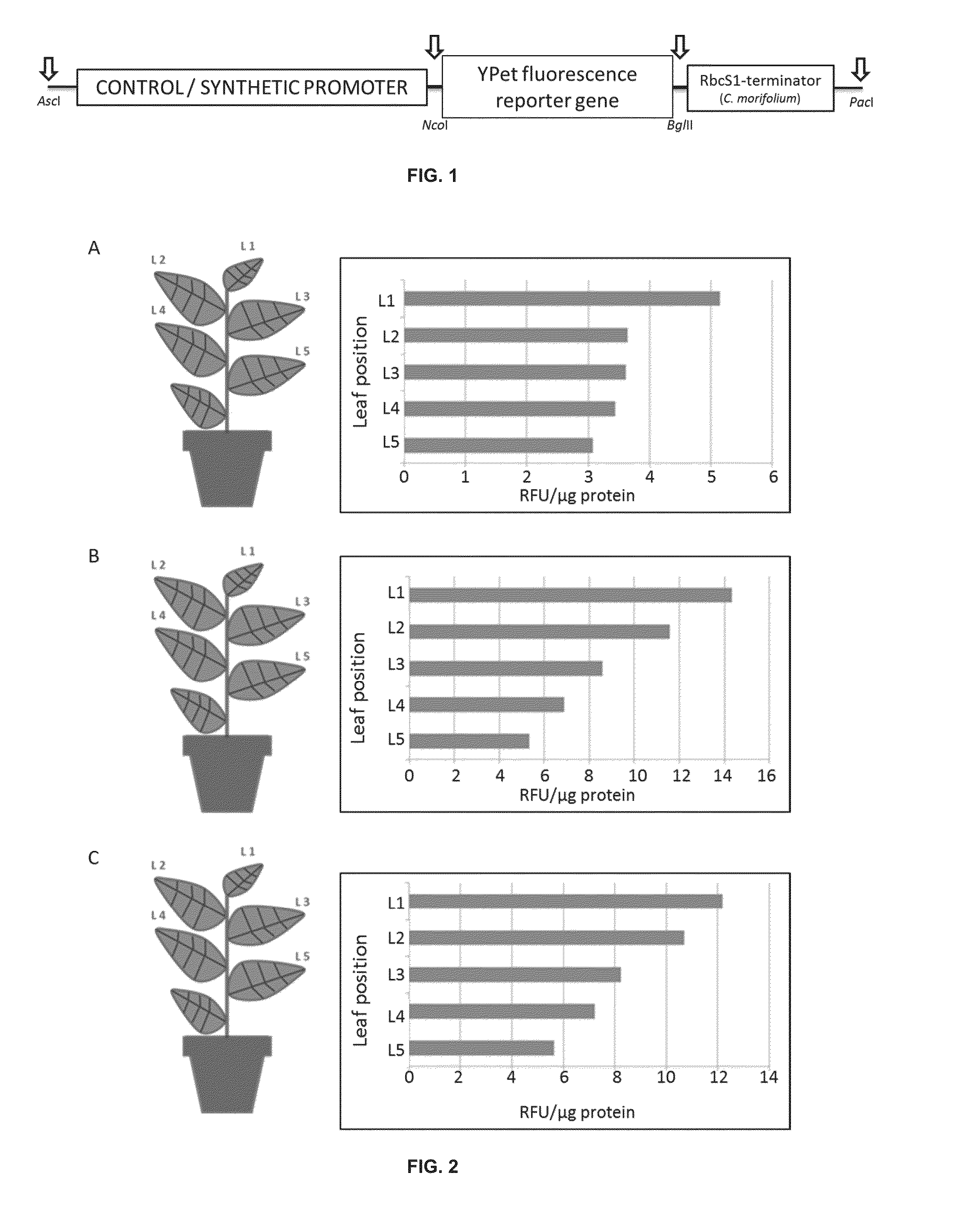
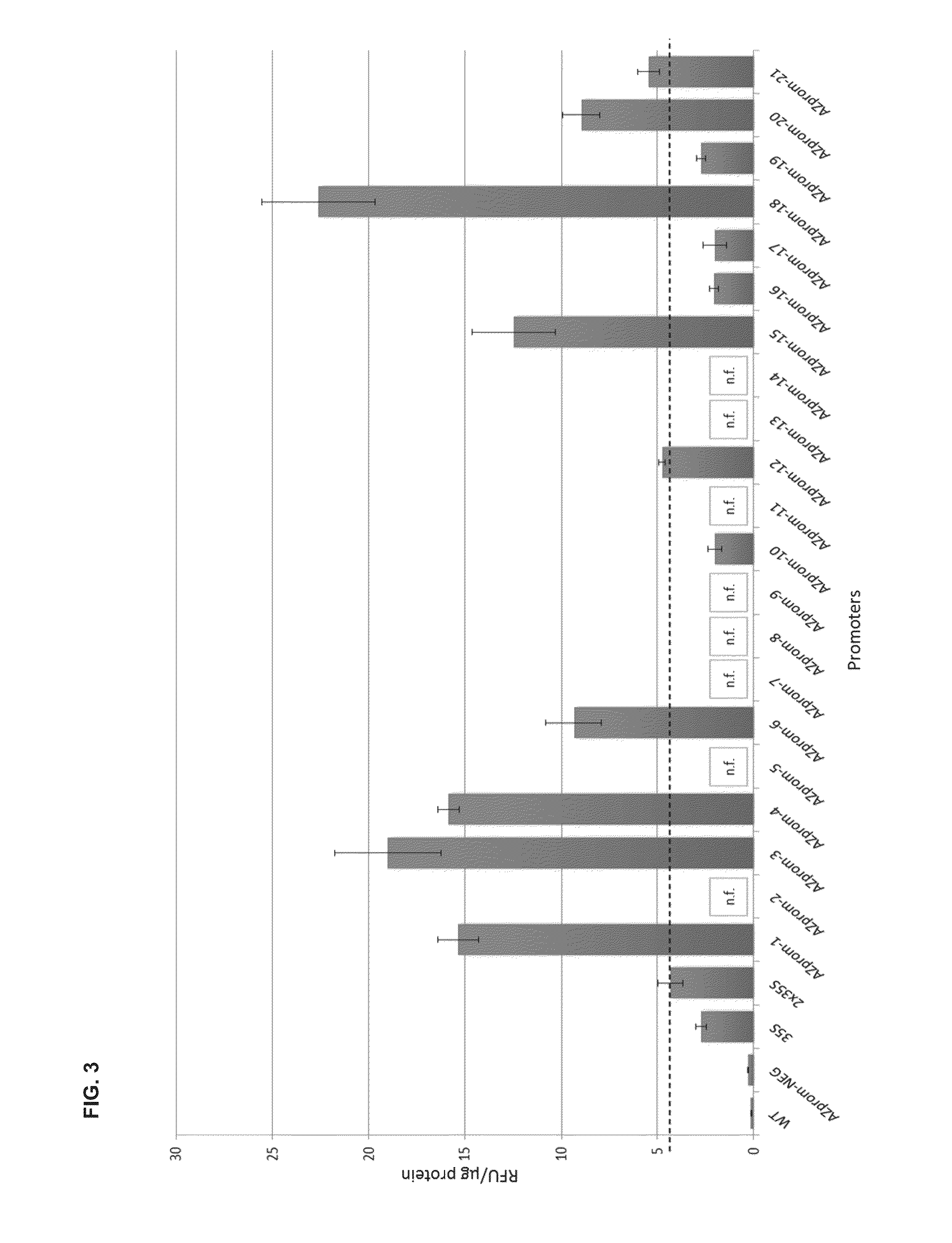
Synthetic promoter construct for transgene expression
The invention is directed to synthetic promoter constructs for enhanced transgene expression in plants and to expression cassettes comprising the synthetic promoter constructs. The expression cassettes may include various elements for improved expression, stability of the expressed protein or efficient purification of the expressed protein, including signal sequences, protease cleavage sites for release of the target protein, trafficking peptides for trafficking of the expressed protein to various plant compartments, and / or various tags. The invention further relates to methods of expression of transgenic proteins in plants with the use of the synthetic promoter constructs and expression cassettes.
Owner:AZARGEN BIOTECHNOLOGIES (PTY) LTD
Method and device for detecting transgenic ingredients in food
ActiveCN104569432AIncrease the number of timesIncrease the areaBiological testingFiberTransgelin Gene
The invention discloses a method for detecting transgenic ingredients in food. The method comprises the steps of step I. extracting total proteins of a raw material; step II. injecting the total protein obtained in the step I into a detection device, and enabling the total protein to pass through the detection device, wherein the detection device comprises a sample feeding device, a plurality of hollow fiber polypropylene films and a first porous plate body, each hollow fiber polypropylene film is provided with a first antibody which is specifically combined with the transgenic protein in an attaching manner, the sample feeding device comprises a second porous plate body and a cone, and two ends of each hollow fiber polypropylene film are respectively fixed on the first porous plate body and the second porous plate body and are respectively communicated with holes in the first and second porous plate bodies; step III. washing; step IV. injecting a second antibody which is specifically combined with the transgenic protein and provided with marks into sample feeding holes of the detection device, and enabling the second antibody to pass through the detection device; step V. washing; and step VI, detecting whether the hollow fiber polypropylene film has the mark or not, and correlating the detection result with whether the transgenic ingredient exists in the raw material.
Owner:通标标准技术服务有限公司
Mycobacterial vaccine vectors and methods of using the same
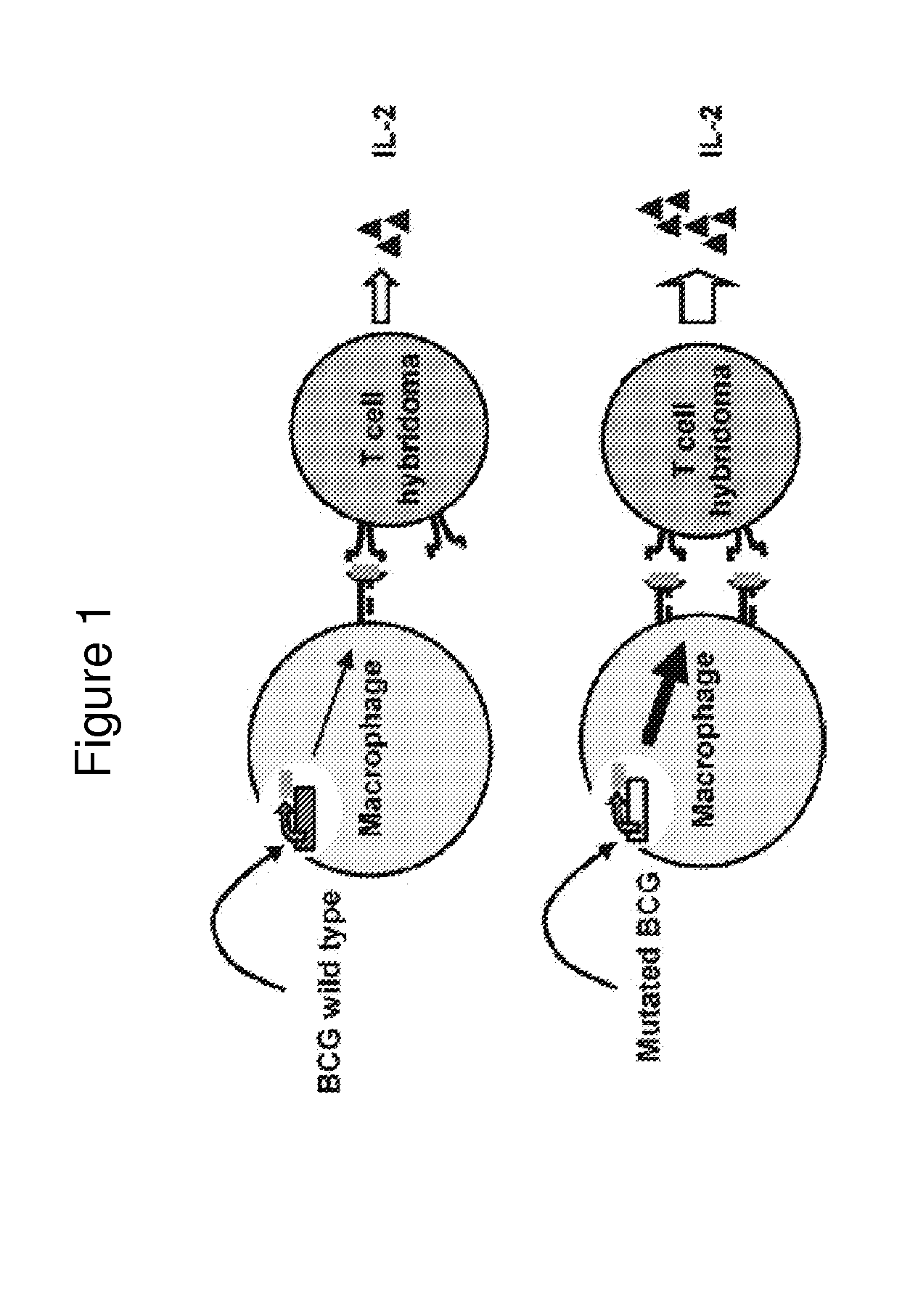
Mycobacterium bovis BCG is a potent stimulator of the cellular immune response and has potential as a recombinant vaccine vector. Disclosed are BCG strains that generate greater MHC class I presentation of a transgenic protein, relative to the unmutated parental strain, and that improve a recipient's CD8+ T cell response against the transgenic protein following vaccination. The mycobacterial constructs and their respective t / ansposon mutant BCG strains exhibit increased immunogenicity that is several times greater than responses generated by the parental strain and other existing modified rBCG strains. Furthermore, upon introducing the SIV gag gene into these novel strains, we observed that these strains primed for increased CD8+ T cell responses in a heterologous prime / boost regimen, comparable to levels generated by plasmid DNA vaccines. Creation of a second generation rBCG vector that may be utilized as a vaccine vector for immunizing against a variety of pathogens.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Plant bioreactors
A plant expressing a cytokine and an autoantigen is disclosed. An example of a cytokine is a contra-inflammatory cytokine, for example IL-4 and IL-10, and an example of autoantigen is GAD. A novel method for the production of transgenic proteins of interest suitable for oral administration is also disclosed. Further, non-food crop plants expressing one or more proteins of interest are disclosed, as is a method involving the preparation of a protein of interest suitable for oral administration within a non-food crop plant comprising, transforming the non-food crop plant with a suitable vector containing a gene of interest and appropriate regulatory regions to ensure expression of the gene of interest within the non-food crop plant, such that the non-food crop plant is characterized as being non-toxic, non-addictive, palatable, and requiring minimal or no processing prior to oral administration. An example of a non-food crop plant is low alkaloid tobacco. Proteins of interest may include pharmaceutically active proteins such as growth regulators, insulin, interferons, interleukins, growth hormone, erythropoietin, G-CSF, GM-CSF, hPG-CSF, M-CSF, Factor VIII, Factor IX, tPA, antibodies, antigens and combinations and / or derivatives thereof.
Owner:LONDON HEALTH SCI CENT RES +1
Quantitative analysis of transgenic protein 5-enolpyruvylshikimate-3-phosphate synthase
The invention relates to methods for quantitative multiplex analysis of complex protein samples from plants using mass spectroscopy. In some embodiments, the disclosure concerns methods for maintaining a transgenic plant variety, for example by analyzing generations of a transgenic plant variety for selective and sensitive quantitation of multiplexed transgenic proteins.
Owner:DOW AGROSCIENCES LLC
Auxiliary method for detecting whether genetically modified protein exists in food
The invention discloses an auxiliary method for detecting whether genetically modified protein exists in food. The method comprises steps as follows: total protein is extracted; the total protein is injected to sample adding holes of a detection device, holes in a first perforated plate are closed, the detection device comprises the first perforated plate, a sample adding device and a hollow fiber polypropylene film tube, the sample adding device comprises a second perforated plate, a cone and multiple sample adding channels, the hollow fiber polypropylene film tube is arranged between the first perforated plate and the second perforated plate, and a first antibody specifically bound with to-be-detected genetically modified protein is attached to the inner wall; the holes in the first perforated plate are opened, and washing is performed; the holes in the first perforated plate are closed again, and a second antibody is injected into the sample adding holes in the detection device; the holes in the first perforated plate are opened again, and washing is performed; whether a mark of the second antibody exists in a hollow fiber polypropylene film is detected, and if the mark exists, the genetically modified protein exists in the total protein and existence of a genetically modified ingredient in the raw material is determined.
Owner:黎志春
Epinephelus anti-transgenosis protein and nucleotide sequence and use thereof
InactiveCN1640891AImprove reproductive performanceImprove controllabilitySugar derivativesPeptide/protein ingredientsTransgelin GeneOpen reading frame
The present invention discloses one kind of rockfish sex reversal relative gene sequence, vector and recombinant strain containing the gene and their use. The gene has cDNA of 608 bp length, one open reading frame of 252 bp (54-305), 83 coded amino acids, 5' non-coding region of 53 bp length and starting at one ANNATG motif included inside vertebrate initiator codon, and 3' non-coding region of 303 bp length and with polypeptide tailing signal (AAUAAA) and polypeptide tail. The vector and recombinant strain containing the gene and protoyon expressed fusion protein are used in propagation regulation, sex gland differentiation and sex conversion of rockfish and the feed additive preparation.
Owner:INST OF AQUATIC LIFE ACAD SINICA
Multiplex analysis of stacked transgenic protein
The invention relates to methods for multiplex analysis of complex protein samples from plants using mass spectroscopy. In some embodiments, the disclosure concerns methods for maintaining a transgenic plant variety, for example by analyzing generations of a transgenic plant variety for presence and concentration of multiplexed transgenic proteins.
Owner:CORTEVA AGRISCIENCE LLC
Application of polypeptide and small molecules in regulating protein expression level in rice or wheat
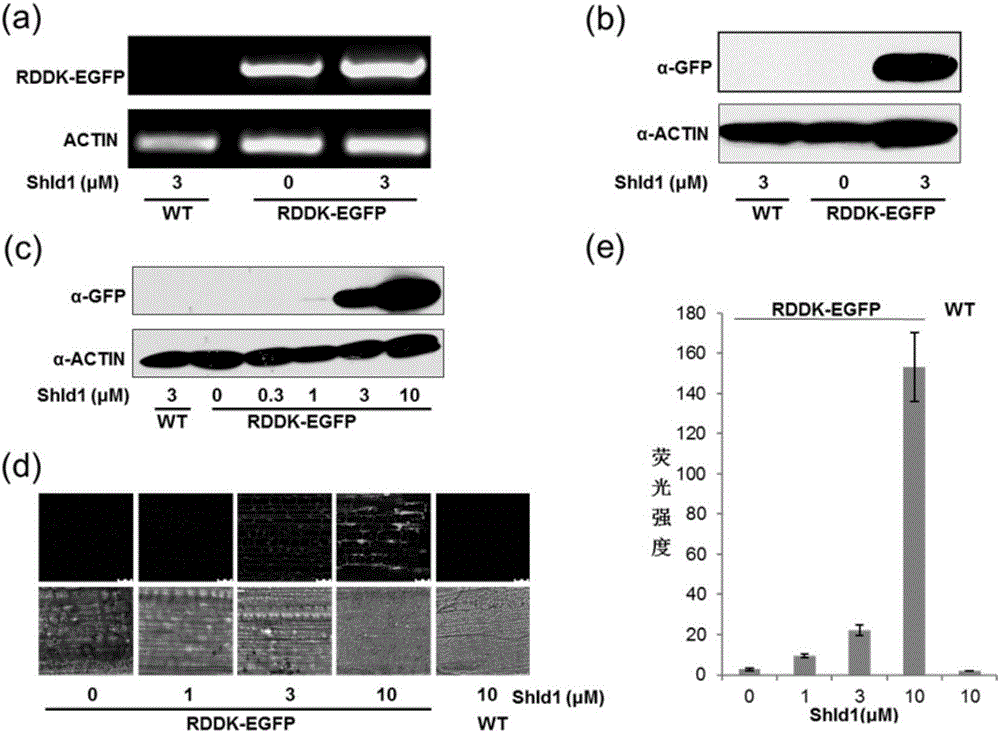
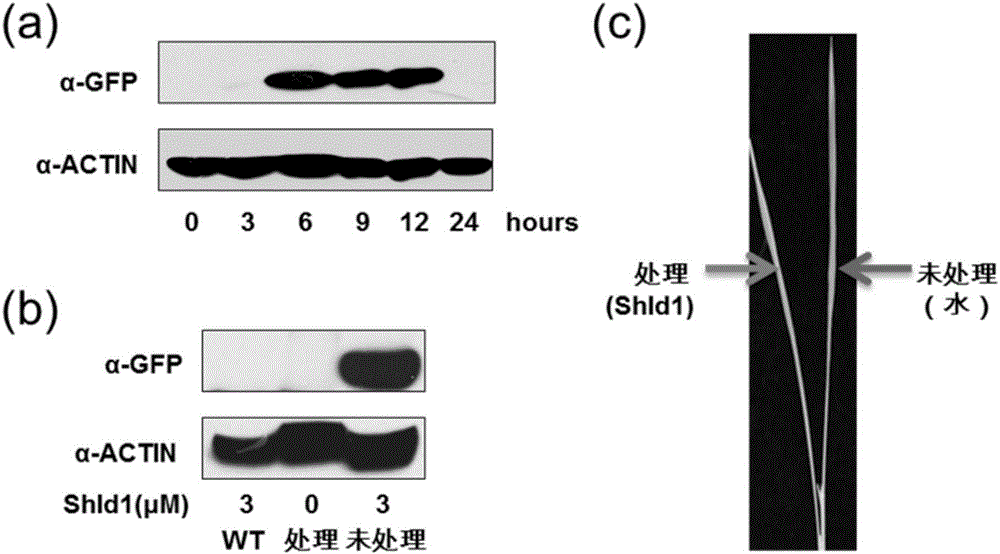
The invention discloses application of polypeptide and small molecules in regulating a protein expression level in rice or wheat. The invention provides application of a polypeptide related biomaterial and shield1 in cultivating genetically modified crops. The polypeptide related biomaterial is any one of polypeptide RDDK or RDDKHA, a polypeptide RDDK encoding geneor or a polypeptide RDDKHA encoding gene, an expression vector containing the polypeptide RDDK encoding gene or an expression vector containing the polypeptide RDDKHA encoding gene, and a fusion protein and a fusion protein encoding gene formed by fusing the polypeptide RDDK encoding gene or the polypeptide RDDKHA encoding gene and a functional protein gene. The experiments provided by the invention show that an efficient space-time controlled chemical induction system of the transgene protein expression level is built in the rice and the wheat for the first time, and finally a powerful technical support is provided for crop function gene excavation and transgene molecule breeding.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Apparatus for aided detection of existence of transgenic protein in food
The invention discloses an apparatus for aided detection of the existence of a transgenic protein in a food. The apparatus comprises: a first porous plate body; a sample adding device comprising a second porous plate body, a cone and a plurality of sample adding channels, wherein the diameter of the cone gradually decreases from bottom to top, the free end of the cone forms a sample adding hole, the plurality of sample adding channels correspond to pores in the second porous plate one to one, one ends of the plurality of sample adding channels are connected with the sample adding holes, and the other ends the plurality of sample adding channels are connected with the pores in the second porous plate; and hollow fiber polypropylene membrane tubes arranged between the first porous plate body and the second porous plate body, wherein the inner wall of every hollow fiber polypropylene membrane tube is attached with an antibody specifically binding to a transgenic protein to be detected, the upper ends of the hollow fiber polypropylene membranes tubes are fixed in the pores of the second porous plate body, the lower ends of the hollow fiber polypropylene membrane tubes are fixed in the pores of the first porous plate body, and the inner wall of every hollow fiber polypropylene membrane tube is provided with a plurality of projections and recesses.
Owner:黎志春
Test strip, preparation method and application thereof in transgenic protein AM79 EPSPS detection
InactiveCN112526133AEasy to operateIncreased sensitivityBiological material analysisBiological testingBiotechnologyTransgelin Gene
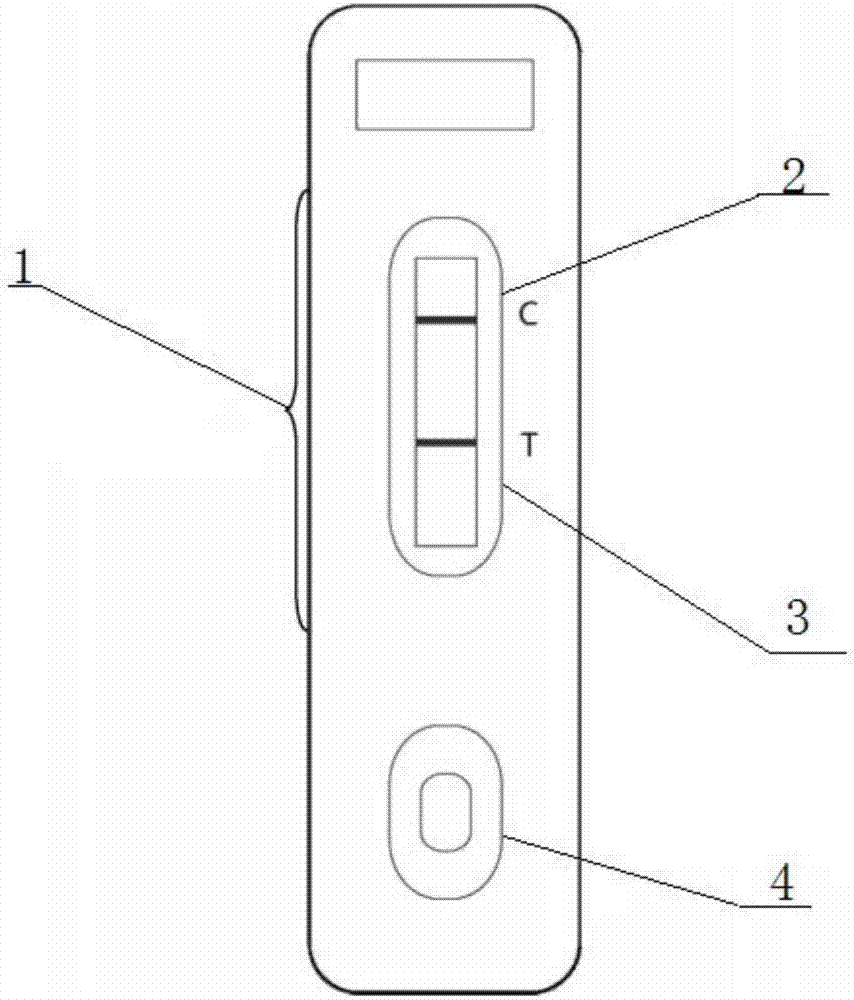
The invention relates to the technical field of biochemical detection, in particular to a test strip, a preparation method of the test strip and application of the test strip in transgenic protein AM79 EPSPS detection. The handheld colloidal gold rapid test paper for the transgenic protein AM79 EPSPS provided by the invention can be widely applied to rapid detection of the transgenic protein AM79EPSPS, and has the following specific advantages that: (1) the test paper is simple to operate, does not need special instruments and equipment, and can be conveniently and directly used for detectionin fields and other fields; (2) the detection time is short (5-8 minutes); the result judgment standard is unified, i.e. for a negative result (-), only one C line appears; and for a positive result(+), T line and C line appear at the same time; and (3) the test paper is verified to have good sensitivity, and the lowest detection limit can reach 0.1microg / mL.
Owner:ZHEJIANG XINAN CHEM INDAL GROUP
Thionine labeled antibody immunochromatography testing strip for visually and rapidly detecting transgenic protein
PendingCN113687065AShorten the timeAccurate detectionBiological material analysisBiological testingTransgelin GeneCellulose
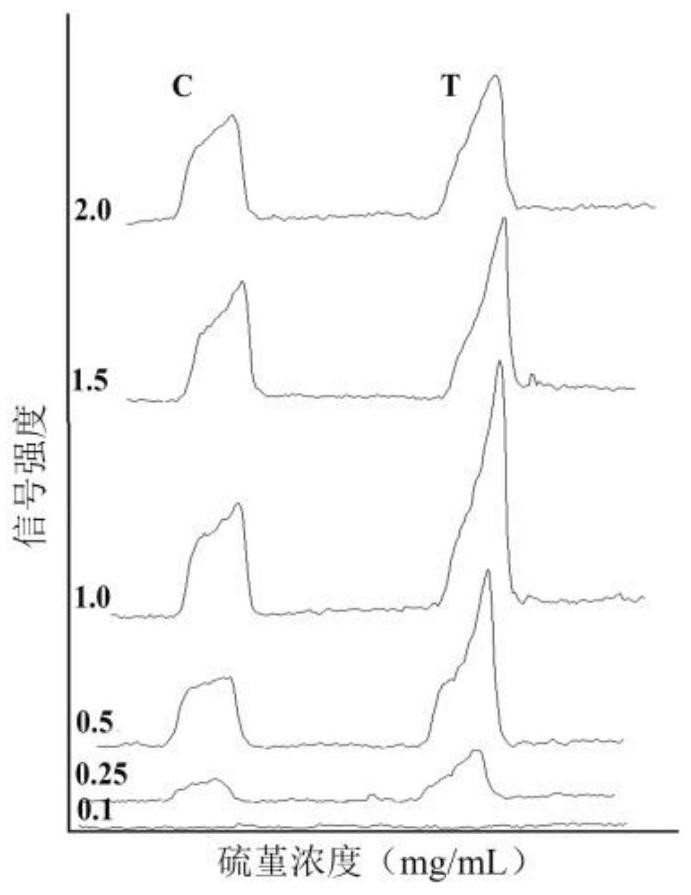
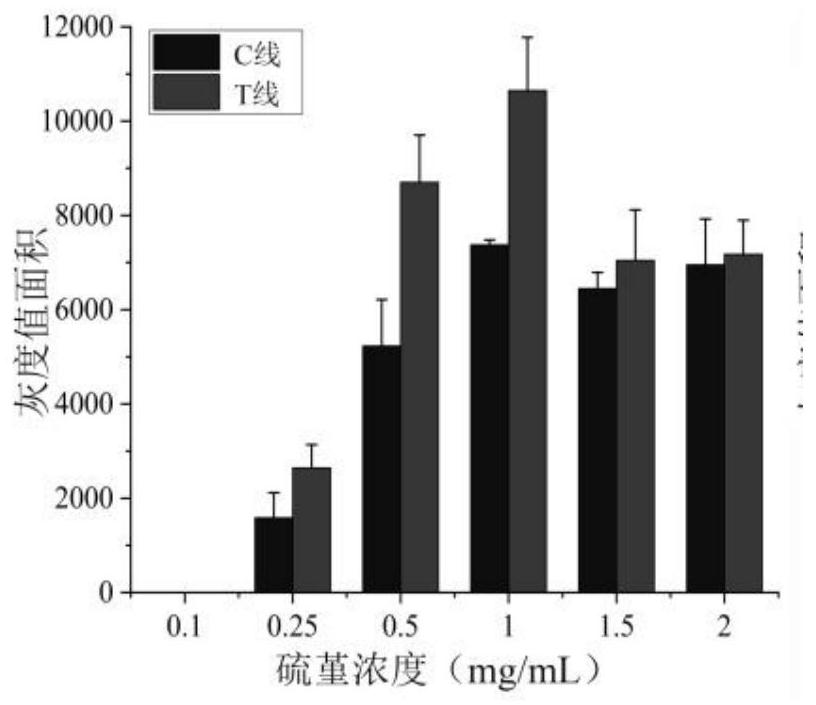
The invention relates to a thionine labeled antibody immunochromatography testing strip for visually and rapidly detecting transgenic protein, the thionine labeled antibody immunochromatography testing strip comprises a bottom plate, and a sample pad, a combination pad, a nitrocellulose membrane and absorbent paper which are fixed on the bottom plate and are connected in sequence, and a labeled antibody labeled by thionine is fixed in the combination pad; a Thi-mAb1 compound which is formed by coupling the thionine with a monoclonal antibody mAb1 through glutaraldehyde. The immunochromatography testing strip provided by the invention takes Thi as a signal marker, does not need a nano material, does not need a complex synthesis process, utilizes a conventional cross-linking agent to be rapidly coupled with an antibody through a covalent bond, breaks through the tedious preparation process of colloidal gold, greatly shortens the time for marking the antibody, simplifies the preparation process, and can rapidly detect transgenic protein; the sensitivity is high; and the specificity, the stability and the reproducibility are good.
Owner:SHANGHAI ACAD OF AGRI SCI +1
Microfluidic protein chip for detecting genetically modified crops and kit of microfluidic protein chip
InactiveCN102759628BDecreased probe activityHigh sensitivityBiological testingTransgelin GenePhosphate
The invention discloses a microfluidic protein chip for high-through detecting genetically modified crops. The microfluidic protein chip comprises a nucleic acid chip which is synthesized on the basis of mu ParafloTM microfluidic protein chip and contains six nucleotide sequences and 6 types of antibody-oligonucleotide composite probes, thereby simultaneously detecting 6 transgenetic proteins of trans-bacillus thuringiensis Bt insecticidal crystal protein (Cry1Ac), trans-bacillus thuringiensis Bt insecticidal crystal protein (Cry1c), trans-bacillus thuringiensis Bt insecticidal crystal protein (Cry1F), trans-bacillus thuringiensis Bt insecticidal crystal protein (Cry1Ab), soybean trypsin (SBTI) and neomycin phosphate transferase II gene (NPTII). The invention further provides a transgenic assay kit which comprises a mu ParafloTM microfluidic deoxyribose nucleic acid (DNA) chip which contains six types of probes, six types of antibody-oligonucleotide composite probes, six types of detection antibodies and the like.
Owner:ZHEJIANG UNIV
Preparation method and application of fluorescent biosensor for detecting transgenic protein
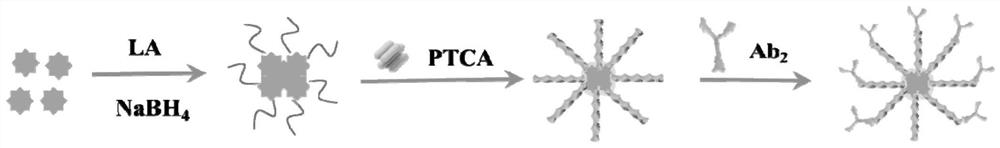
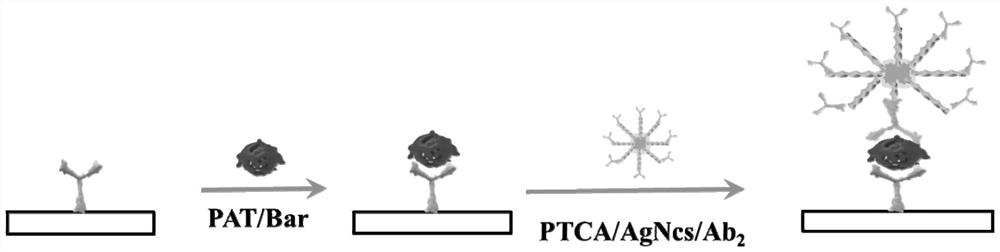
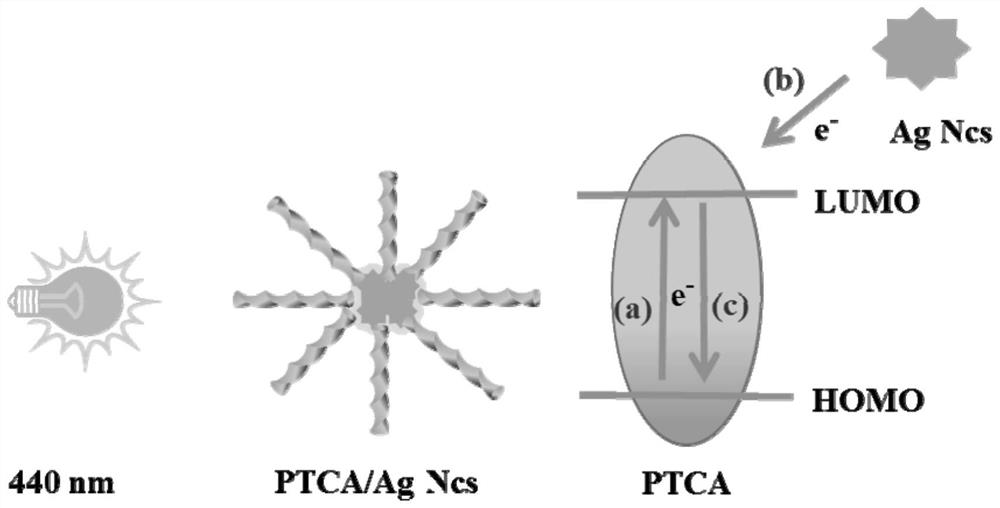
ActiveCN114354914AStrong fluorescent signalLarge FL signal strengthBiological material analysisBiological testingTransgelin GeneCarboxylic acid
The invention discloses a preparation method and application of a fluorescent biosensor for detecting transgenic protein, and the preparation method is characterized by comprising the following steps: mixing a 1 mg / mL 3, 4, 9, 10-perylenetetracarboxylic acid solution and a silver nano-cluster solution according to a volume ratio of 5: 1, and stirring at normal temperature for 4 hours to obtain an AgNCs / PTCA solution; adding the EDC / NHS mixed solution into the AgNCs / PTCA solution in equal volume, keeping out of light at room temperature, continuously stirring for 6 hours, adding a second antibody Ab2 of the transgenic protein to be detected, which is equal to the EDC / NHS mixed solution in volume, incubating at 37 DEG C for 2 hours, centrifuging to remove the free second antibody Ab2, washing the product with ultrapure water for three times, re-dispersing into the PBS buffer solution, which is equal to the EDC / NHS mixed solution in volume, and centrifuging to remove the free second antibody Ab2; the supermolecular high-fluorescence material has the advantages of strong specificity, high sensitivity and good accuracy.
Owner:NINGBO UNIV
Protein production in transgenic alfalfa plants
InactiveCN1237175CImmunoglobulin superfamilyMicrobiological testing/measurementTransgelin GeneClinical trial
This invention is directed to characterizing a host system suitable for the production of functional transgenic proteins, such as anti-human IgG, for use in applications requiring government regulatory approval. It is well known that regulatory agencies required stable, consistent master cell banks and master cell lines for the production of transgenic proteins in order to ensure sufficient material for appropriate characterization, clinical trials, and potential sales. Current plant production systems require the establishment of seed banks for this purpose. However, there are many draw backs related to such a system for the production of a continuous reliable transgenic protein source. An aspect of this invention is directed to characterizing a plant production system suitable for transgenic proteins that meet the stringent regulatory requirements. Another aspect of this invention exemplifies the production and characterization of an anti-human IgG for use as a blood grouping reagents, through the expression of corresponding genes in transgenic alfalfa plants. The cDNAs of the heavy and light chains of a human IgG-specific IgG2a(kappa) murine mAb (C5-1) were transferred into alfalfa through Agrobacterium infection. Transgenic plants expressing the light- and heavy-chain encoding mRNAs were obtained and plants from the Fl progeny (obtained by sexual crossing) were found to express fully assembled C5-1. Furthermore, the transgenic protein was stable in vivo, as well as during extraction and purification procedures. Purification yielded a unique H2L2 form with a reactivity indistinguishable from hybridoma-derived C5-1 in standardized serological tests. Results indicate that plant-derived transgenic proteins, such as mAbs can be used as diagnostic reagents as effectively as hybridoma-derived mAbs, and demonstrates the usefulness of the transformed alfalfa system to produce large amounts of proteins, including multimeric proteins such as mAbs.
Owner:AGRI & AGRI FOOD +2
Protein production in transgenic alfalfa plants
InactiveCN1290302AImmunoglobulin superfamilyMicrobiological testing/measurementTransgelin GeneIn vivo
This invention is directed to characterizing a host system suitable for the production of functional transgenic proteins, such as anti-human IgG, for use in applications requiring government regulatory approval. It is well known that regulatory agencies required stable, consistent master cell banks and master cell lines for the production of transgenic proteins in order to ensure sufficient material for appropriate characterization, clinical trials, and potential sales. Current plant production systems require the establishment of seed banks for this purpose. However, there are many draw backs related to such a system for the production of a continuous reliable transgenic protein source. An aspect of this invention is directed to characterizing a plant production system suitable for transgenic proteins that meet the stringent regulatory requirements. Another aspect of this invention exemplifies the production and characterization of an anti-human IgG for use as a blood grouping reagents, through the expression of corresponding genes in transgenic alfalfa plants. The cDNAs of the heavy and light chains of a human IgG-specific IgG2a(kappa) murine mAb (C5-1) were transferred into alfalfa through Agrobacterium infection. Transgenic plants expressing the light- and heavy-chain encoding mRNAs were obtained and plants from the Fl progeny (obtained by sexual crossing) were found to express fully assembled C5-1. Furthermore, the transgenic protein was stable in vivo, as well as during extraction and purification procedures. Purification yielded a unique H2L2 form with a reactivity indistinguishable from hybridoma-derived C5-1 in standardized serological tests. Results indicate that plant-derived transgenic proteins, such as mAbs can be used as diagnostic reagents as effectively as hybridoma-derived mAbs, and demonstrates the usefulness of the transformed alfalfa system to produce large amounts of proteins, including multimeric proteins such as mAbs.
Owner:AGRI & AGRI FOOD +2
Method for producing transgene protein medicine of mammary gland expression using gland virus as carrier mammary
InactiveCN1869217BAvoid harmReduce R&D costsPeptide/protein ingredientsRecombinant DNA-technologyTransgelin GeneMammary gland structure
The invention discloses a method to produce transgene albumen medicine that takes galactophore expression using adenovirus as carrier. The feature is that: using copying defect type adenovirus framework plamid vector to carry different target albumen gene sequence, and gaining reconstructed albumen carrier from packaging cell in E1A sequence containing Ad5 adenovirus gene group, and gaining instantaneous high expression external target gene medicine albumen. The operation process includes the following steps: separating and cloning target medicine albumen gene sequence; constructing confluence eukaryon expression carrier to testing target albumen to gain external expression right medicine albumen gene; rebuilding the target albumen gene with the adenovirus gene group frame molecule to gain the adenovirus carrier of rebuilt target medicine albumen gene; inducing into animal galactophore; excreting the needed target medicine albumen from latex. The rebuilt albumen could be directly usedto develop kinds of health products and medicine.
Owner:李青旺 +2
Method for constructing rat model for verifying property of drug for treating cerebral infarction ischemia
InactiveCN107904258APromote recoveryGood treatment effectGenetically modified cellsNervous system cellsTransgelin GeneTherapeutic effect
The invention belongs to the medical field and discloses a method for constructing a rat model for verifying the property of a drug for treating cerebral infarction ischemia. NSC is separated from theforebrain tissue of a neonatal rat, the self-renewal and pluripotency differentiation characteristics are shown through in vitro culture and expansion by means of a flow cytometry and the single cellcloning technology, and through rAAV-mediated EPO gene and LacZ gene transfer, an NSC transfection system which stably expresses the EPO gene and the LacZ gene is established, and the in vitro and invivo transgene protein expression conditions of the NSC transfection system are analyzed; the NSC transfection system modified by the EPO gene and the LacZ gene is transplanted into cerebral infarction ischemia lesions of the rat, and the repairing effect of the EPO gene and the LacZ gene on the cerebral infarction ischemia lesions is analyzed. The EPO-modified NSC transplantation effectively promotes the recovery of the neurological function during the recovery from cerebral ischemia and improves the therapeutic effect.
Owner:FENGXIAN CENT HOSPITAL
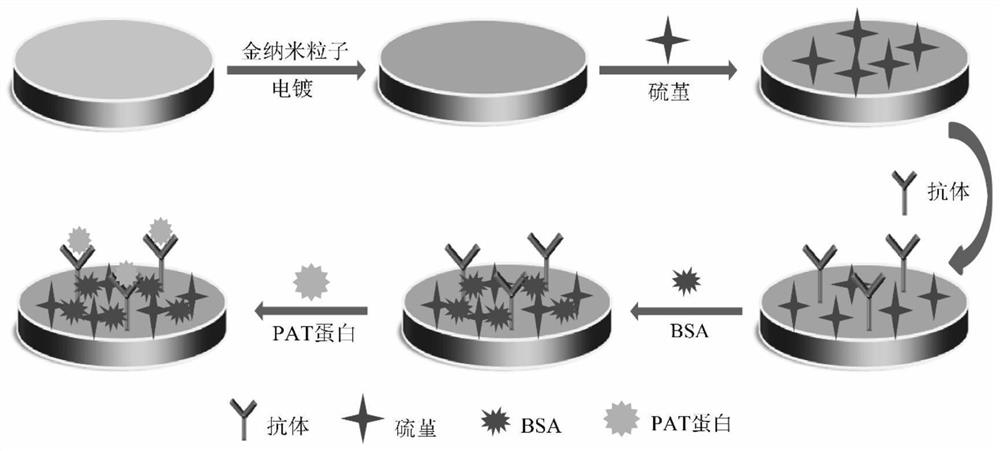
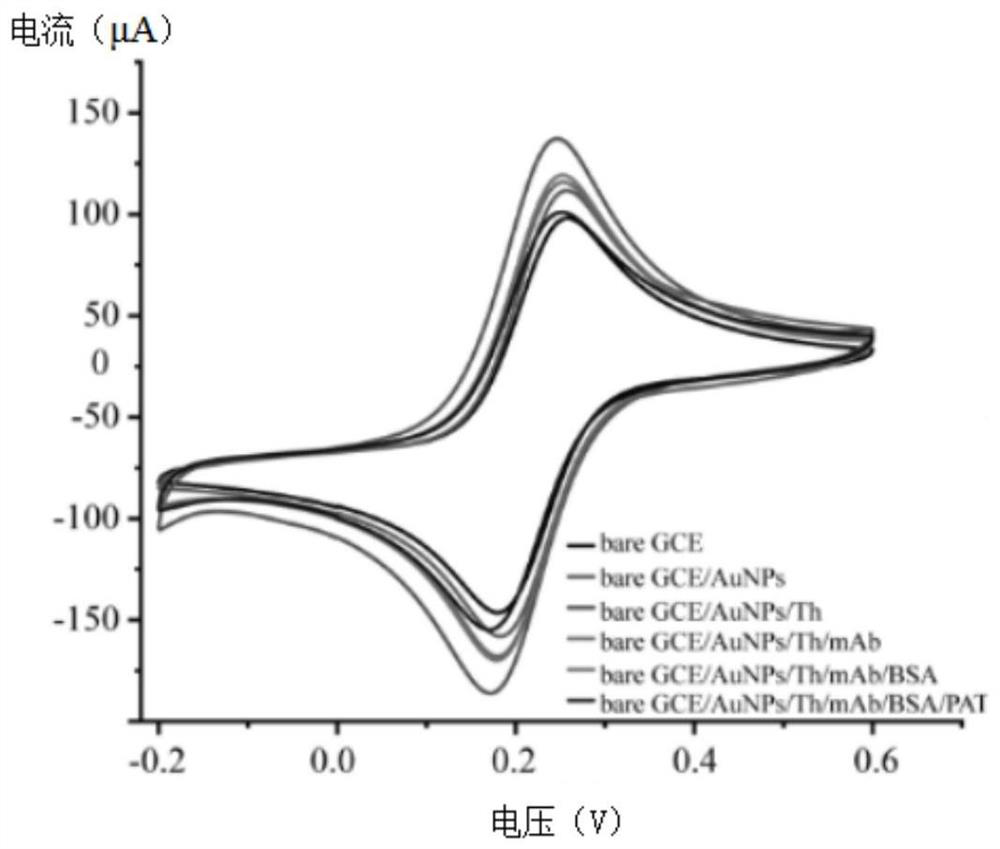
Unmarked AuNPs-Thi electrochemical immunosensor for detecting transgenic protein and a preparation method thereof
PendingCN113655103AQuick analysisAccurate analysisBiological testingMaterial electrochemical variablesTransgelin GeneNanoparticle
The invention discloses an unmarked AuNPs-Thi electrochemical immunosensor for detecting transgenic protein and a preparation method thereof. The preparation method comprises the following steps: electrodepositing gold nanoparticles AuNPs on the surface of a glassy carbon electrode, and stably adsorbing thionine Thi on the surface of the AuNPs to form an AuNPs-Thi film; and binding the transgenic protein-mAb antibody covalently to the AuNPs-Thi membrane, and then using BSA (bovine serum albumin) for sealing. According to the method, after AuNPs is electrodeposited on the surface of the electrode, a proper amount of Thi is adsorbed, an electrochemical signal is remarkably enhanced, an antibody of transgenic protein is connected to an AuNPs-Thi film through the amino cross-linking effect, the long-term stability and sensitivity of the sensor are facilitated, the method is simple and rapid, the amount of needed antibodies is small, the sensitivity is high, and the specificity and reproducibility are good; and the invention provides a novel method for electrochemical immunodetection of transgenic protein in transgenic crops.
Owner:SHANGHAI ACAD OF AGRI SCI +1
Rapid detection device for genetically modified ingredients in grain and oils
The invention specifically discloses a rapid detection device for genetically modified ingredients in grain and oils. The rapid detection device comprises a sample feeding device, a plurality of hollow cellulose polypropylene membranes and a porous plate body, wherein primary antibodies, which are specifically combined with genetically modified proteins in total proteins, are attached to the hollow cellulose polypropylene membranes, so that the genetically modified proteins are combined with the primary antibodies and are attached onto the hollow cellulose polypropylene membranes. The rapid detection device disclosed by the invention is simple in design, convenient to use and easy to operate. According to the rapid detection device disclosed by the invention, the antibodies of genetically modified expression proteins are fixed on the cellulose polypropylene membranes, so that whether the genetically modified proteins exist in raw materials or not is detected through enabling the total proteins of raw materials to be detected to flow through the detection device, and whether the genetically modified proteins exist or not can be effectively detected. The rapid detection device is provided with two sample feeding holes at the same time and two groups of the hollow cellulose polypropylene membranes are arranged, so that the device can be used for detecting two groups of samples at the same time.
Owner:上海为然环保科技有限公司
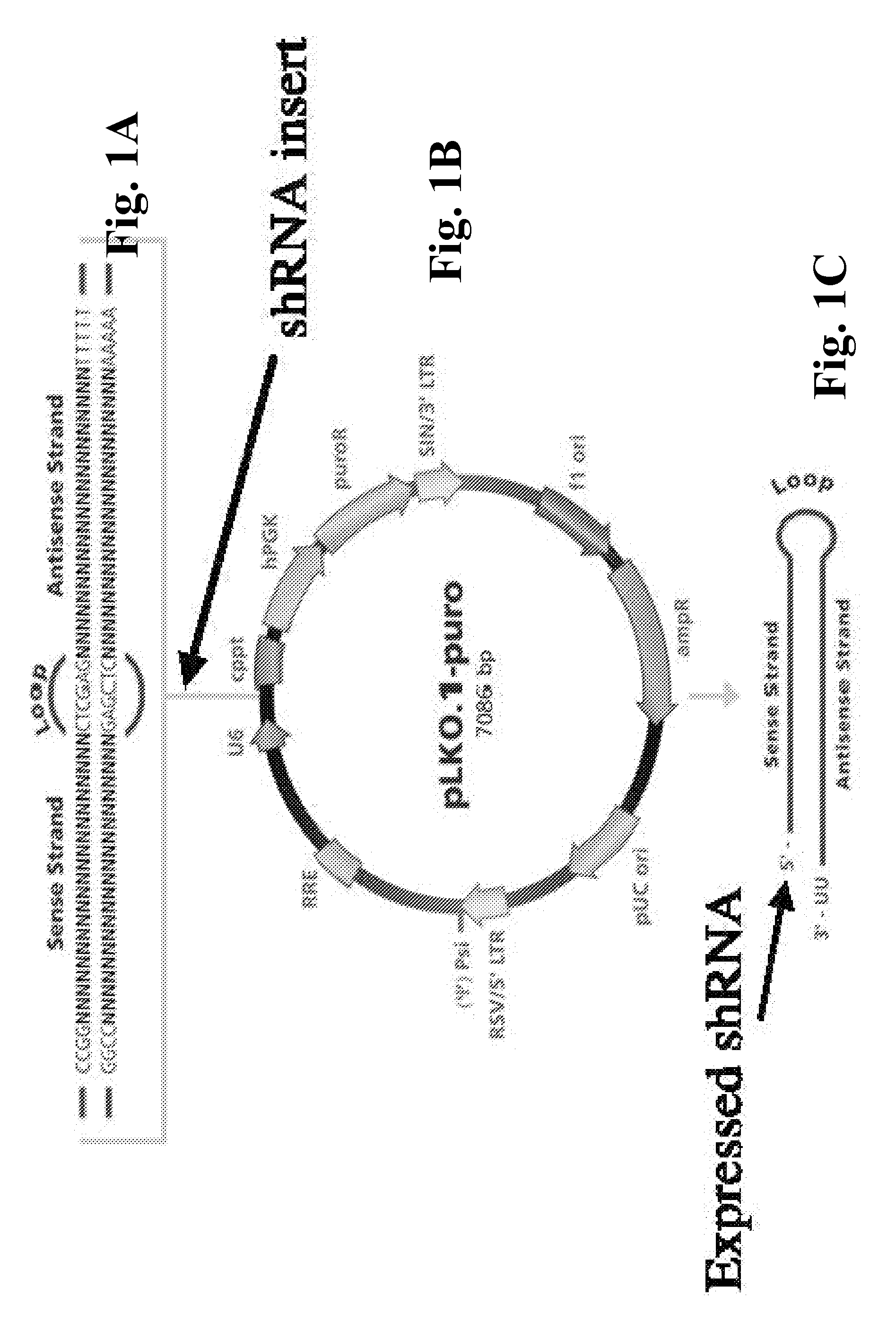
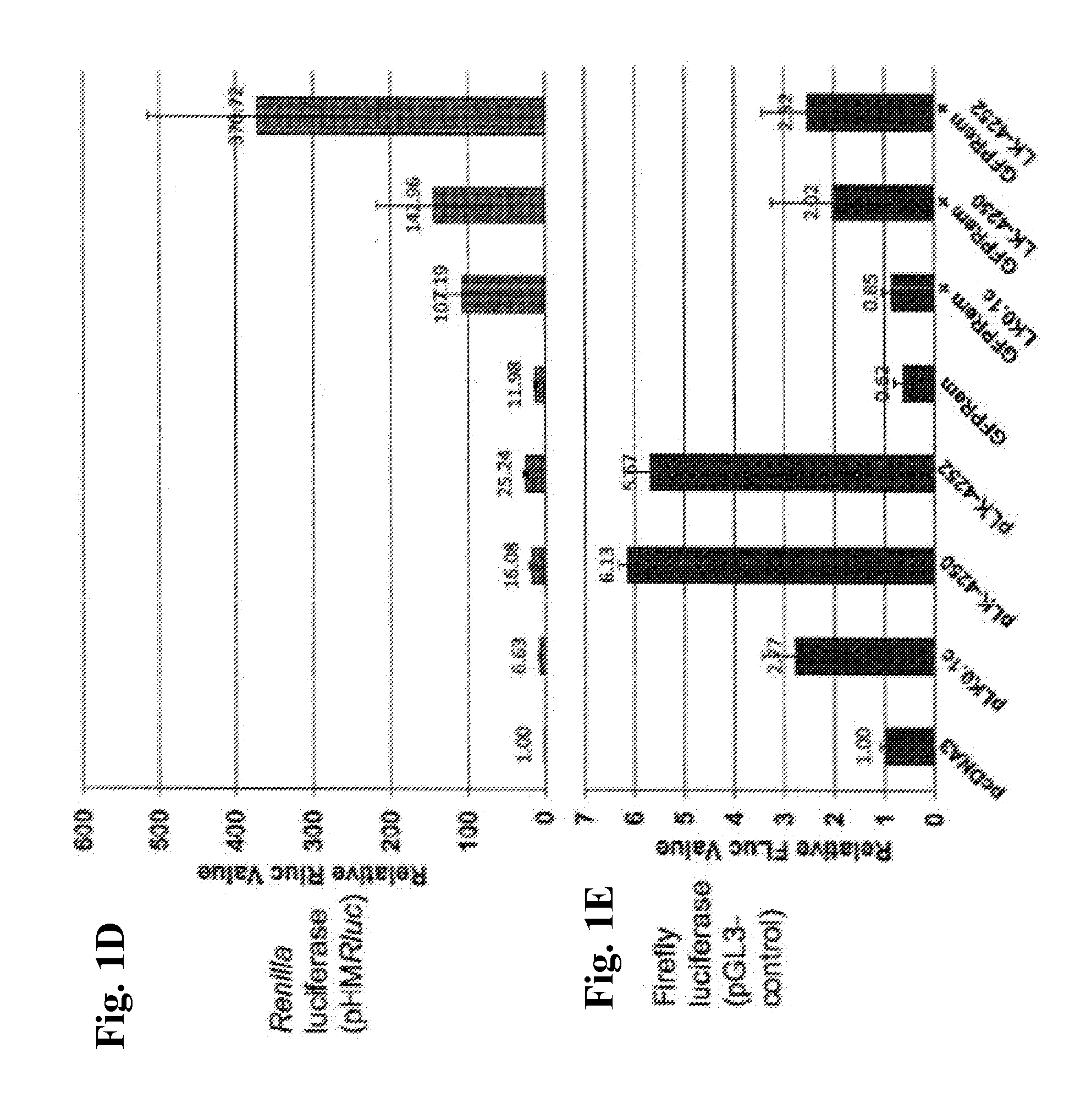
Compositions And Methods To Enhance Protein Expression
InactiveUS20160002643A1Low amountIncrease gene expressionAnimal cellsVirusesTransgelin GeneEukaryotic plasmids
The present invention relates to methods, compositions and kits for increasing expression of transfected genes through the use of super-induction nucleic acid sequences, including highly structured RNA. In particular, co-transfection of super-induction nucleic acid sequences including, for example, shRNA encoding sequences, retroviral elements, and (in other embodiments) VA-RNA encoding sequences, is contemplated for enhancing the expression of co-transfected transgenes in a cell. More specifically, the super-induction sequences are contemplated for use, in one embodiment, with mammalian expression plasmids and gene therapy vectors for increasing transgenic protein activity levels in cells and tissues. In one embodiment, the super-induction sequences of the present inventions are contemplated for use with known DNA vaccination vectors for enhancing therapy or enhancing protective immunity.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
A method for detection of transgenic proteins based on antibody modification and terahertz spectroscopy
ActiveCN103528987BImprove accuracyAvoid the Fluorescence StepMaterial analysis by optical meansTime domainTransgelin Gene
The invention relates to a transgenic protein detection method combining antibody modification and terahertz spectrum. The invention aims to provide the method with the characteristics of high accuracy and rapid and convenient detection. According to a technical scheme involved in the invention, the transgenic protein detection method combining antibody modification and terahertz spectrum comprises the steps of: (1) quartz slide cleaning; (2) quartz slide surface modification; (3) quartz slide surface activation; (4) antibody immobilization; (5) quartz slide surface redundant site close; (6) transgenic protein immobilization; (7) acquisition of the terahertz time-domain spectrums of a to-be-measured point on a quartz slide and a reference point; and (8) comparison of the terahertz time-domain spectrums of the to-be-measured point on the quartz slide and the reference point.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com