Protein production in transgenic alfalfa plants
A plant, alfalfa technology, applied in the direction of anti-animal/human immunoglobulin, plant products, genetic engineering, etc., can solve problems such as infeasibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] To exemplify the usefulness of alfalfa in expressing proteins, including multimeric proteins such as mAbs, a high affinity mAb (C5 -1). When tested with erythrocytes weakly sensitized with blood group antibodies, the C5-1 mAb gave reactivity similar to that of commercial rabbit polyclonal reagents.
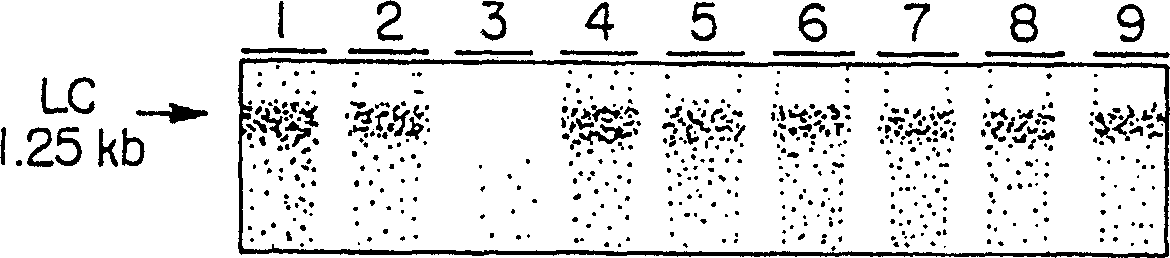
[0101] Integration and expression of transgene in alfalfa
[0102] The cDNAs encoding the C5-1 light and heavy chains were transferred into alfalfa plants following the protocol of Desgagnes et al. (1995). 15 plants and 25 plants of κ chain and γ chain were obtained respectively. The mRNA levels of kappa chain and gamma chain were monitored by RNA hybridization. The probe specifically hybridized to approximately 1.25 kbp kappa chain mRNA (Fig. 1A) and 1.75 kbp gamma chain mRNA (Fig. 1B). The alfalfa-derived mRNA was approximately 250 bp longer than the murine cDNA, indicating that in both cases the polyadenylation signal of gene 7 was used. Among the 29 F1 plants analy...
Embodiment 2
[0106] affinity purification
[0107] Peptide content and stability of the alfalfa-derived C5-1 mAb. Recovery of C5-1 mAb from leaf extracts of transgenic alfalfa plants by affinity purification ( Figure 3A ). Quantitative assays using leaf extracts from individual F1 double transgenic plants showed that C5-1 antibody levels ranged from 0.13-1.0% of total soluble protein. SDS-PAGE analysis of purified protein under reducing conditions ( Figure 3B) showed that both strands were detected by Coomassie blue staining and that their mobility was identical to that of their hybridoma-derived counterparts. These results suggest that plant-derived C5-1 is protected by glycosylation to the same extent as C5-1 produced with hybridoma cells.
Embodiment 3
[0109] Stability of transgenic proteins produced in and extracted from alfalfa
[0110] Proteolysis of recombinant IgG has been shown to occur in Nicotiana tabacum and Arabidopsis thaliana (Hiatt et al., 1989; Ma et al. 1994). Although non-truncated IgG is synthesized in plants with a signal peptide that facilitates targeting to the inert extracellular matrix (De Wilde et al. 1996), it has been observed that IgG can be degraded by endogenous proteases upon extraction ( Hiatt et al. (1989), During et al. (1990), Ma et al. (1995), Ma and Hein (1995), Schouten (1996)). Since the present invention concerns the preparation of monomeric or multimeric proteins such as mAb C5-1 in alfalfa, the stability of such preparations was examined.
[0111] 1) Protein stability in alfalfa extract compared to tobacco extract
[0112] To establish whether the protein is stable in crude extracts of alfalfa tissue, plant-derived or hybridoma-derived C5-1 were co-extracted in water in the presence ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com