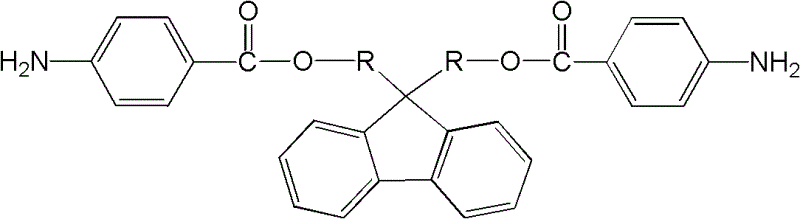
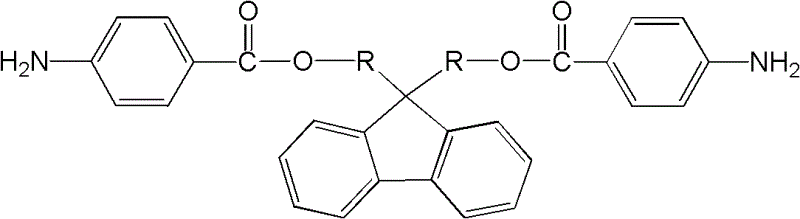
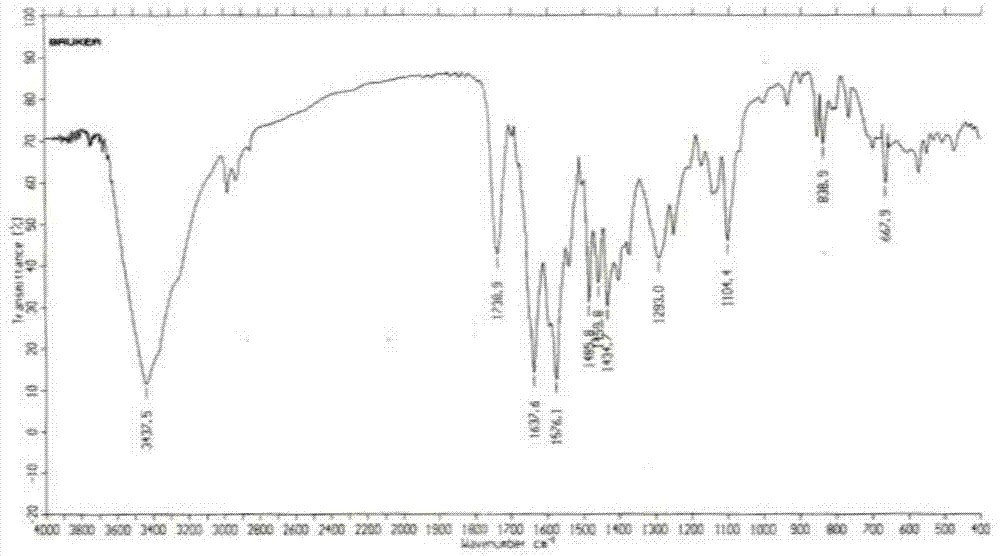
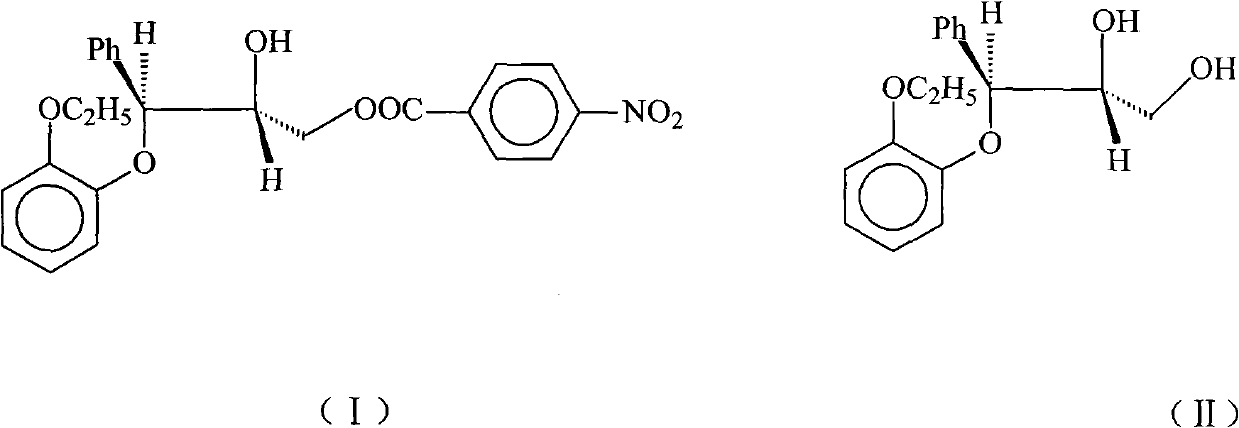
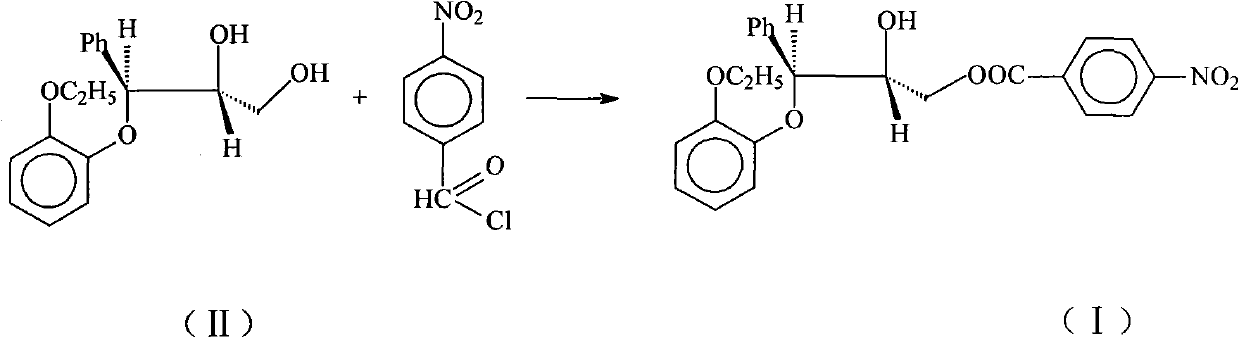
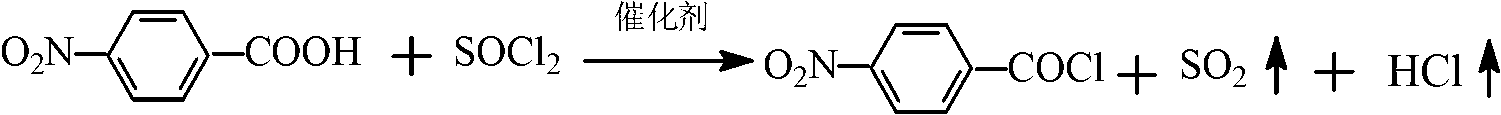Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
69 results about "P-nitrobenzoyl chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
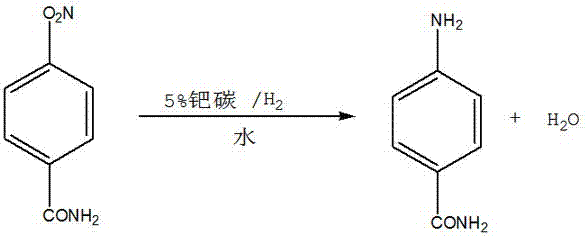
P-NITROBENZOYL CHLORIDE is incompatible with bases (including amines), with strong oxidizing agents, and with alcohols. May react with reducing agents. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Method for preparing folic acid
The invention relates to a preparation method of folic acid. The process steps are as follows: firstly, the intermediate product N-p-aminobenzoyl glutamic acid is prepared by using p-nitrobenzoyl chloride and sodium glutamate; Guanidine and sodium methoxide were used to prepare the intermediate product 6-hydroxy-2,4,5-triaminopyrimidine sulfate; finally, the above two intermediate products were used to prepare crude folic acid, which was purified to obtain pure folic acid. The beneficial effects of the invention are that the production cost is greatly reduced, the product efficiency is improved, and the pollution to the environment is reduced.
Owner:潘福星
Unsymmetrical fragrant diamine containing fluorine, preparation and application in synthesizing polyimide thereof
InactiveCN101270059AHigh purityImprove performanceOrganic chemistryOrganic compound preparationSolubilityOptical transparency
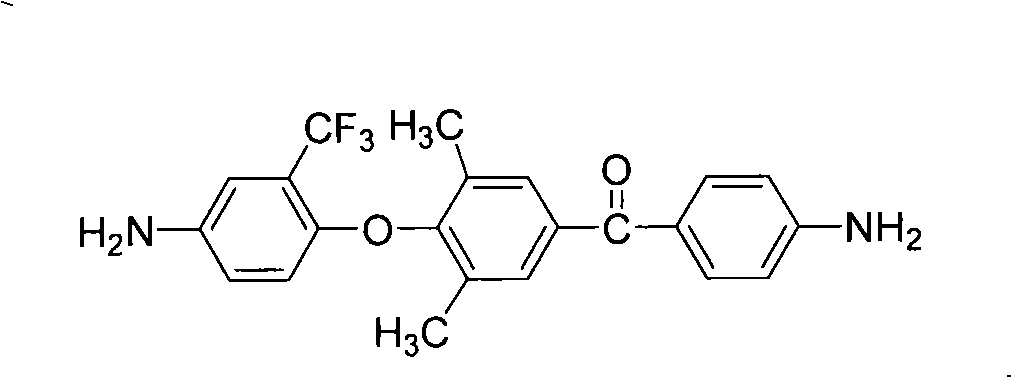
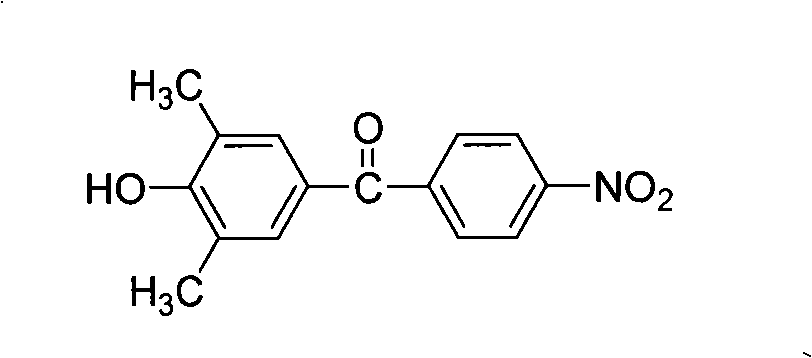
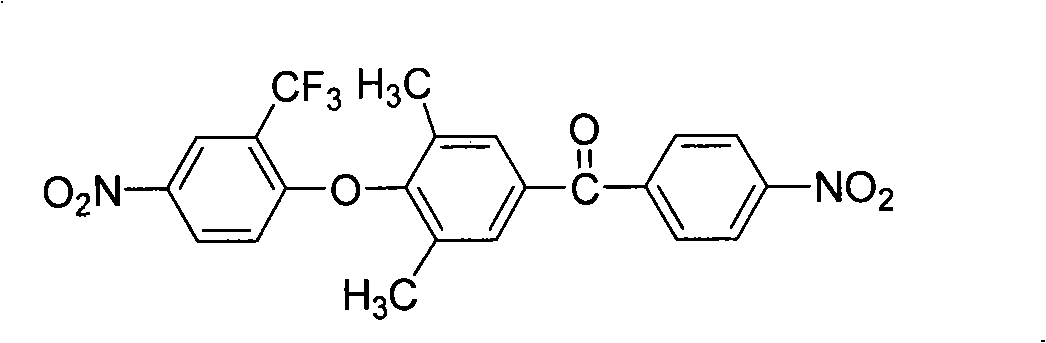
The present invention relates to fluorine-containing asymmetric aromatic diamine, a preparation method and an application thereof in the synthesis of polyimide. The compound has a constitutional formula as shown in the right. The preparation method comprises the following steps: (1) 2, 6-dimethyl phenol, paranitrobensoyl chloride and alchlor have catalytic reaction in an organic solvent to prepare (4'-hydroxy-3', 5'-dimethyl benzene)-(4-nitrophenyl) ketone; (2) the (4'-hydroxy-3', 5'-dimethyl benzene)-(4-nitrophenyl) ketone reacts with 2-chlorine-5-nitro trifluorotoluene with alkaline, to prepare (3', 5'-dimethyl-4'-(4''-nitro-2''-trifluoromethyl phenoxy) benzene)-(4-nitrophenyl) ketone; (3) the prepared product in the second step is reduced by reducing agent with organic solvents and catalysts; thus the fluorine-containing asymmetric aromatic diamine can be prepared. The fluorine-containing asymmetric aromatic diamine can be used for preparing fluorine-containing polyimide. The fluorine-containing asymmetric aromatic diamine prepared in the method has high purity and can keep stable at the room temperature; the polyimide made of the fluorine-containing asymmetric aromatic diamine has excellent solubility, film forming capability, optical transparency, mechanical properties and heat resistance.
Owner:DONGHUA UNIV
2-amino-fluorene containing ester group and preparation method thereof
InactiveCN102617382AGood flexibilityNo thermal degradationOrganic compound preparationAmino-carboxyl compound preparationHydrazine compoundRoom temperature
The invention provides 2-amino-fluorene containing an ester group and a preparation method thereof. The preparation method includes reacting 2-hydroxy-fluorene with p-nitrobenzoyl chloride at a room temperature for 1-5 hours, wherein the mass ratio of the 2-hydroxy-fluorene and the p-nitrobenzoyl chloride is 1:(2-2.2), subjecting the mixture to a reaction under a reflux condition for 10-24 hours to obtain 2-nitrofluorene containing the easter group; and subjecting the 2-nitrofluorene containing the easter group to reduction through hydrazine hydrate to obtain the white 2-amino-fluorene containing the ester group in the presence of a catalyst for 24-60 hours of reflux reaction, wherein the mass ratio of the 2-nitrofluorene containing the easter group and the hydrazine hydrate is 1:(3.2-4.8). According to the 2-amino-fluorene containing the ester group, flexibility of molecules is improved while thermal performance cannot be reduced greatly due to the induction of the ester group; and the 2-amino-fluorene containing the ester group can serve as a raw material for a curing agent for epoxy resin, polyimide and benzoxazine.
Owner:HARBIN ENG UNIV
Synthesis method for paranitrobenzoyl chloride
InactiveCN102796004AAvoid lossAvoid blockageOrganic chemistryOrganic compound preparationDistillationSynthesis methods
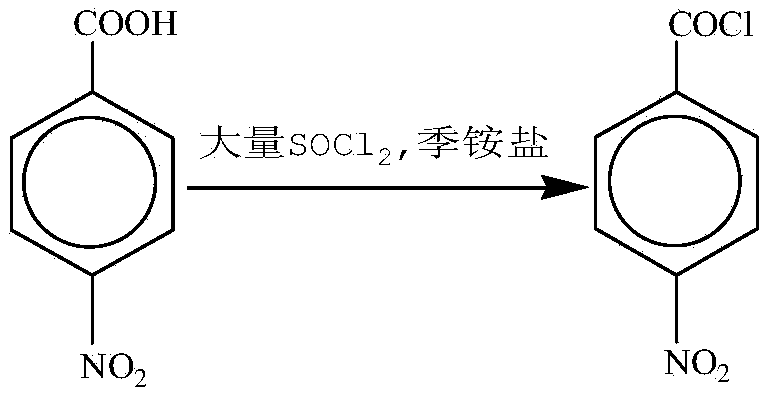
The invention relates to a synthesis method for paranitrobenzoyl chloride and belongs to the field of fine chemical industry. The method is characterized by comprising the following steps: directly reacting p-nitrobenzoic acid with sulfoxide chloride under the catalytic action of quaternary ammonium salt; distilling and recovering the residual sulfoxide chloride; and distilling under reduced pressure to obtain high-purity paranitrobenzoyl chloride. The sulfoxide chloride serves as an acyl chlorination reagent and a solvent and organic solvents such as carbon tetrachloride are not required, so recycling of solvents and materials and energy loss are avoided, the obtained product has high purity and does not need to be refined, the chromatograph content of the product obtained by primary distillation can reach over 99.5 percent, production cost is reduced, the yield of equipment is increased, blockage of pipelines is avoided, and burden is reduced for the subsequent procedure.
Owner:SHANDONG KAISHENG NEW MATERIALS
Preparation method for p-aminobenzamide
ActiveCN104193646AEmission reductionSimple processOrganic compound preparationCarboxylic acid amides preparationFerric hydroxideP-nitrobenzoic acid
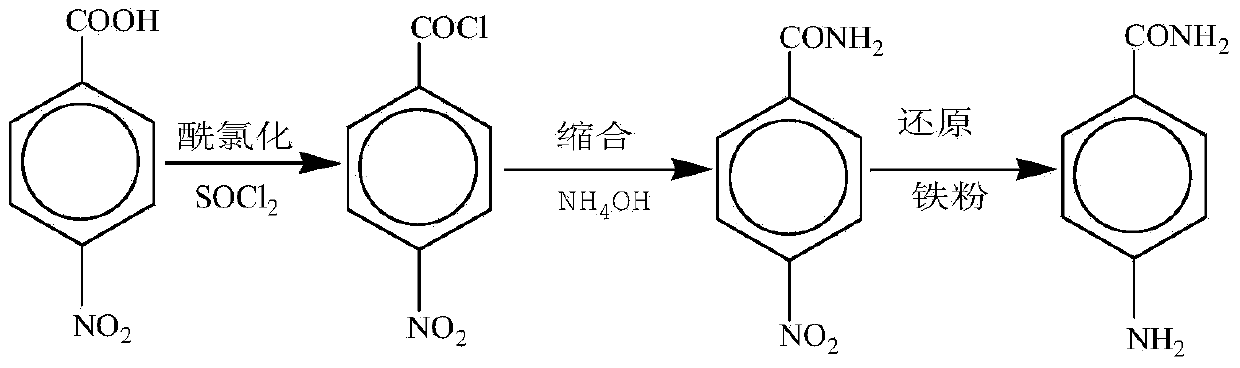
The invention relates to a preparation method for p-aminobenzamide. The preparation method comprises the following steps: (1) enabling p-nitrobenzoic acid to react with thionyl chloride in the presence of an organic base catalyst to obtain paranitrobenzoyl chloride liquor which is directly used for next-step reaction; (2) dropwise adding the paranitrobenzoyl chloride liquor into 10wt%-30wt% ammonium hydroxide, reacting and carrying out suction filtration on the mixture to obtain p-nitrobenzamide; and (3) enabling p-nitrobenzamide and hydrazine hydrate to carry out reaction in a solvent in the presence of ferric hydroxide or a material attached with ferric hydroxide to obtain p-aminobenzamide, wherein filtrate produced in the step is recycled for the next batch of reactions. The preparation method is high in product yield, low in cost, good in quality, capable of satisfying green and environment-friendly production requirements, simple and convenient to operate, and convenient for industrialization.
Owner:内蒙古美力坚科技化工有限公司
Method for preparing 2-( p-aminophenyl) benzimidazole-5-amine
InactiveCN101397275AIncrease productivityShort process routeOrganic chemistryBenzoyl chlorideContamination
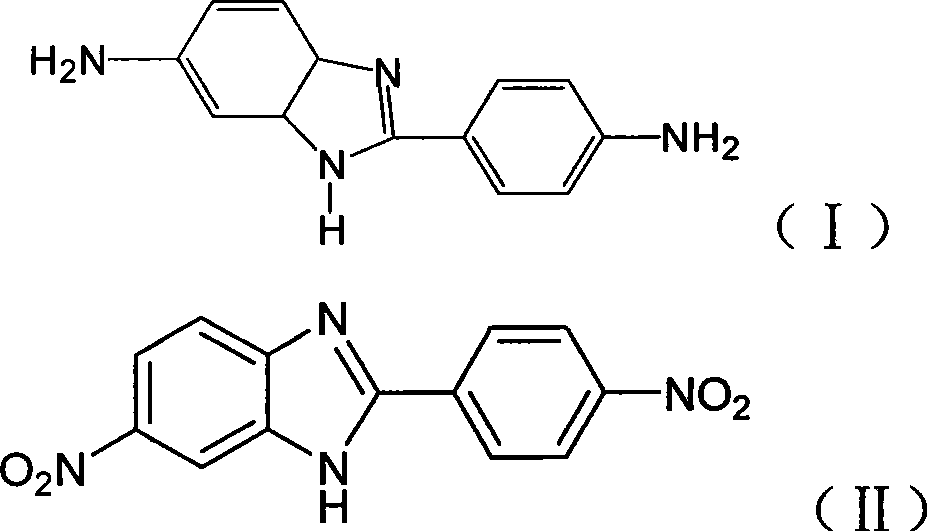
The invention relates to a preparation method of 2-(p-amino phenyl) benzimidazole-5-amic, including the following steps: (A) p-Nitro o-phenylendiamine and p-Nitro benzoyl chloride are adopted as materials which are processed through condensation, cyclization and dehydration one-pot method for synthesizing the benzimidazole compound shown as the formula (II); and (B) the compound of the formula (II) is subjected to catalytic hydrogenation to obtain the final product. The preparation method is characterized in that the manufacturing efficiency is high; and the synthesizing cycle is short and the reducing technique is environment-friendly. As a green synthesizing technique, the method overcomes the contamination problem troubling people for years.
Owner:ZHEJIANG DRAGON CHEM CO LTD +1
Preparation method of 4-aminobenzoyl-N-(4-aminobenzoyl) amine
InactiveCN1869003ASimple processHigh yieldOrganic compound preparationCarboxylic acid amides preparationOrganic solventIron powder
A process for preparing 4-aminobenzoyl-N-(4-aminobenzoyl) amine includes such steps as dissolving nitrobenzoyl chloride in organic solvent, adding it to ammonia water, ammonolysis reaction to obtain p-nitrobenzoylamine, iron powder reducing reaction or hydrocatalytic nitro reducing reaction in alcohol-water solution to obtain p-amino benzoylamine, condensating reaction on p-nitrobenzoyl chloride in organic solvent under existence of acid binding agent to obtain 4-nitrobenzoyl-N-(4-aminobenzoyl) amine, and iron powder reducing reaction of hydrocatalytic nitro reducing reaction in solvent.
Owner:SHENYANG RES INST OF CHEM IND +1
Fluorescent probe for detecting CO (carbon monoxide) in cells and preparation method and application of fluorescent probe
ActiveCN105623647AFaster and more sensitiveHigh selectivityMicrobiological testing/measurementPalladium organic compoundsNitriteSynthesis methods
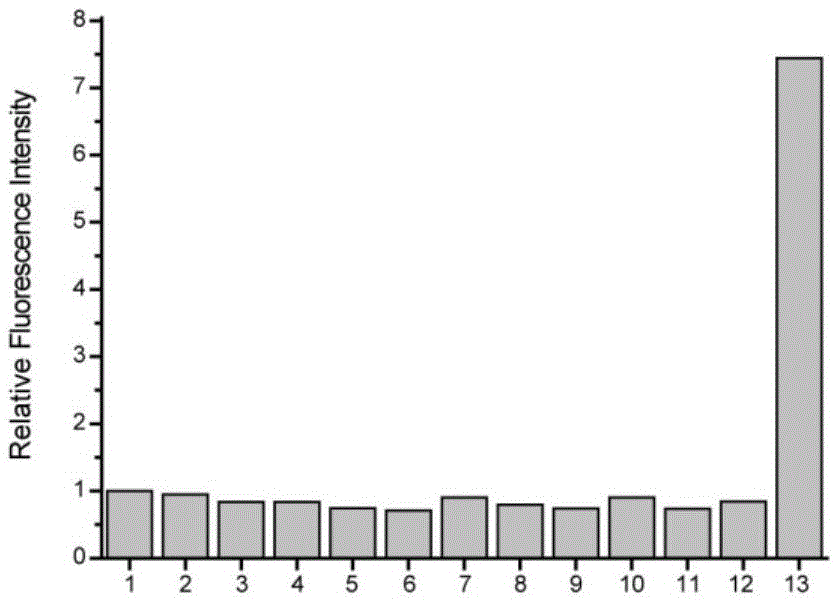
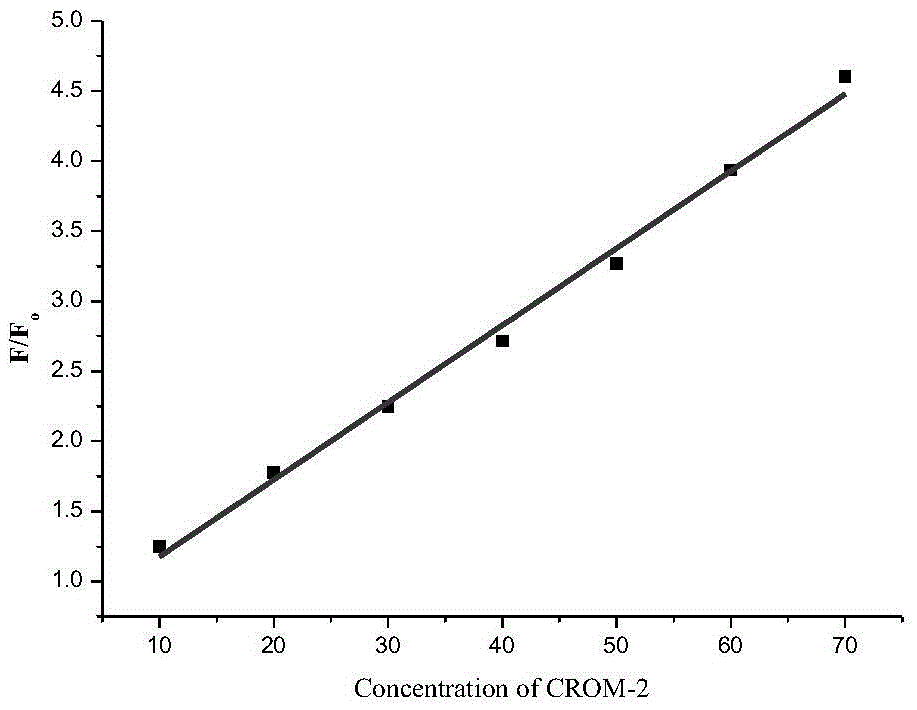
The invention discloses a fluorescent probe for detecting CO (carbon monoxide) in cells and a preparation method and application of the fluorescent probe. The fluorescent probe for detecting the CO in the cells is shown as the structural formula below, wherein R refers to H, -CH3, -CH2CH3 or -COOEt. According to the invention, a novel cyclopalladation radical group shown in the description below is synthesized and capable of responding to the CO more rapidly and sensitively, and after the novel cyclopalladation radical group is combined with BODIPY, selectivity for the CO is improved and the CO can be detected more rapidly and sensitively. The preparation method includes enabling paranitrobenzoyl chloride, dimethyl pyrrole and boron trifluoride diethyl etherate or the paranitrobenzoyl chloride, dimethyl pyrrole derivatives and the boron trifluoride diethyl etherate to react to generate nitrophenyl BODIPY, reducing nitro in the nitrophenyl BODIPY to amino, adding nitrite and 3,5-dimethylphenol to undergo diazotization, and adding palladium salt to cyclopalladation so as to obtain a target product, namely, the fluorescent probe for detecting the CO in the cells (ACP-CO). The preparation method is simple in synthesis method and applicable to industrial production.
Owner:SHANDONG NORMAL UNIV
Synthesis method of 4-aminobenzamide
InactiveCN106946726AReasonable settingHigh yieldOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsPhosgene
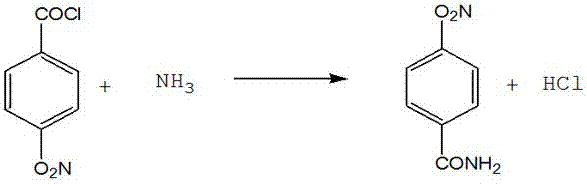
The invention provides a method for synthesizing p-aminobenzamide, comprising the steps of: 1) taking a container, adding p-nitrobenzoic acid, triphosgene and a solvent, after the reaction is completed, collecting the solvent to obtain p-nitrobenzoyl chloride; ) take another reaction vessel, add ammonia and phase transfer catalyst, drop p-nitrobenzoyl chloride toluene solution to obtain p-nitrobenzamide; 3) take the reaction kettle, add p-nitrobenzamide, and add water, Catalyst, obtains described p-aminobenzamide. In the method, the amount of raw materials used is small and the reaction speed is fast. After the hydrogen chloride gas produced by the reaction is absorbed into hydrochloric acid by water, it can be used for other purposes, which effectively reduces the production cost. In the reduction reaction, the iron powder method is replaced by the hydrogenation method, so that the production The cost is reduced, no solid waste is generated, the labor load is greatly reduced, the process is environmentally friendly, and the economic benefit is good.
Owner:连云港恒贸化工有限公司
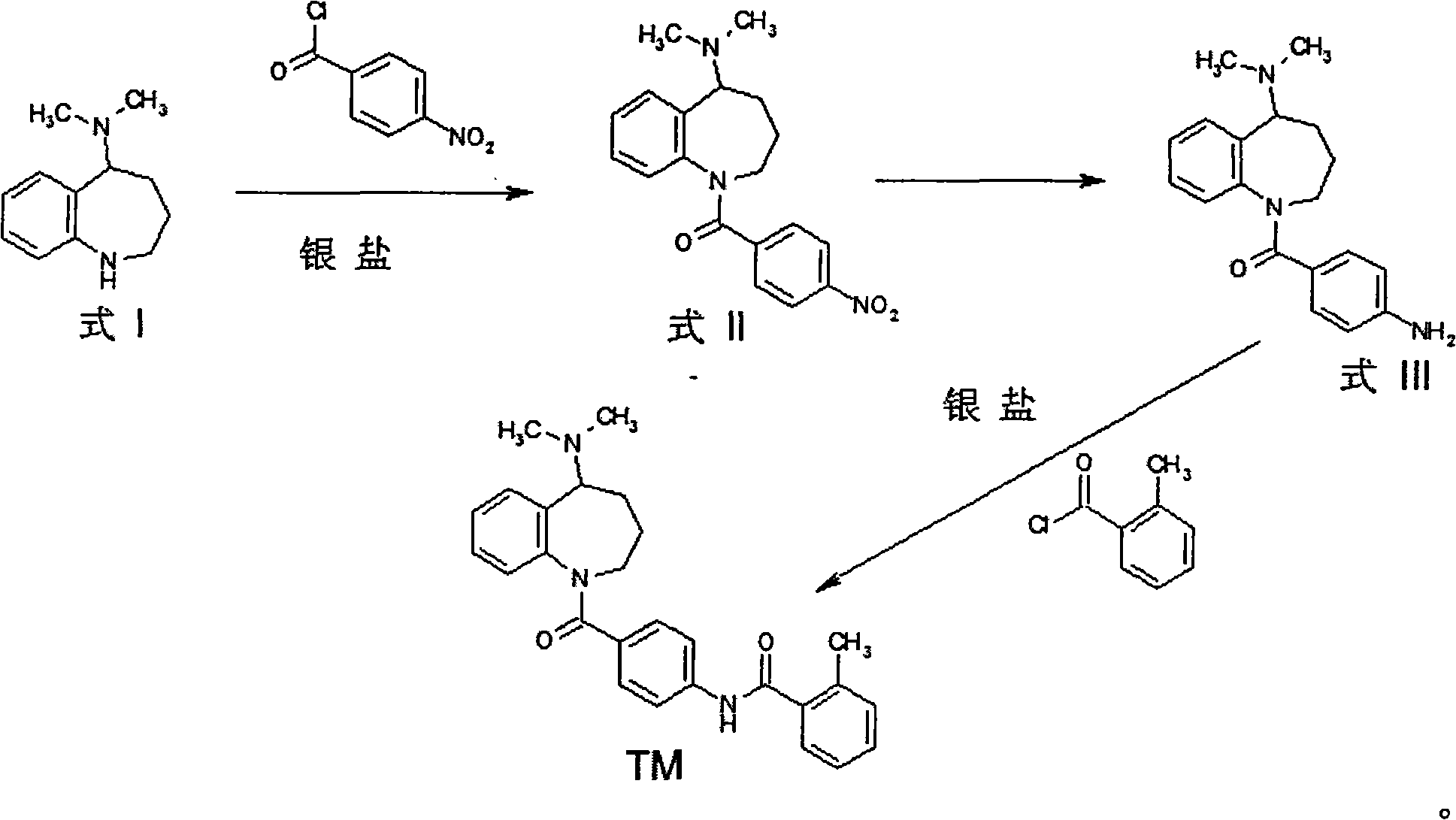
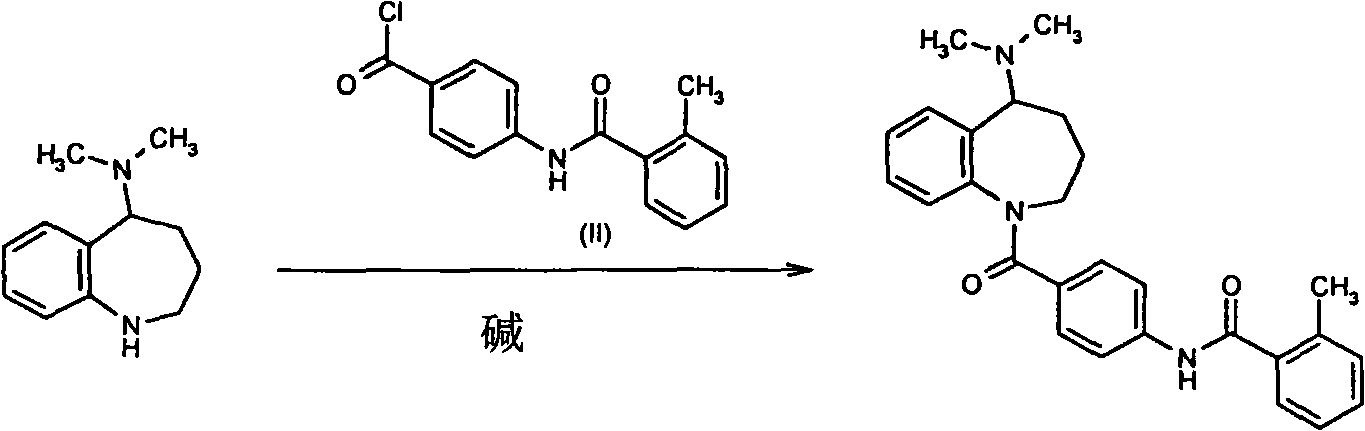
Method for preparing mozavaptan
InactiveCN101570511AShort reaction timeSimple post-processingOrganic chemistryBenzoyl chlorideProtic solvent
The invention discloses a method for preparing mozavaptan, which comprises the following steps: dissolving reactants of 5-dimethylamino-2,3,4,5-tetrahydro-1H-benzodiazepine and 4-(ortho-methyl-benzamido) benzoyl chloride in an alkylogen solvent; adding an anhydrous aprotic solvent dissolved with a silver salt to the mixture; and reacting the mixture at a temperature of between 10 and 30 DEG C. The invention also discloses another method which comprises the following steps: reacting the 5-dimethylamino-2,3,4,5-tetrahydro-1H-benzodiazepine with paranitrobenzoyl chloride under the action of a soluble silver salt; and after deoxidizing nitro groups, reacting the mixture with ortho-methyl benzoyl chloride under the action of the soluble silver salt. The methods have short reaction time without column chromatography purification on a product, the product can be purified through recrystallization after the solvent is reclaimed, the post-treatment is simple, the reaction yield is high, and the yield after the recrystallization can reach more than 70 percent.
Owner:NANJING UNIV OF TECH
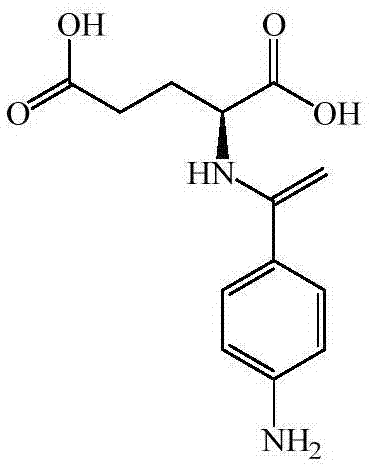
Preparation method of N (4-aminobenzoyl)-L-glutamic acid
ActiveCN105439895AFacilitates controlled absorption processingMild responseOrganic compound preparationCarboxylic acid amides preparationP-nitrobenzoic acidSodium Glutamate
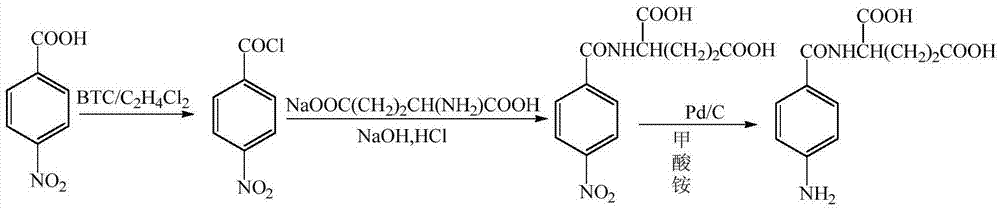
The invention provides a preparation method of N (4-aminobenzoyl)-L-glutamic acid. According to the method, p-nitrobenzoic acid is taken as a raw material, BTC / C2H4Cl1 is taken as an acylating chlorination agent, DMF (dimethyl formamide) is added to serve as an initiator, and p-nitrobenzoyl chloride is prepared through reaction at a reflux temperature; p-nitrobenzoyl chloride and sodium glutamate have condensation, and N-(4-nitrobenzoyl)-L-glutamic acid is prepared; N-(4-nitrobenzoyl)-L-glutamic acid is reduced by Pd / C / HCO2NH4, and N (4-aminobenzoyl)-L-glutamic acid is prepared. The preparation method has mild reaction conditions and is simple in process, easy to operate and suitable for industrial production; few three wastes are generated, and a product has high purity and high yield.
Owner:ZHEJIANG ESIGMA BIOTECH CO LTD
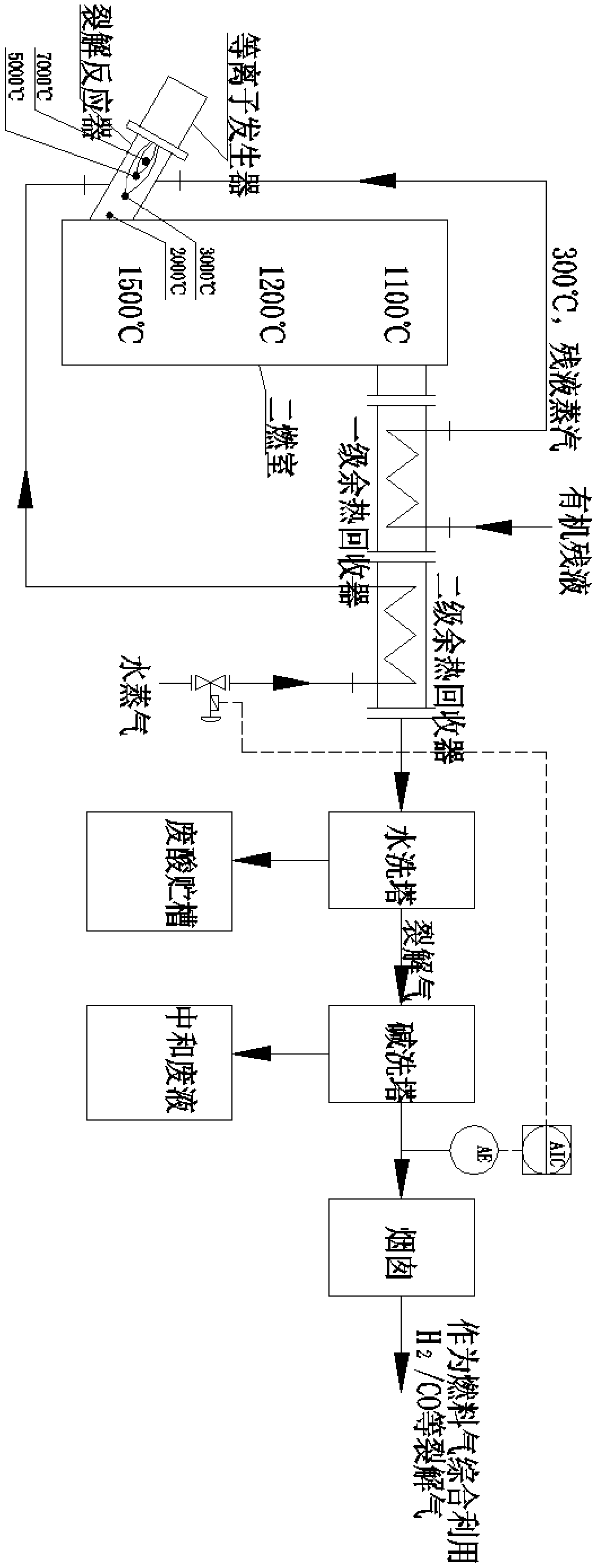
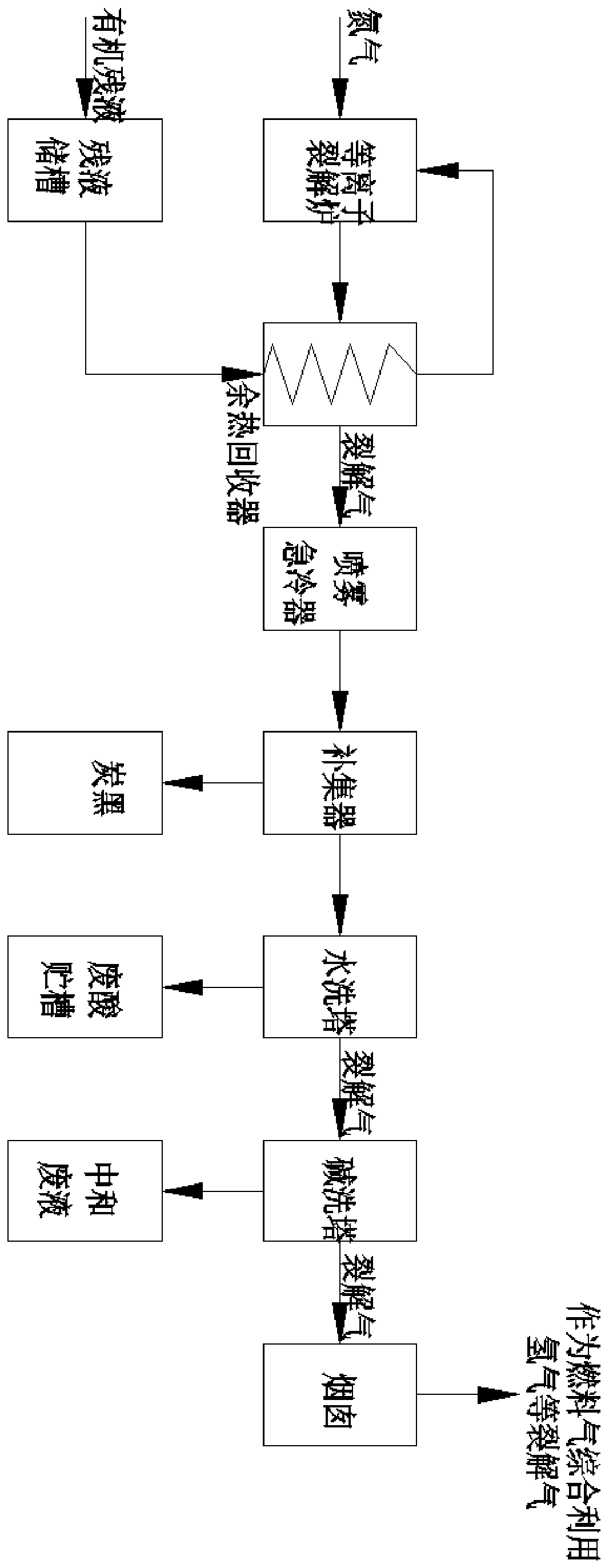
Method for preparing water gas from p-nitrobenzoyl chloride raffinate
The invention discloses a method for preparing water gas from p-nitrobenzoyl chloride raffinate. The method comprises the following steps: (1) vaporizing the raffinate in a primary waste heat recoverer; (2) cracking the vaporized raffinate steam in a plasma cracking tubular reactor; (3) combusting cracked gas obtained in step (2) in a secondary combustion chamber; and (4) enabling the cracked gasobtained in step (3) to sequentially pass through the primary waste heat recoverer, a secondary waste heat recoverer, a water washing tower and an alkaline washing tower, and discharging obtained tailgas through a chimney. An environmentally-friendly plasma system is adopted to treat the raffinate, the system is a set of sealed circulation and non-incineration process, chemical bonds of wastes are decomposed and recombined through a high-energy dense plasma field to form valuable commodities, dioxin is not generated, and zero emission of pollutants can be achieved.
Owner:江苏帕斯玛环境科技有限公司
Method for preparing folic acid
The present invention relates to a preparation method of folic acid, the technical steps of which are as follows: firstly, p-nitrobenzoyl chloride and sodium glutamate are used to prepare for a mediumproduct N-p-amino-benzoyl glutamic acid; then, methyl cyanoacetate, guanidine nitrate and sodium methoxide are used to prepare for the medium product 6-hydroxyl-2, 4, 5-triamino pyrimidine sulfate; finally, the two kinds of medium products are used to prepare for a crude product of the folic acid which is refined to obtain the pure product of the folic acid. The present invention has the beneficial effects of reducing the production cost greatly, improving the product efficiency and reducing the pollution to the environment.
Owner:潘福星
Production process of paranitrobenzoyl chloride intermediate
InactiveCN105254506AReduce generationSimple processOrganic chemistryOrganic compound preparationImpurityCrystallization
The invention provides a production process of a paranitrobenzoyl chloride intermediate. The production process comprises the following steps that a xylene solution is added to a reaction kettle, then paranitrotoluene is added to the xylene solution, and air is fed into the reaction kettle; concentrated sulfuric acid is drop added to the reaction kettle again; an NaOH solution is added to the reaction kettle; finally, water is added to the reaction kettle, and exhausting, filtering and crystallization are performed, so that the paranitrobenzoyl chloride intermediate is obtained. Materials can be further diluted, products can be separated favorably, and the product yield is improved. With the adoption of the production process, the process is simpler, control is easy, the production amount of impurities is reduced in the production process, the reaction time is saved, and accordingly, the production efficiency is improved.
Owner:ANHUI GUANGXIN AGROCHEM
Refining process of paranitrobenzoyl chloride intermediate
InactiveCN105254504AReduce generationSimple processOrganic chemistryOrganic compound preparationNitrogenSodium dichromate
The invention provides a refining process of a paranitrobenzoyl chloride intermediate. The refining process comprises the following steps that a xylene solution is added to a reaction kettle, then paranitrotoluene is added to the xylene solution, air is fed into the reaction kettle, and sodium dichromate is added to the reaction kettle; concentrated sulfuric acid is drop added to the reaction kettle; stirring of the reaction kettle is stopped, an NaOH solution is added to the reaction kettle until the PH of the reaction kettle is 7; the solution is transferred to a rectifying kettle, the heated solution is heated, nitrogen is fed for exhausting, then the solution after exhausting is cooled, and crystallization is performed, so that the paranitrobenzoyl chloride intermediate is obtained. With the adoption of the production process, the process is simpler, control is easy, the production amount of impurities is reduced in the production process, the reaction time is saved, and accordingly, the production efficiency is improved.
Owner:ANHUI GUANGXIN AGROCHEM
Preparation method of N-p-aminobenzoyl-L-glutamic acid
ActiveCN108147977AReduce pollutionSimple and fast operationOrganic compound preparationCarboxylic acid amides preparationHydrazine compoundOxalyl chloride
The invention discloses a preparation method of N-p-aminobenzoyl-L-glutamic acid. The preparation method comprises the following steps of: (1) adopting p-nitrobenzoic acid as a starting material, adopting oxalyl chloride as an acylating chlorination reagent, adopting tetrahydrofuran and DMF (Dimethyl Formamide) as a mixed solvent, and carrying out acylating chlorination reaction to prepare paranitrobenzoyl chloride; (2) carrying out condensation reaction of the paranitrobenzoyl chloride prepared in the step (1) and sodium glutamate to prepare N-p-nitrobenzoyl-L-glutamic acid; (3) adopting hydrazine hydrate as a reducing agent, adopting ferric trichloride hexahydrate as a catalyst, and carrying out reducing reaction of the N-p-nitrobenzoyl-L-glutamic acid prepared in the step (2) to preparethe N-p-aminobenzoyl-L-glutamic acid. The preparation method disclosed by the invention has the beneficial effects that the acylating chlorination reaction selects the oxalyl chloride as the acylating chlorination reagent in the mixed solvent of the tetrahydrofuran and the DMF, the reducing reaction selects the hydrazine hydrate as the reducing agent and selects the ferric trichloride hexahydrateas the catalyst, and finally the N-p-aminobenzoyl-L-glutamic acid with the purity being more than or equal to 99.9% can be obtained.
Owner:江苏尚莱特医药化工材料有限公司
Preparation method of p-amino benzamide glutamic acid
InactiveCN104478751AReduce pollutionHigh reaction conversion rateOrganic compound preparationCarboxylic acid amides preparationFiltrationDistilled water
The invention discloses a preparation method of p-amino benzamide glutamic acid. The preparation method comprises the following steps of (1) adding 180g / ml glutamic acid and a paranitrobenzoyl chloride condensate into a water solution, and stirring until the 180g / ml glutamic acid and the paranitrobenzoyl chloride condensate are sufficiently dissolved to prepare a p-amino benzamide glutamic acid water solution with the weight ratio of 35%; (2) regulating the pH value to 7.3, adding 52g / ml ammonium chloride, heating to 55 DEG C, adding 63g / ml gray cast iron powder, heating to 75 DEG C, pre-corroding for 2.5 hours, heating to 85 DEG C, and preserving the heat for 3.5 hours; and (3) filtering to remove iron mud, washing with distilled water, adding carbon for discoloring, crystallizing, carrying out suction filtration, washing, and drying to obtain a finished product of p-amino benzamide glutamic acid. By using the preparation method of p-amino benzamide glutamic acid, disclosed by the invention, the reaction conversion rate is increased, and the environment pollution is reduced.
Owner:CHANGSHU HUAGANG PHARMA
Synthesis method of paranitrobenzoyl chloride
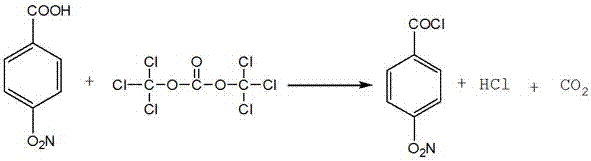
InactiveCN105254507ALow toxicityRaw material stabilityOrganic chemistryOrganic compound preparationDistillationSynthesis methods
The invention discloses a synthesis method of paranitrobenzoyl chloride. The synthesis method comprises the steps that p-nitrobenzoic acid and triphosgene in a molar ratio of 1:(0.5-1.1) are placed in a three-mouth bottle provided with a reflux condensing tube, a dried catalyst is drop added, the mixture is subjected to magnetic stirring and oil-bath heating after dried methylbenzene is taken as a solvent for dissolving, and a crude product is obtained after 5-12 hours of reaction; a reaction by-product of hydrogen chloride is guided out by the reflux condensing tube, alkali liquor is used for absorption, and high-purity paranitrobenzoyl chloride is obtained after the solvent is removed from the crude product through reduced pressure distillation; compared with a traditional process, raw materials are stable, non-toxic and easy to preserve; the toxicity of the by-product is small, the by-product is easy to remove, and the equipment cost is saved; the reaction acyl chlorination effect is good, and the selectivity and the yield are high; the reaction is simple, the time is short, the use is convenient, the process energy consumption is low, and the synthesis method is an ideal preparation method of the paranitrobenzoyl chloride no matter from the angle of green chemistry or atom economy.
Owner:ANHUI GUANGXIN AGROCHEM
Preparation method of tetracaine hydrochloride
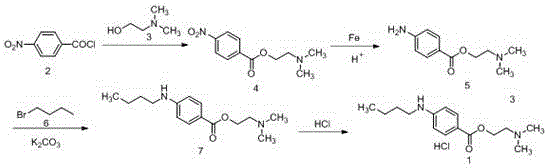
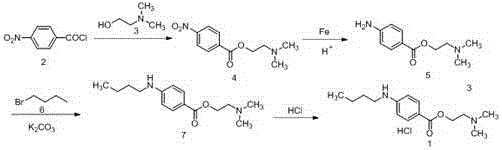
InactiveCN106518697AHigh yieldOrganic compound preparationAmino-carboxyl compound preparationP-nitrobenzoic acidP-Aminobenzoic acid
The invention relates to the technical field of preparation method of tetracaine hydrochloride. The preparation method comprises the preparation steps: carrying out a reaction of p-nitrobenzoyl chloride (2) and 2-dimethylamino-1-ethanol (3) to generate p-nitrobenzoic acid-2-dimethylamino ethyl (4), reducing the compound (3) to obtain p-aminobenzoic acid-2-dimethylamino ethyl (4), generating pontocaine (7) from a compound (5) and 1-bromobutane (6) under alkaline conditions, and finally carrying out a reaction of the pontocaine (7) with HCl to generate tetracaine hydrochloride (1).
Owner:BEIJING VENTUREPHARM BIOTECH
Method for preparing carbon black by plasma cracking of p-nitrobenzoyl chloride residual liquid
The invention discloses a method for preparing carbon black by plasma cracking of p-nitrobenzoyl chloride residual liquid, which comprises the following steps: (1) heating and gasifying the p-nitrobenzoyl chloride residual liquid; (2) directly spraying the gasified residual liquid into a plasma torch of a plasma cracking reactor, and cracking at high temperature to form carbon black and cracking gas; (3) sequentially introducing the carbon black and cracking gas generated in the step (2) into a waste heat recovery device and a spray quench device, and introducing the carbon black and the cracking gas into a supplementary collector after the spray quench device is cooled, and the supplementary collector collecting the carbon black; (4) successively introducing the cracking gas discharged from the replenishment collector in the step (3) into a water washing tower and an alkali washing tower, and then discharging from a chimney. The invention adopts an environment-friendly plasma system to treat waste liquid. The system is a set of sealed circulation and non-incineration process. The chemical bonds of waste are decomposed and reorganized by using a high-energy intensive plasma field to convert the waste into valuable commodities without dioxin generation and zero emission of pollutants.
Owner:江苏帕斯玛环境科技有限公司
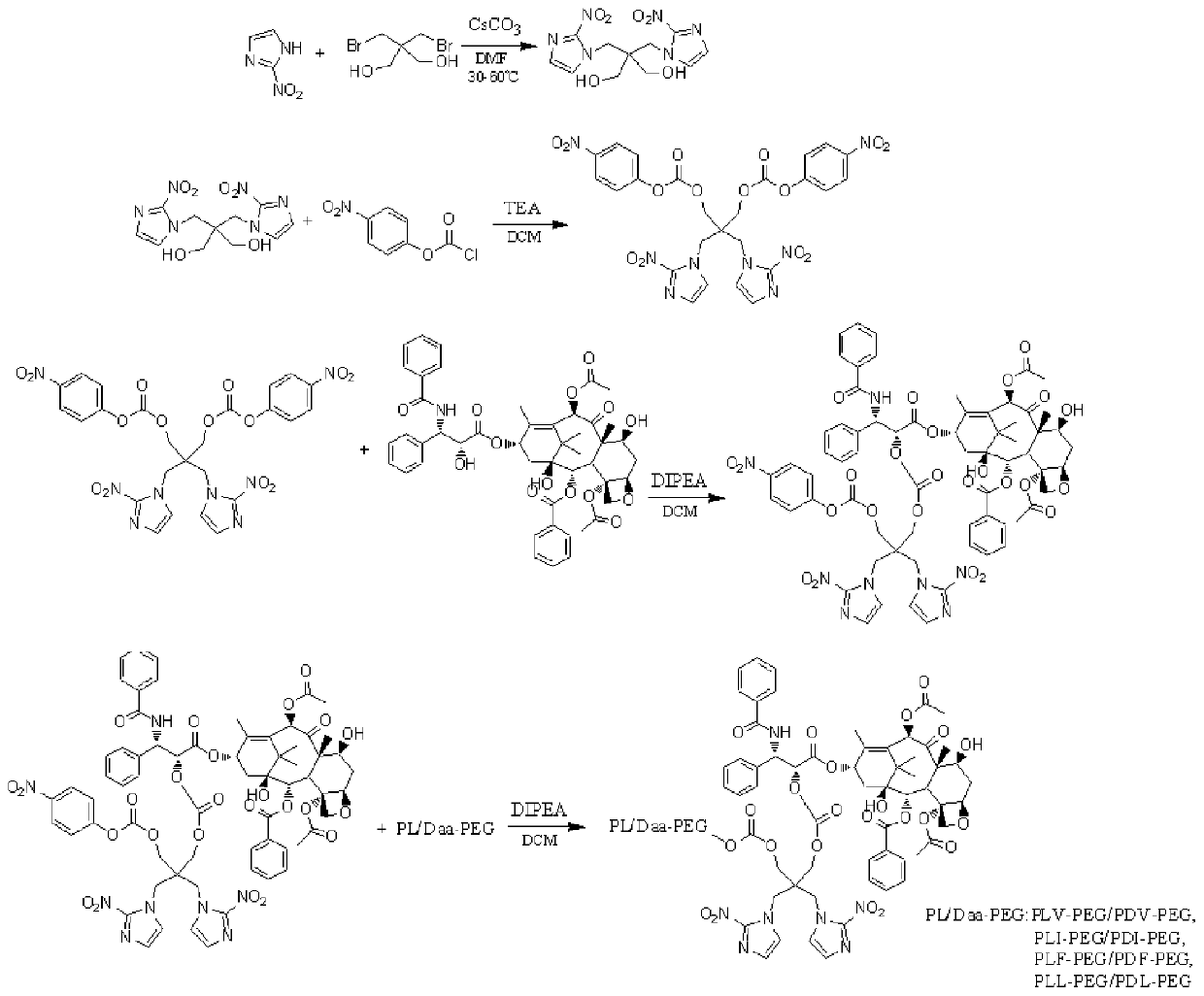
Low-oxygen-responsive polyamino acid-PEG stereo drug-loaded micelle and preparation method thereof
ActiveCN110652494ALow CMC valueGood dispersion and solubilityOrganic active ingredientsPharmaceutical non-active ingredientsMicelleTriethylamine
The present invention discloses a low-oxygen-responsive polyamino acid-PEG stereo drug-loaded micelle and a preparation method thereof. The preparation method comprises the following steps: firstly selecting 2-nitroimidazole and dibromo neopentyl glycol to conduct reaction under catalysis of cesium carbonate to obtain 2,2-bis[(2-nitro-1H-imidazole-1-yl)methyl]-1,3-propylene glycol, then conductingacyl halide with p-nitrobenzoyl chloride in presence of triethylamine to obtain 2,2-bis[(2-nitro-1H-imidazole-1-yl)methyl]propane-1,3-diylbis(4-nitrophenyl)carbonate, then conducting reaction with paclitaxel under catalysis of N,N-diisopropylethylamine, then adding poly L-amino acid-PEG or poly D-amino acid-PEG to respectively obtain bis 2-nitroimidazole-paclitaxel-poly L amino acid-PEG and bis 2-nitroimidazole-paclitaxel-poly D amino acid-PEG, respectively, finally mixing the two materials at equal amount in a PBS buffer, conducting homogenization at high speed, and conducting extruding witha filter membrane with a pore size of 100 nm to obtain a finished product. The prepared drug-loaded micelle has high encapsulation efficiency and also low-oxygen-responsive characteristics, can be effectively accumulated in tumor tissues to achieve a very good antitumor effect, and reduces distribution of drugs in other normal tissues to reduce toxic and side effects.
Owner:NANTONG UNIVERSITY
Preparation method of N-tert-butyl-4-aminobenzamide
PendingCN110305032AReduce usageImprove condensation reaction yieldOrganic compound preparationCarboxylic acid amides preparationTert-ButylamineHydrogen
The invention discloses a preparation method of N-tert-butyl-4-aminobenzamide. The preparation method of the N-tert-butyl-4-aminobenzamide comprises the steps that a condensation reaction is carried out on p-nitrobenzoyl chloride and tert-butylamine to obtain N-tert-butyl-4-nitrobenzamide, and further, the N-tert-butyl-4-aminobenzamide is obtained by catalytic hydrogenation, wherein the condensation reaction is carried out in the presence of a mixed solvent, the mixed solvent is toluene-water, and the condensation reaction comprises the steps that reaction is carried out at 5-10 DEG C for 0.5-2 h, and then reaction is carried out at room temperature for 0.5-2 h, and finally heating is carried out to reflux for reaction for 1-4 h. According to the condensation reaction of the preparation method of the N-tert-butyl-4-aminobenzamide, toluene and water are used as the mixed solvent, then reaction is carried out at room temperature for a certain period of time, and then heating is carried out to reflux to continue the reaction for a certain period of time, so that the yield of the condensation reaction can be significantly increased; and the reduction reaction avoids the use of hydrazine hydrate, and environmental-protection is achieved.
Owner:CHANGZHOU YONGHE FINE CHEM
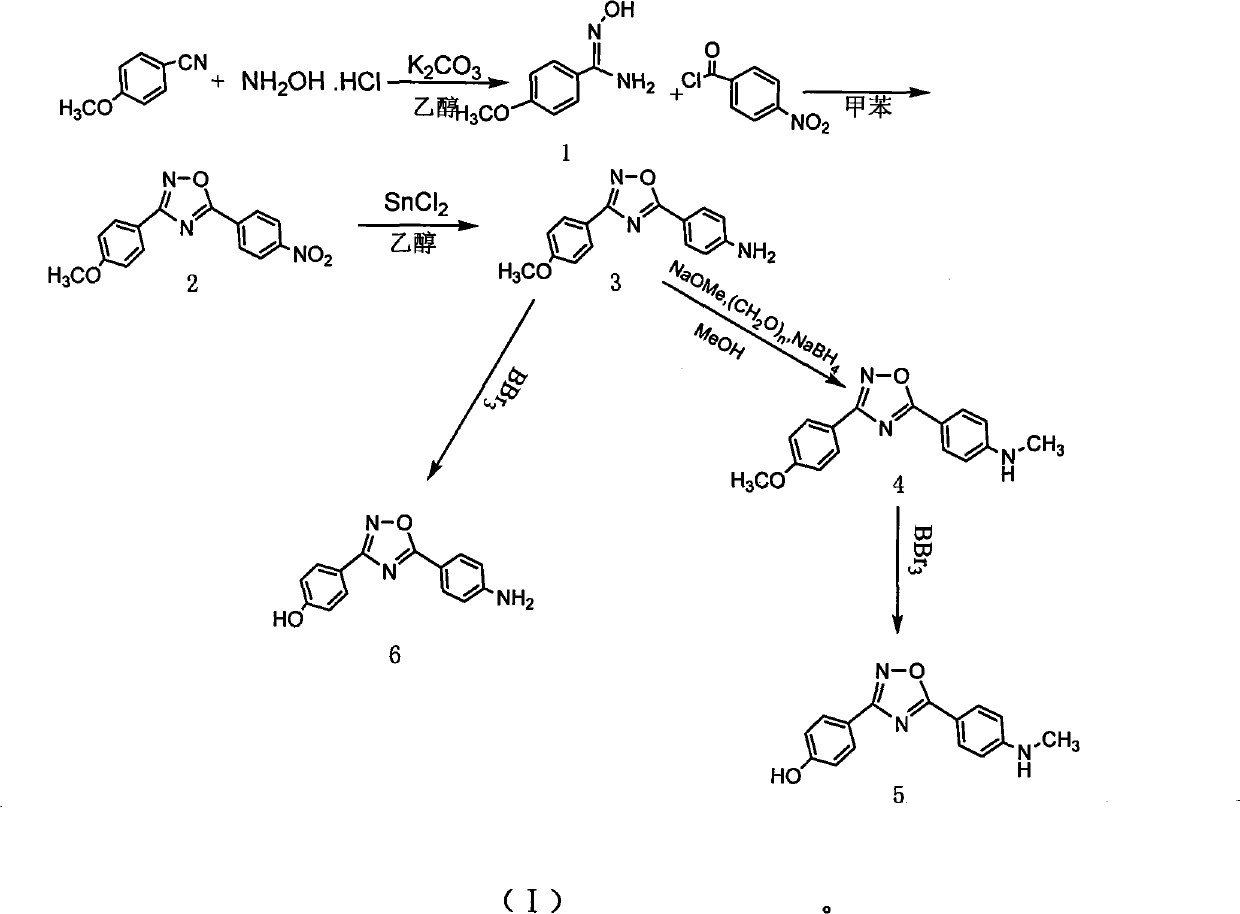
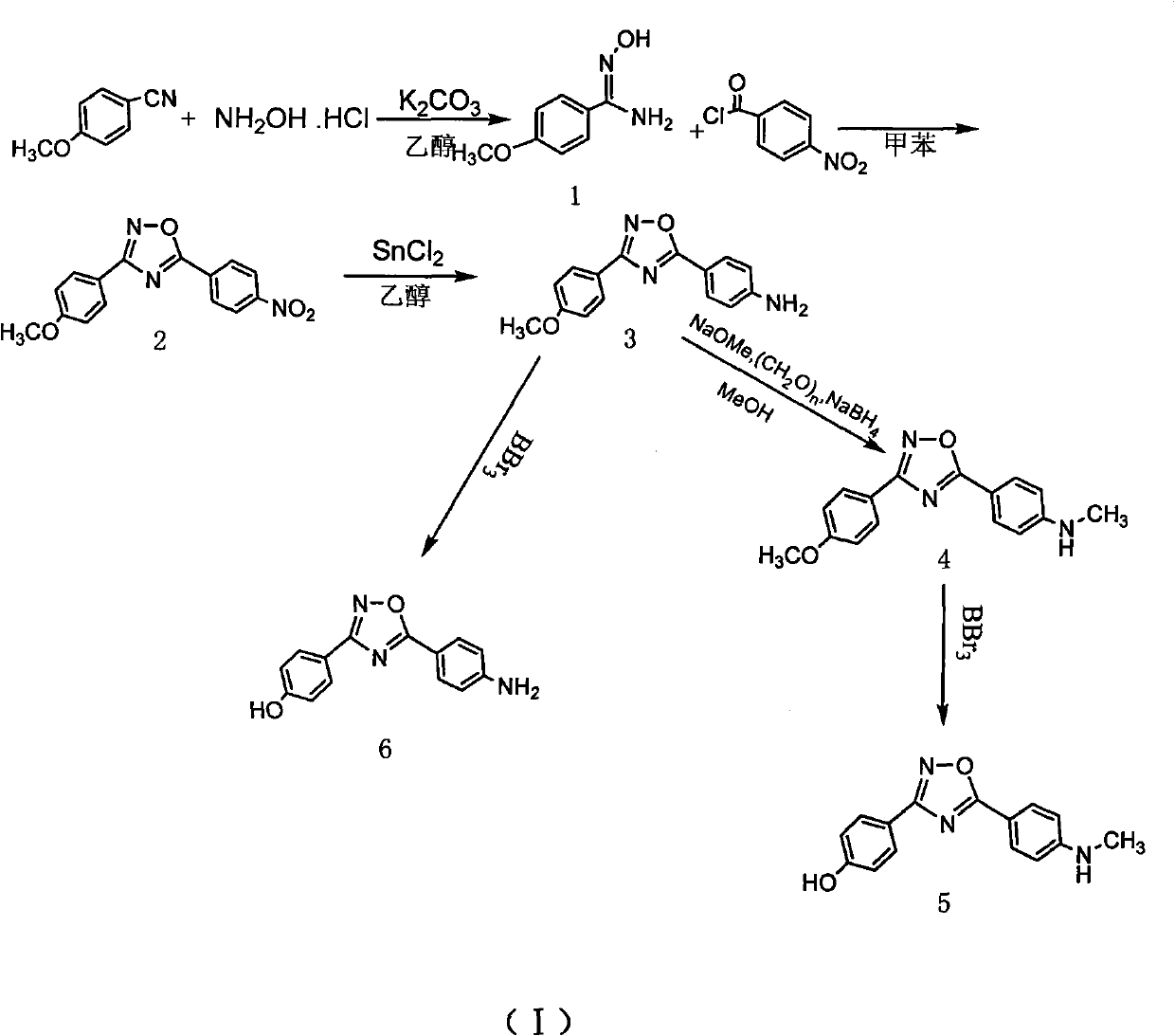
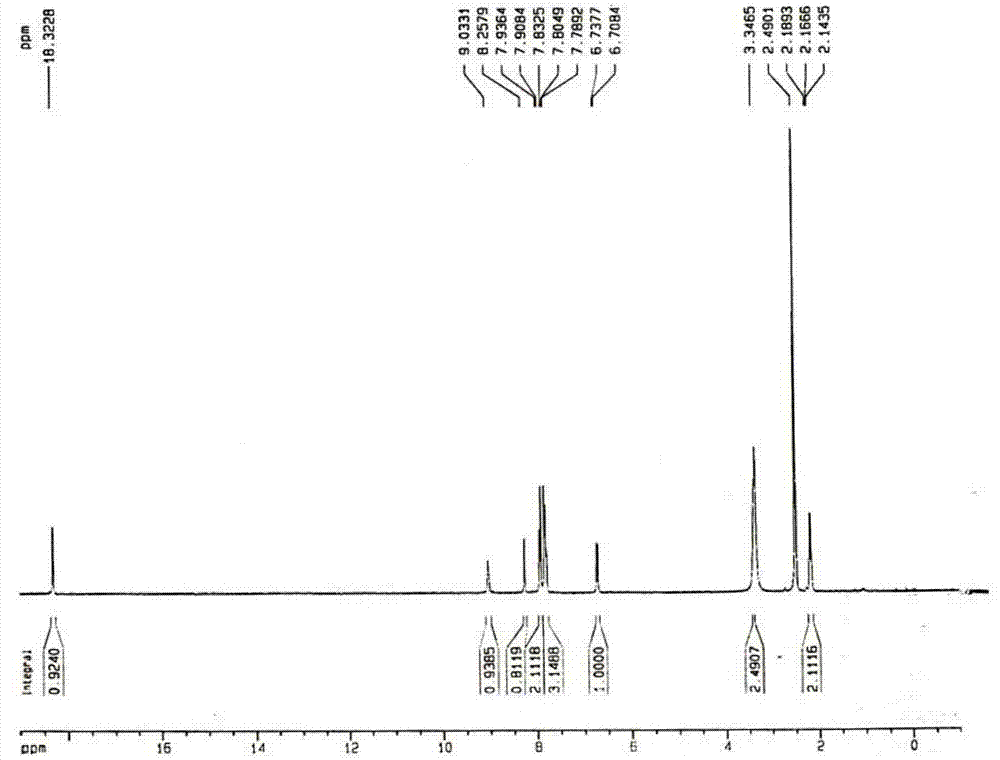
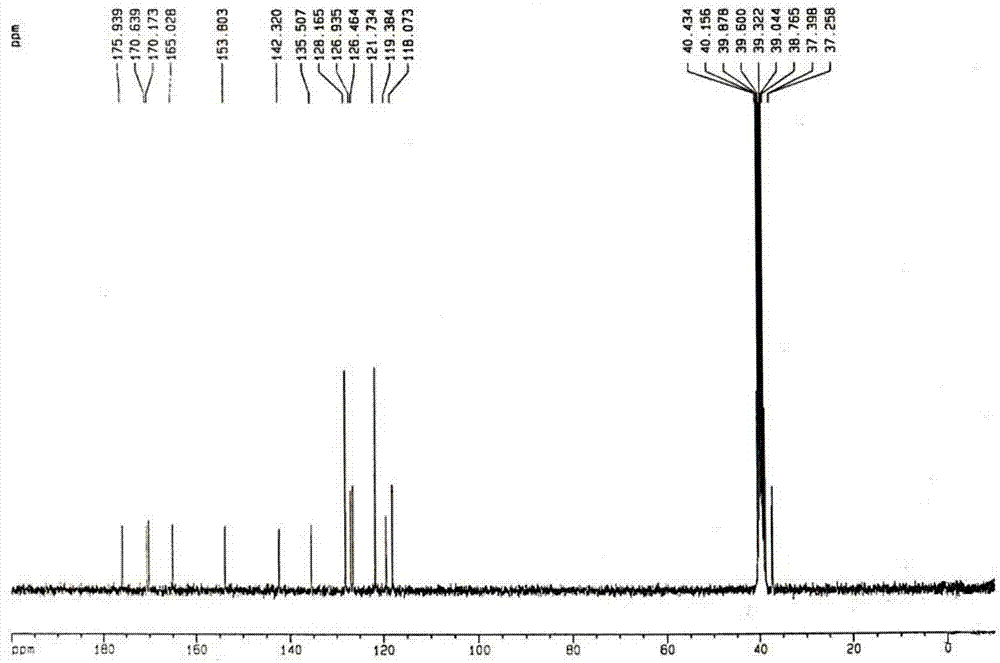
Method for preparing 4-(3-(4-hydroxylphenyl)-1,2,4-oxadiazole-5-yl)-aniline
The invention belongs to the field of chemical synthesis and relates to a preparation technology of a PET (Polyester Terephthalate) imaging agent, in particular to a synthesis method for a diphenyl-substituted oxadiazole derivative of a labeled precursor of a positron medicament Abeta plaque agent, i.e. 4-(3-(4-hydroxyphenyl)-1,2,4-oxadiazole-5-yl)-aniline and a reference substance of 4-(3-(4-hydroxyphenyl)-1,2,4-oxadiazole-5-yl)-N-methylaniline thereof. In the invention, the method comprises the following steps of: condensing cyanobenzene and hydroxylamine hydrochloride as raw materials to obtain p-methoxybenzamidoxime; cyclizing the p-methoxybenzamidoxime and paranitrobenzoyl chloride to obtain 3-(4-methoxyphenyl)-5-(4-nitrophenyl)-1,2,4-oxadiazole; reducing the 3-(4-methoxyphenyl)-5-(4-nitrophenyl)-1,2,4-oxadiazole and removing hydroxymethoxyl to obtain the labeled precursor; and methylating a reduzate of amine and then removing hydroxymethoxyl to obtain the reference substance. By adopting the method, the yield of obtaining the labeled precursor of a target product reaches 20%, and the whole reaction process has mild condition and is easy to operate.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
2, 4, 4'-trinitrobenzene formanilide preparation method
ActiveCN102633674AImprove protectionHigh yieldOrganic compound preparationCarboxylic acid amides preparationMolten stateDistillation
The invention discloses a 2, 4, 4'-trinitrobenzene formanilide preparation method, which includes the steps: firstly, adding 2, 4-dinitroaniline and paranitrobenzoyl chloride into a reaction device in a nitrogen atmosphere, stirring and increasing the temperature to 178-185 DEG C for reaction of 10h-15h, wherein the molar ratio of the 2, 4-dinitroaniline to paranitrobenzoyl chloride is 1:1.1-1:1.5; secondly, increasing the temperature to 195-200 DEG C, and distilling to recover unreacted paranitrobenzoyl chloride; and thirdly, cooling to 140-150 DEG C, so that 2, 4, 4'-trinitrobenzene formanilide is obtained by means of reduced pressure distillation. By the method, the reaction temperature can be controlled to be higher than the melting points of the two kinds of raw materials, so that condensation reaction can be performed in the molten state, solvent is not needed, environmental protection is benefited, yield is greatly increased, and the method is suitable for large-scale industrial production.
Owner:大连新阳光材料科技有限公司 +1
A kind of preparation method of balsalazide sodium
InactiveCN104974061BImprove operational safetyHigh yieldOrganic compound preparationCarboxylic acid amides preparationBalsalazideHydrazine compound
The invention relates to a preparation method of balsalazide disodium, which includes following steps: (1) with paranitrobenzoyl chloride as a raw material, performing a reaction with [beta]-alanine in a NaOH water solution to obtain paranitrobenzoyl-[beta]-alanine; (2) with hydrazine hydrate as a reduction agent, FeCl3.6H2O as a catalyst and water as a solvent, performing a reduction to the paranitrobenzoyl-[beta]-alanine to obtain paraminobenzoyl-[beta]-alanine; (3) performing a diazo-reaction and a coupling reaction to the paraminobenzoyl-[beta]-alanine to obtain balsalazide acid; and (4) performing a salt forming reaction to the balsalazide acid to obtain balsalazide disodium. In the invention, the reduction reaction is carried out with hydrazine hydrate as the reduction agent, FeCl3.6H2O as the catalyst and water as the solvent, so that an expensive Pd-C catalyst is replaced and usage of hydrogen is canceled, so that the preparation method is greatly improved in operation safety and reduced in synthetic cost.
Owner:辽宁省药物研究院
Preparation method of (2RS, 3RS)-3-(2-ethoxy phenoxy)-2-hydroxy-1-(4-nitrobenzoyloxy)-3-phenyl propane
InactiveCN102101831ALow costEasy to operateOrganic chemistryOrganic compound preparationBenzene4-nitrobenzoyl chloride
The invention relates to a preparation method of (2RS, 3RS)-3-(2-ethoxy phenoxy)-2-hydroxy-1-(4-nitrobenzoyloxy)-3-phenyl propane as expressed in the formula (I), which is an intermediate of reboxetine. In the method the compound as expressed in the formula (II) and 4-nitrobenzoyl chloride are employed as raw materials; aliphatic caboxylic ester, lower halogenated hydrocarbon, benzene, or toluene is employed as solvent; triethylamine is employed as an acid-binding agent; and the target product (2RS, 3RS)-3-(2-ethoxy phenoxy)-2-hydroxy-1-(4-nitrobenzoyloxy)-3-phenyl propane is obtained by reaction of the above-mentioned compounds. Compared with the prior methods, the method in the present invention requires inexpensive raw materials, can be simply operated, needs no special apparatus and is suitable for industrial production.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Preparation method of p-aminobenzoyl glutamic acid
InactiveCN110668968AReduce pollutionMild responseOrganic compound preparationCarboxylic acid amides preparationBenzoic acidMonosodium glutamate
The invention discloses a preparation method of p-aminobenzoyl glutamic acid. The preparation method comprises the following specific steps: adding nitrobenzoic acid, oxalyl chloride, dichloroethane and DMF into a reaction container to react to obtain p-nitrobenzoyl chloride; reacting p-nitrobenzoyl chloride with sodium glutamate to obtain p-nitrobenzoyl glutamic acid; and carrying out reduction,separation, acid precipitation, refining and drying unit processes on the obtained p-nitrobenzoylglutamic acid in the presence of a polar solvent and a catalyst to obtain a finished product, wherein in the reduction process, pH value is adjusted to 6-10 with sodium hydroxide, and then hydrogenation and hydrazine hydrate reduction are carried out. The method is mild in reaction, simple and convenient to operate, high in process safety, low in production cost, green and environmentally friendly, the obtained product is high in purity and yield, the catalyst can be repeatedly used, the solvent can be recycled after being distilled and recycled, a small amount of waste liquid can be discharged up to the standard after being subjected to alkali treatment, environmental pollution is small, and therefore the method is suitable for industrial production.
Owner:SHE COUNTY JINDONG ECONOMIC & TRADE CO LTD
Exhaust gas treatment process for paranitrobenzoyl chloride
ActiveCN105384640AAvoid pollutionLow costOrganic chemistryOrganic compound preparationMolecular sieveBenzene
The invention provides an exhaust gas treatment process for paranitrobenzoyl chloride. The process comprises the following steps: adding a toluene solution into a reaction vessel, introducing phosgene into the reaction vessel, and meanwhile, continuing to introduce the phosgene; dropwise adding paranitrobenzoic acid into the reaction vessel, and carrying out stirring while carrying out dropwise adding; introducing nitrogen gas into the solution for first-time gas purging; drying gas subjected to first-time gas purging with a molecular sieve; introducing the dried gas into a recovery kettle, introducing chlorine gas into the recovery kettle, meanwhile, arranging an activated charcoal layer in the recovery kettle, and enabling a mixture of the dried gas and the chlorine gas to pass through the activated charcoal layer; introducing nitrogen gas into the recovery kettle for second-time gas purging, and then, carrying out phosgene extraction on gas subjected to second-time gas purging with a toluene or benzene solution; and introducing the extracted gas into a NaOH solution. According to the process, by adopting the process, exhaust gases, such as carbon monoxide, resulting from reactions can subject to a reaction again to produce the phosgene, and then, the phosgene is recovered.
Owner:ANHUI GUANGXIN AGROCHEM
Production method of balsalazide
InactiveCN104744296AEasy to prepareImprove product qualityOrganic chemistryBalsalazideSalicylic acid
The invention relates to a production method of balsalazide, wherein the production method comprises the following specific steps: a, dissolving beta-alanine and sodium carbonate in water, adding benzyltriethylammonium chloride, dropwise adding a dichloromethane solution of p-nitrobenzoyl chloride, and stirring for 3 h at room temperature; allowing to stand for layering, and acidifying the aqueous layer with hydrochloric acid to pH of 1; carrying out acetone recrystallization, to obtain N-p-nitrobenzoyl-beta-alanine; b, dissolving N-p-nitrobenzoyl-beta-alanine in ethanol, adding activated palladium, and carrying out hydrogenation; and adding ethyl ether, filtering, and thus obtaining N-p-aminobenzoyl-beta-alanine; and c, cooling with ice bath, adding a reduction product to a small amount of concentrated hydrochloric acid to form a paste, adding water to dilute, slowly adding a sodium nitrite solution, and stirring for 1 h; dropwise adding an alkaline solution of salicylic acid, adjusting the pH to 8.2, and stirring for 3 h; and dropwise adding the reaction liquid into an ice-dilute hydrochloric acid mixed solution, filtering, drying, carrying out ethanol recrystallization, and thus obtaining the product balsalazide. According to the production method of balsalazide, the production method is simple, and the product quality is good.
Owner:李磊
Wastewater treatment process for paranitrobenzoyl chloride
InactiveCN105384641AAvoid pollutionIncrease productionOrganic chemistryOrganic compound preparationTolueneNitrogen gas
The invention provides a wastewater treatment process for paranitrobenzoyl chloride. The process comprises the following steps: adding a toluene solution into a reaction vessel, and introducing phosgene into the reaction vessel; dropwise adding paranitrobenzoic acid into the reaction vessel; introducing nitrogen gas into the solution in the reaction vessel for gas purging; transferring the solution subjected to gas purging into a rectifying still; and filtrating the cooled solution, and adding water into filtrated residue for flushing. The process has the beneficial effects that by adopting the production process, waste liquid can be further treated, and the product can be partially recovered, so that environmental pollution caused by direct discharge is avoided, and meanwhile, the yield of the product is increased.
Owner:ANHUI GUANGXIN AGROCHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com