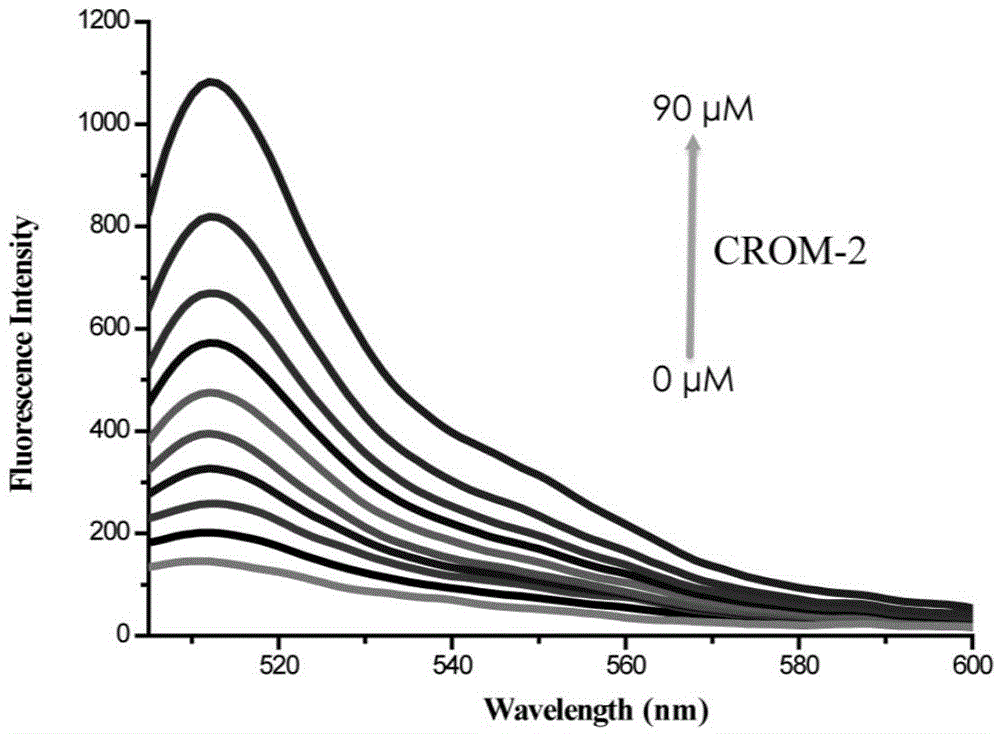
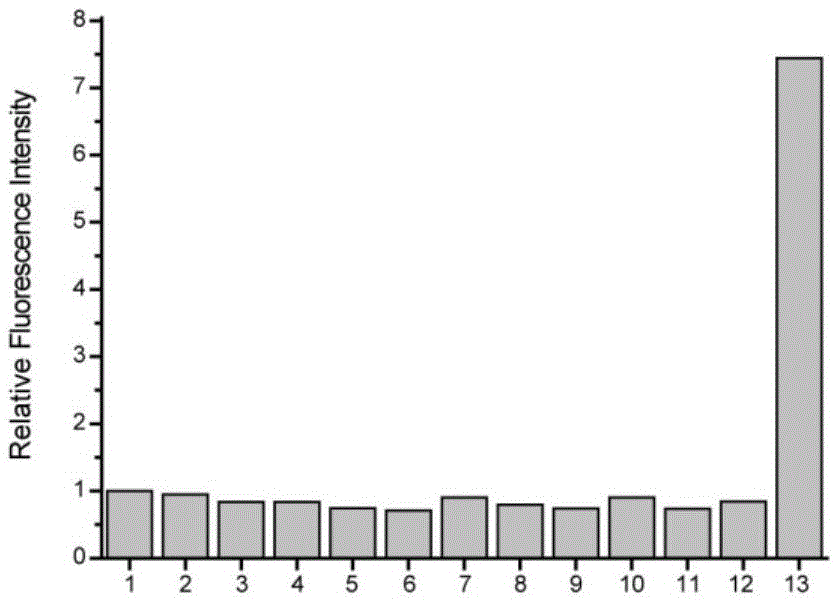
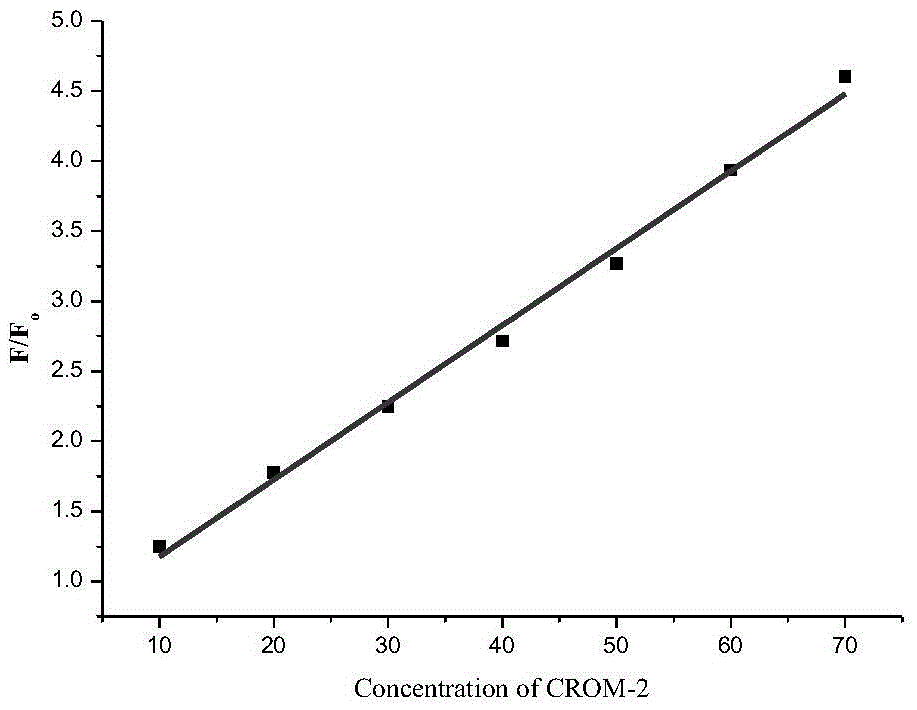
Fluorescent probe for detecting CO (carbon monoxide) in cells and preparation method and application of fluorescent probe
A fluorescent probe and cell technology, applied in the field of detection, can solve problems such as long reaction time, achieve fast detection speed, simple synthesis, and improved selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) Dissolve p-nitrobenzoyl chloride (7g) and 2,4-dimethylpyrrole (7g) in CH 2 Cl 2 (20mL), heated to reflux for 1h. After cooling, triethylamine (3.5 g) and toluene (30 ml) were added. After 15 minutes of reaction, boron trifluoride diethyl ether (3g) was added, the temperature was raised to 50° C. for 3 hours, spin-dried, and separated by chromatographic column (eluent: V 石油醚 :V 二氯甲烷 =5:1), spin-dried to obtain orange solid nitrophenyl BODIPY.
[0045] NMR and mass spectrometry characterization:
[0046] 1 HNMR (400MHz, CDCl 3 ):δ=1.36(s,6H),2.57(s,6H),6.02(s,2H),7.54(d,J=8Hz,2H),8.39(d,J=8Hz,2H)
[0047] ESI-MS: calculated for [M-H] - =368.1, found 368.1.
[0048] (2) Dissolve nitrophenyl BODIPY (5g), ammonium formate (50g) in CH 2 Cl 2 (10mL), then add Pd / C1 2 (1g), stirred at room temperature for 2 hours, filtered, and the filtrate was spin-dried to obtain aminophenyl BODIPY.
[0049] NMR and mass spectrometry characterization:
[0050] 1 HNMR (400MH...
Embodiment 2
[0060] (1) Dissolve p-nitrobenzoyl chloride (0.14g) and 2,4-dimethylpyrrole (0.14g) in CH 2 Cl 2 (2mL), heated to reflux for 1h. After cooling, triethylamine (0.35 g) and toluene (3 ml) were added. After 15 minutes of reaction, add boron trifluoride diethyl ether (0.3 g), heat up to 50° C. for 3 hours, spin dry, and separate with a chromatographic column (eluent: V 石油醚 :V 二氯甲烷 =5:1), spin-dried to obtain orange solid nitrophenyl BODIPY.
[0061] NMR and mass spectrometry characterization:
[0062] 1 HNMR (400MHz, CDCl 3 ):δ=1.36(s,6H),2.57(s,6H),6.02(s,2H),7.54(d,J=8Hz,2H),8.39(d,J=8Hz,2H)
[0063] ESI-MS: calculated for [M-H] - =368.1, found 368.1.
[0064] (2) Dissolve nitrophenyl BODIPY (10g) and ammonium formate (100g) in CH 2 Cl 2 (20mL), then add Pd / C1 2 (2g), stirred at room temperature for 2 hours, filtered, and the filtrate was spin-dried to obtain aminophenyl BODIPY.
[0065] NMR and mass spectrometry characterization:
[0066] 1 HNMR (400MHz, CDCl 3 ...
Embodiment 3
[0076] The present invention is the same as Example 1, except that 2,4-dimethylpyrrole is changed to 2,3,4-trimethylpyrrole.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com