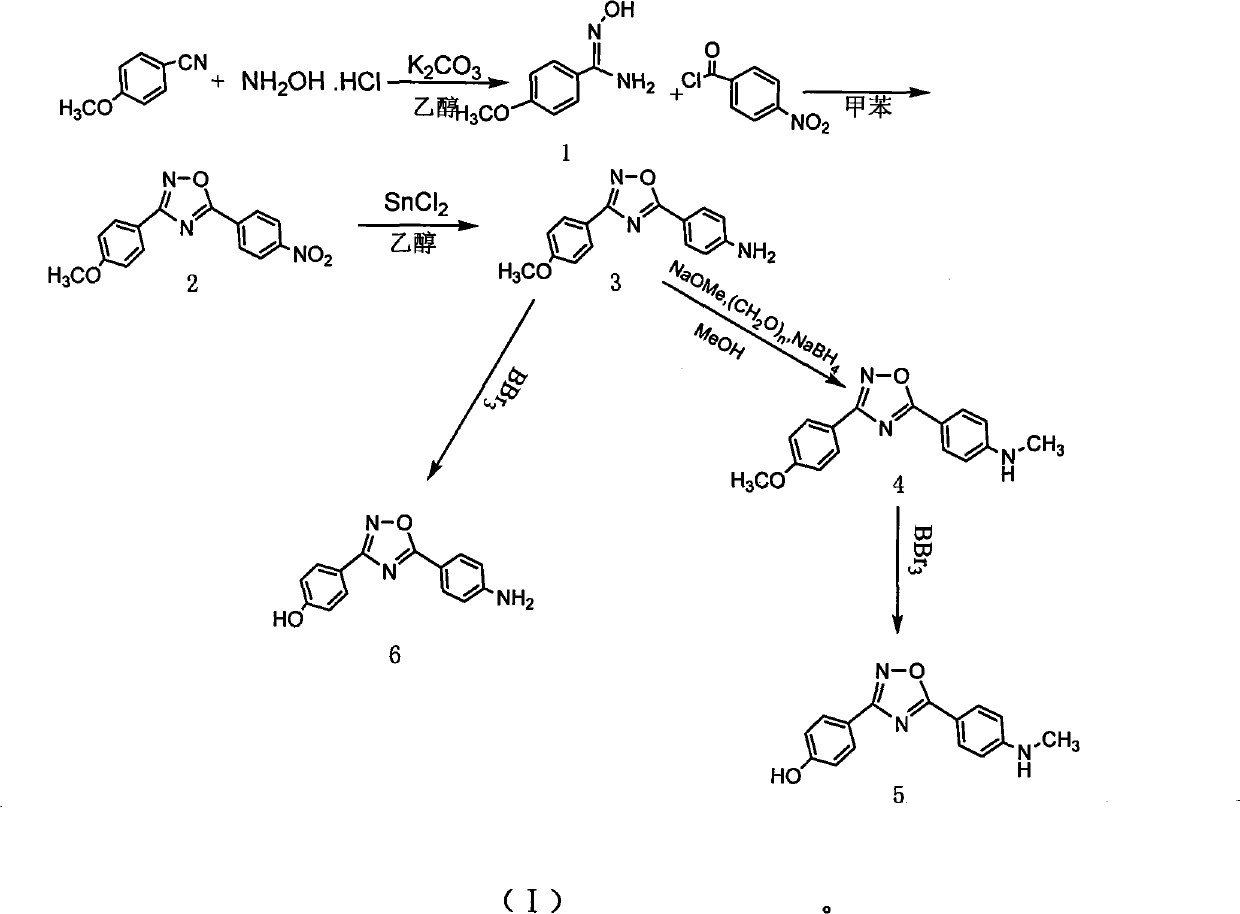
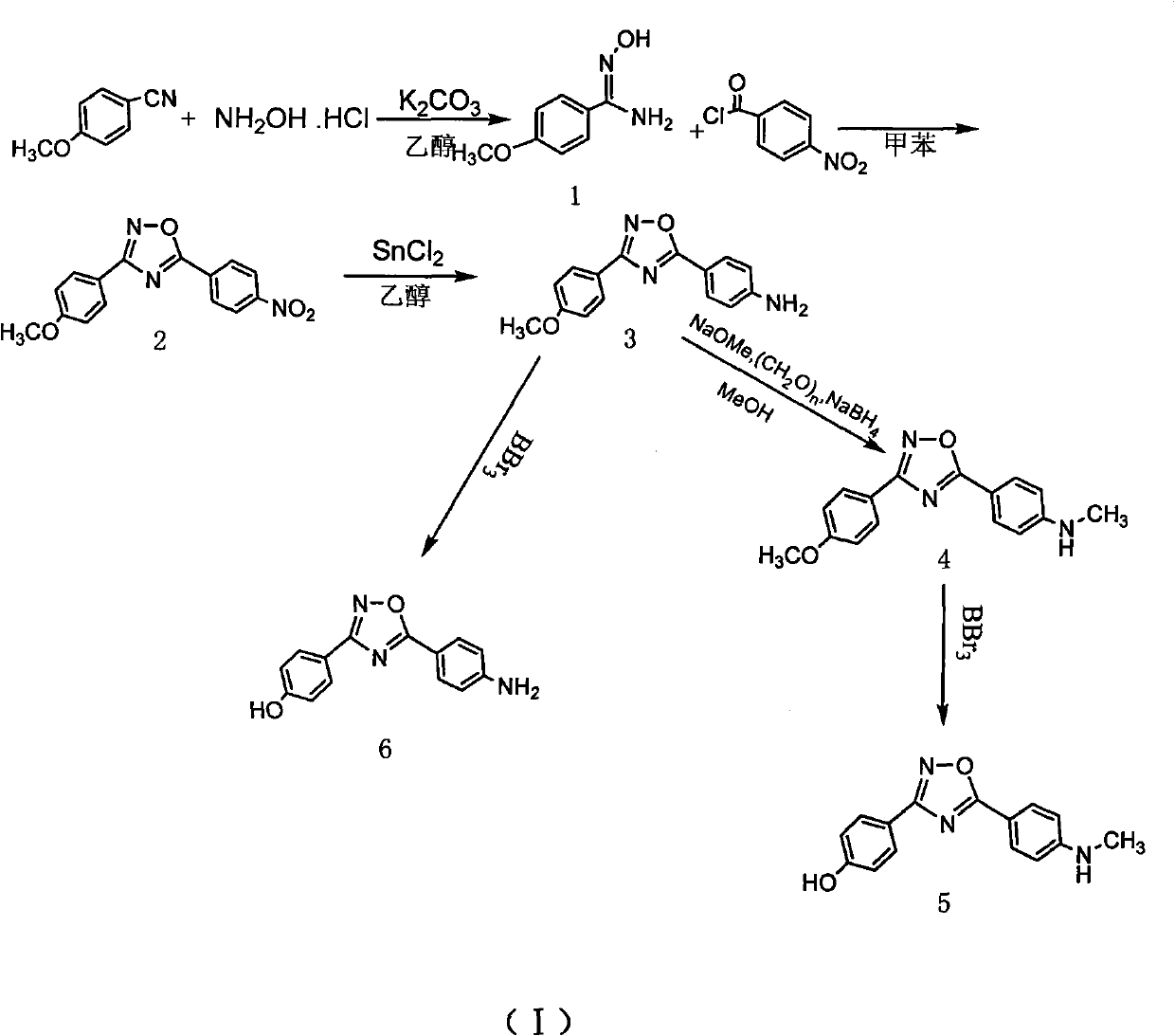
Method for preparing 4-(3-(4-hydroxylphenyl)-1,2,4-oxadiazole-5-yl)-aniline
A technology of hydroxyphenyl and oxadiazole, which is applied in the field of chemical synthesis, can solve problems such as unseen synthetic methods, and achieve the effect of easy operation and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Synthesis of p-methoxybenzamide oxime
[0024] In a 100ml single-necked flask equipped with a reflux condenser, add (3.3mmol, 0.44g) benzonitrile, (3.3mmol, 0.46g) anhydrous K2 CO 3 , (3.3mol, 0.23g) the mixed solvent of hydroxylamine hydrochloride and 50ml 95% ethanol and 15ml water, reflux reaction 12h, detect reaction complete with thin-layer chromatography, stop reaction, cooling, decompression distills off part ethanol, cooling separates out solid, After filtering and drying, a white solid 1 (0.466g, 2.8mmol) was obtained with a yield of 85%.
Embodiment 2
[0026] Synthesis of 3-(4-methoxyphenyl)-5-(4-nitrophenyl)-1,2,4,-oxadiazole
[0027] Under ice-cooling, the mixture of 5mmol p-nitrobenzoyl chloride and 20ml dry toluene was added dropwise to (5mmol, 0.83g) compound 1 in 50ml toluene solution, and the drop was completed in 25min. After dropping, the temperature was raised to reflux for 10 h, and the reaction was detected by thin-layer chromatography. The reaction was stopped, the solid was precipitated by cooling, filtered, and dried to obtain a light yellow solid 2 (1.11 g, 3.75 mmol), with a yield of 75%.
Embodiment 3
[0029] Synthesis of 4-(3-(4-methoxyphenyl)-1,2,4-oxadiazol-5-yl)aniline
[0030] Compound 2 (0.89g, 3mmol) and stannous chloride dihydrate (3.84g, 15mmol) were dissolved in 100ml of ethanol, stirred and refluxed for 4 hours, cooled to room temperature, 300ml of 1M NaOH was added until it was alkaline, 100ml×3 acetic acid Extracted with ethyl ester, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, and evaporated to dryness to obtain compound 3 (0.63 g, 2.37 mmol), with a yield of 79%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com