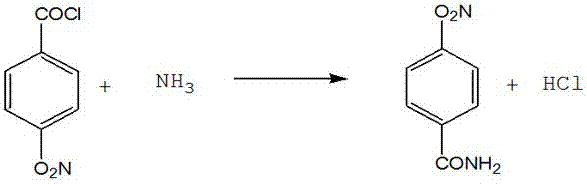
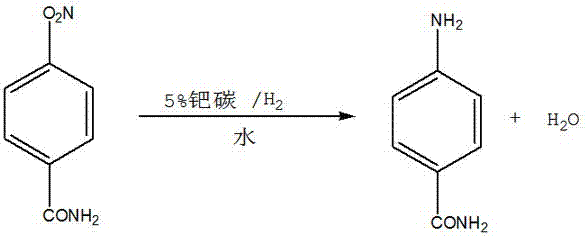
Synthesis method of 4-aminobenzamide
A technology of aminobenzamide and p-nitrobenzoic acid, which is applied in the field of synthesis of p-aminobenzamide, can solve the problems of difficult recovery of thionyl chloride, difficulty of "three wastes" treatment, large amount of waste water discharge, etc., and achieve a synthetic route The effects of reasonable setting, reduced labor load, and faster response speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] The present invention will be described in detail below in conjunction with specific embodiments. The following examples will help those skilled in the art to further understand the present invention, but do not limit the present invention in any form. It should be noted that those skilled in the art can make several modifications and improvements without departing from the concept of the present invention. These all belong to the protection scope of the present invention.
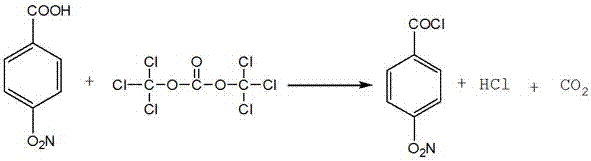
[0030] Acyl chloride reaction, see the reaction equation figure 1 :
[0031] Add 100g of p-nitrobenzoic acid, 213g of triphosgene and 200g of toluene into a 500ml flask, stir and cool down to below 0°C, add 10g of a mixed solution of pyridine and triethylamine (mass ratio 1:9) dropwise, after the dropwise addition is complete, raise the temperature to room temperature, reacted at room temperature for 20 hours, and collected the generated hydrogen chloride gas.
[0032] After the reaction was com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com