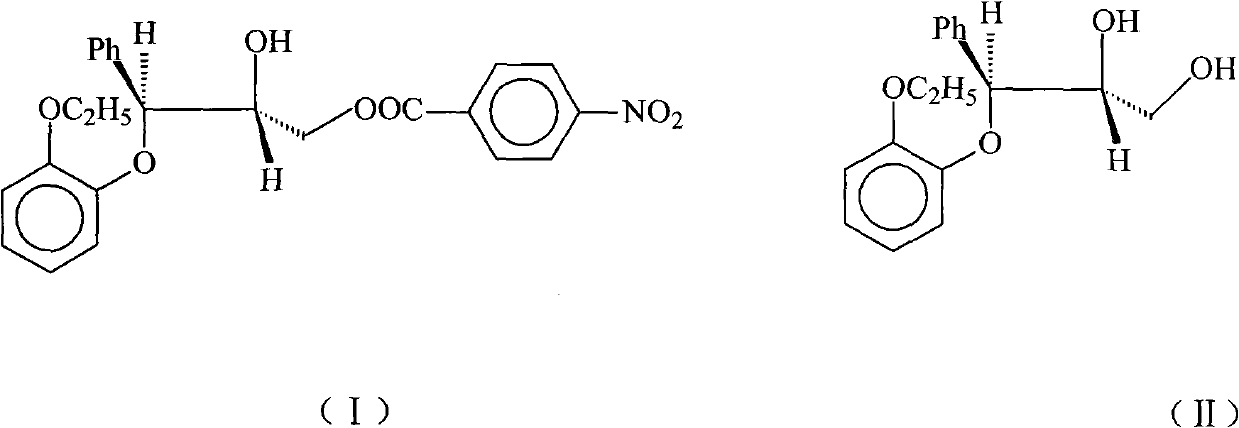
Preparation method of (2RS, 3RS)-3-(2-ethoxy phenoxy)-2-hydroxy-1-(4-nitrobenzoyloxy)-3-phenyl propane
A technology of nitrobenzoyloxy and ethoxyphenoxy, applied in the field of medicinal chemistry, can solve the problems of reducing production capacity, wasting raw materials, expensive pyridine and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
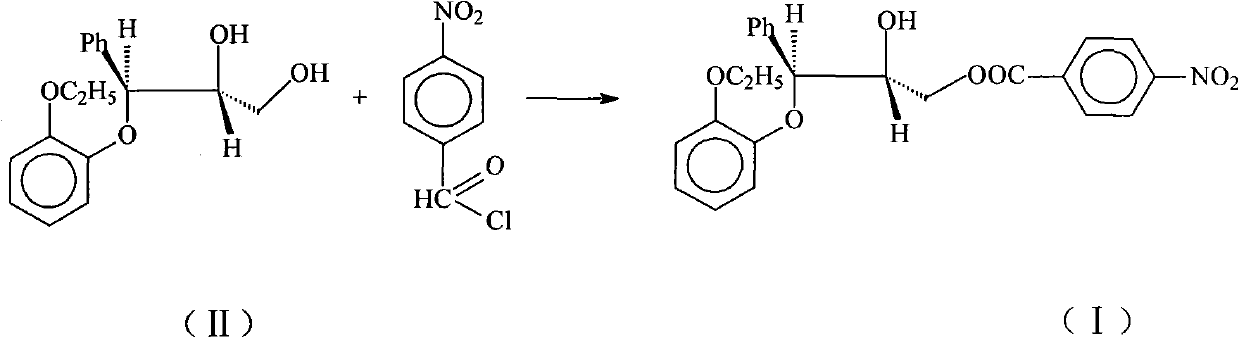
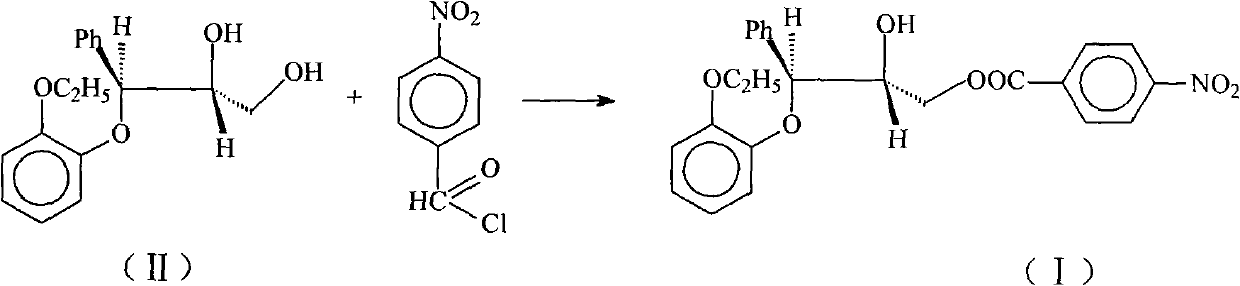
[0013] Dissolve 25g (0.0865mol) of II in 250ml of ethyl acetate, add 32ml of triethylamine (0.22mol), blow in nitrogen, cool to -20°C, and slowly add 16.1g (0.085mol) of 4-nitrate A solution composed of benzoyl chloride and 200ml ethyl acetate was dropped within 1.5h. After reacting for 0.5h, the solution was transferred to a solution composed of 160ml 2mol / L hydrochloric acid and 665g ice, an oily precipitate was produced, the organic phase was separated, the aqueous phase was extracted with ethyl acetate, the combined organic phases were washed with water, and then washed with anhydrous Sodium sulfate was dried and dehydrated, and the solvent was distilled off under reduced pressure. The residue was dissolved in a mixture of isopropyl ether: ethyl acetate = 12:1, and the product gradually solidified to obtain 25.4 g of the product, with a yield of 67.2%. m.p.92-95℃, IR(KBr)cm -1 3540(OH), 1730(CO) 1590, 1500(arom.C=C), 1525, 1350(NO 2 ).
example 2
[0015] Dissolve 25g (0.0865mol) of II in 250ml of ethyl acetate, add 12.5ml of triethylamine (0.0865mol), pass through nitrogen, cool to -20°C, and slowly dropwise add 16.1g (0.085mol) of 4- The solution composed of nitrobenzoyl chloride and 200ml ethyl acetate was dropped within 1.5h. After reacting for 0.5h, the solution was transferred to a solution composed of 160ml 2mol / L hydrochloric acid and 665g ice, an oily precipitate was produced, the organic phase was separated, the aqueous phase was extracted with ethyl acetate, the combined organic phases were washed with water, and then washed with anhydrous Sodium sulfate was dried and dehydrated, and the solvent was distilled off under reduced pressure. The residue was dissolved in a mixture of isopropyl ether: ethyl acetate = 12:1, and the product gradually solidified to obtain 12.1 g of the product, with a yield of 32%. m.p.93-95℃, IR(KBr)cm -1 3540(OH), 1730(CO) 1590, 1500(arom.C=C), 1525, 1350(NO 2 ).
example 3
[0017] Dissolve 25g (0.0865mol) of II in 250ml of toluene, add 32ml of triethylamine (0.22mol), blow in nitrogen, cool to -20°C, and slowly add 16.1g (0.085mol) of 4-nitrobenzene The solution composed of acid chloride and 200ml toluene was dropped within 1.5h. After reacting for 0.5h, the solution was transferred to a solution composed of 160ml 2mol / L hydrochloric acid and 665g ice, an oily precipitate was produced, the organic phase was separated, the aqueous phase was extracted with toluene, the combined organic phases were washed with water, and then washed with anhydrous sodium sulfate After drying and dehydration, the solvent was evaporated under reduced pressure, and the residue was dissolved in a mixture of isopropyl ether: ethyl acetate = 12:1, and the product gradually solidified to obtain 24.8 g of the product, with a yield of 65.6%. m.p.92-95℃, IR(KBr)cm -1 3540(OH), 1730(CO) 1590, 1500(arom.C=C), 1525, 1350(NO 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com