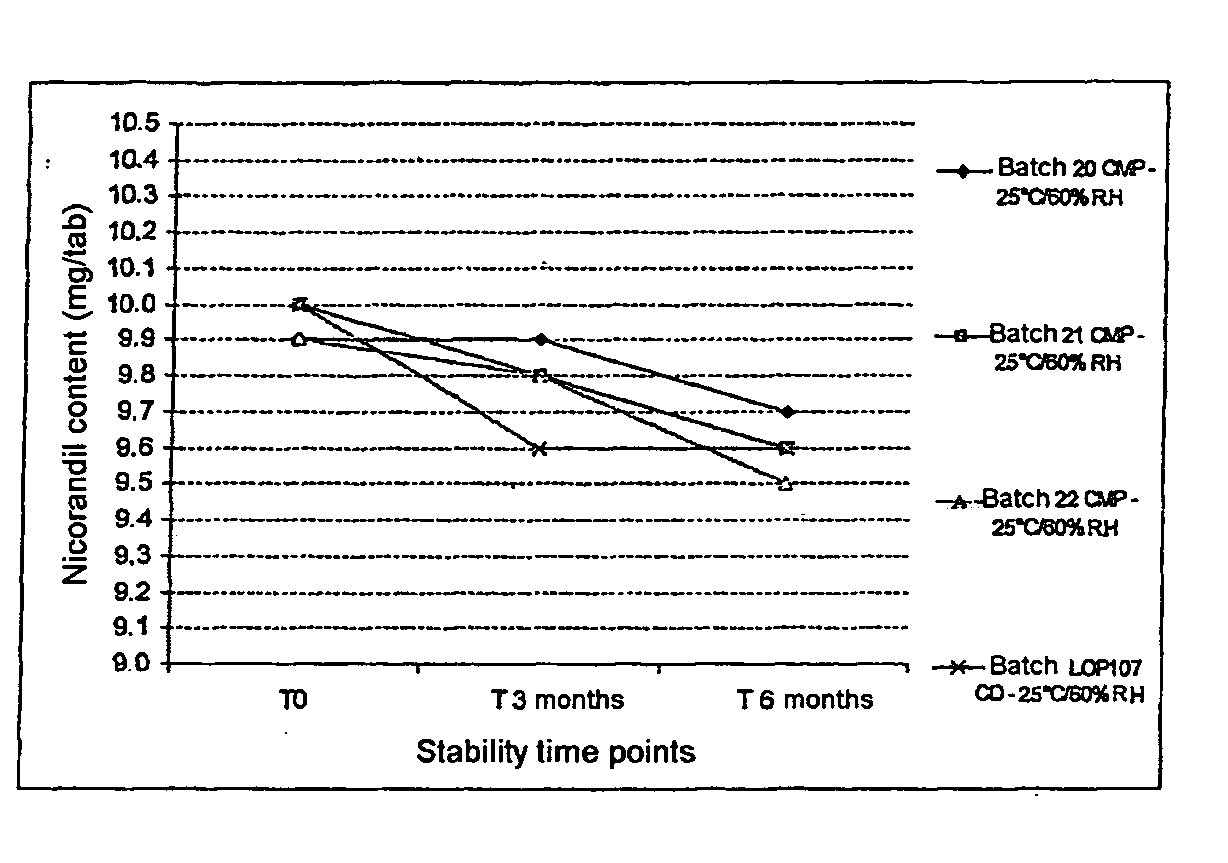
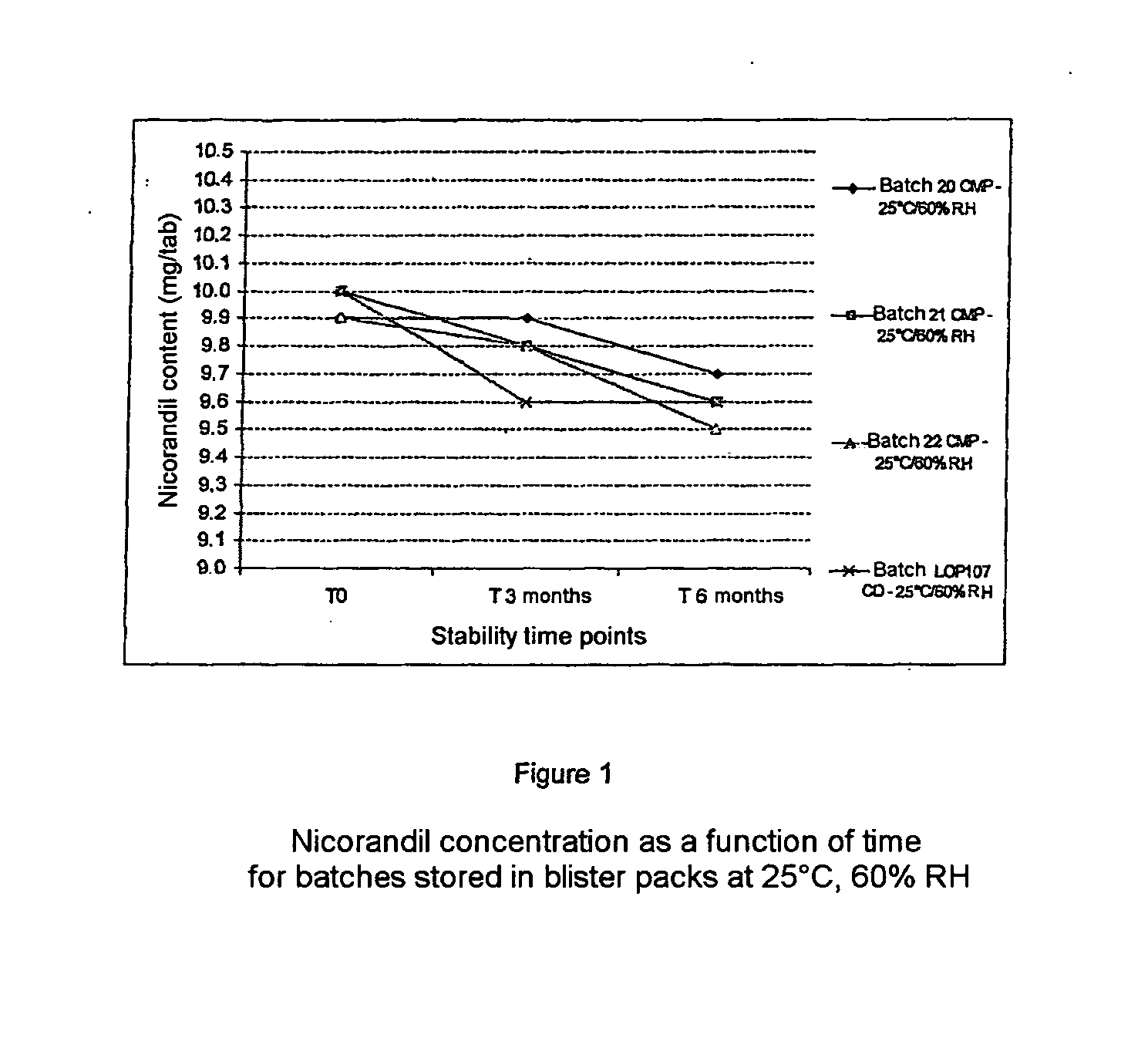
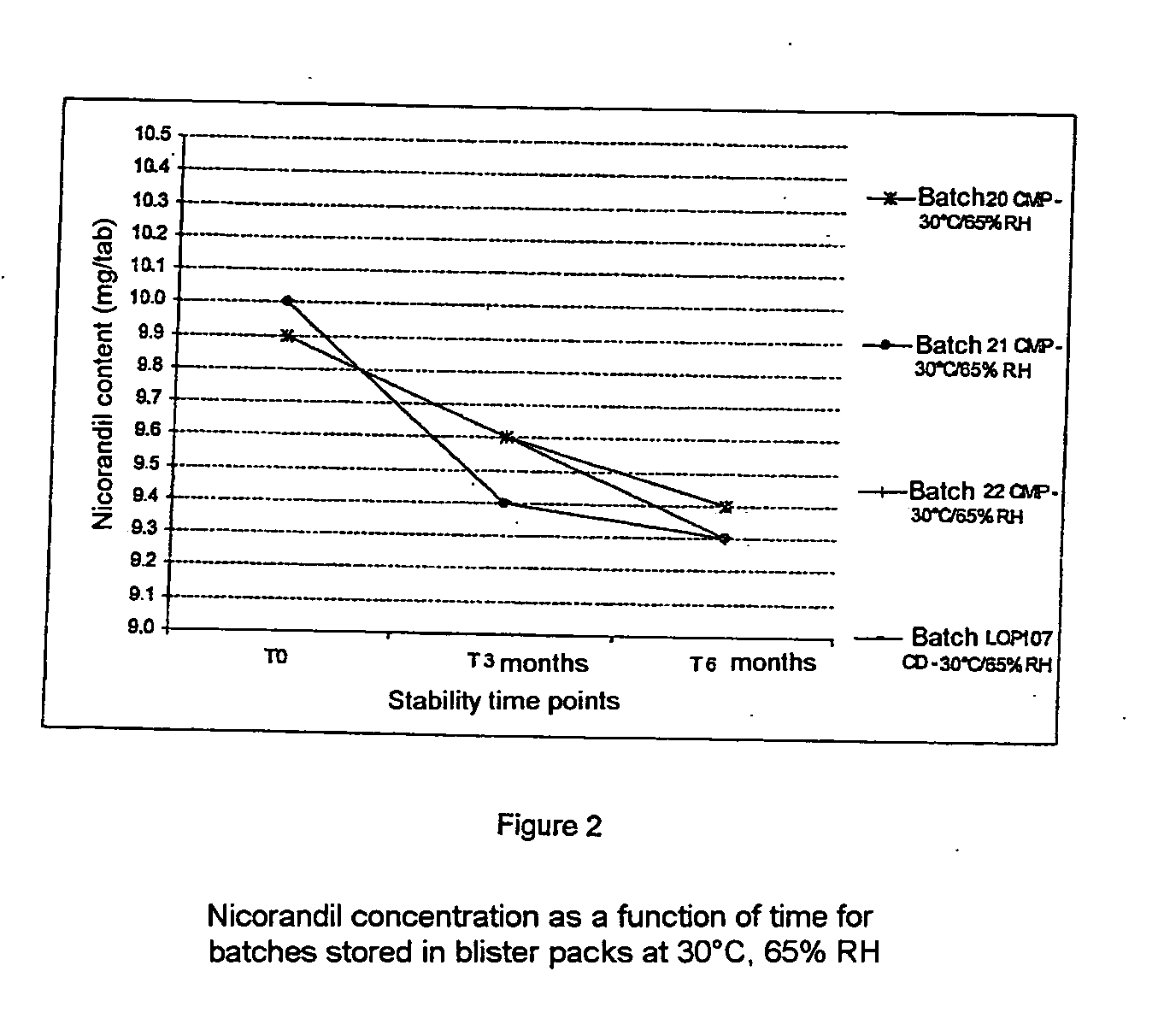
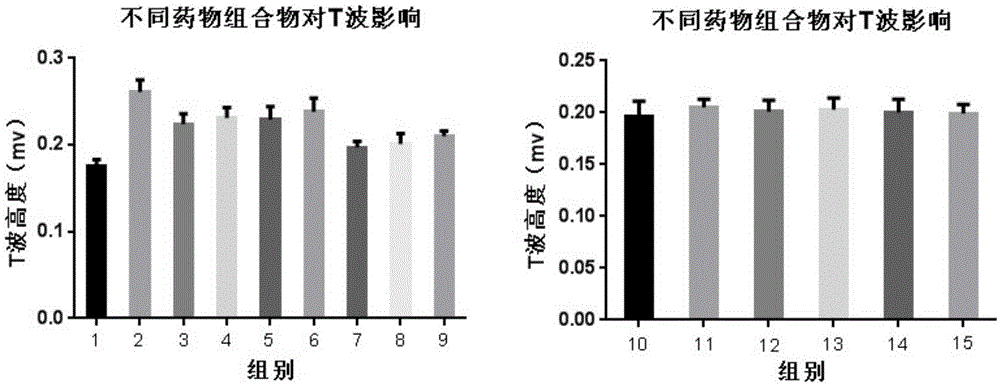
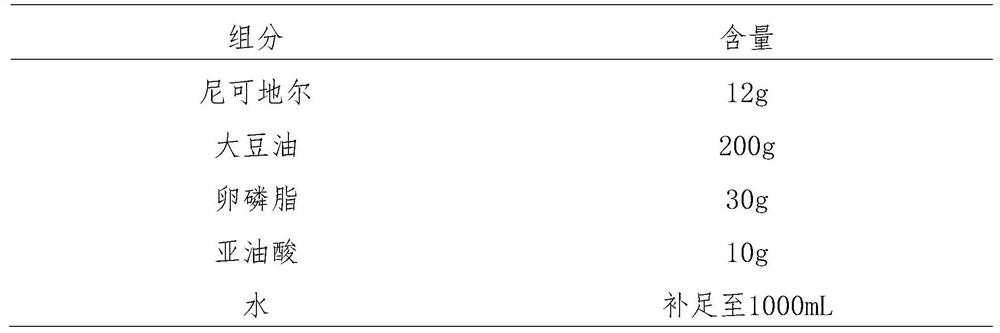
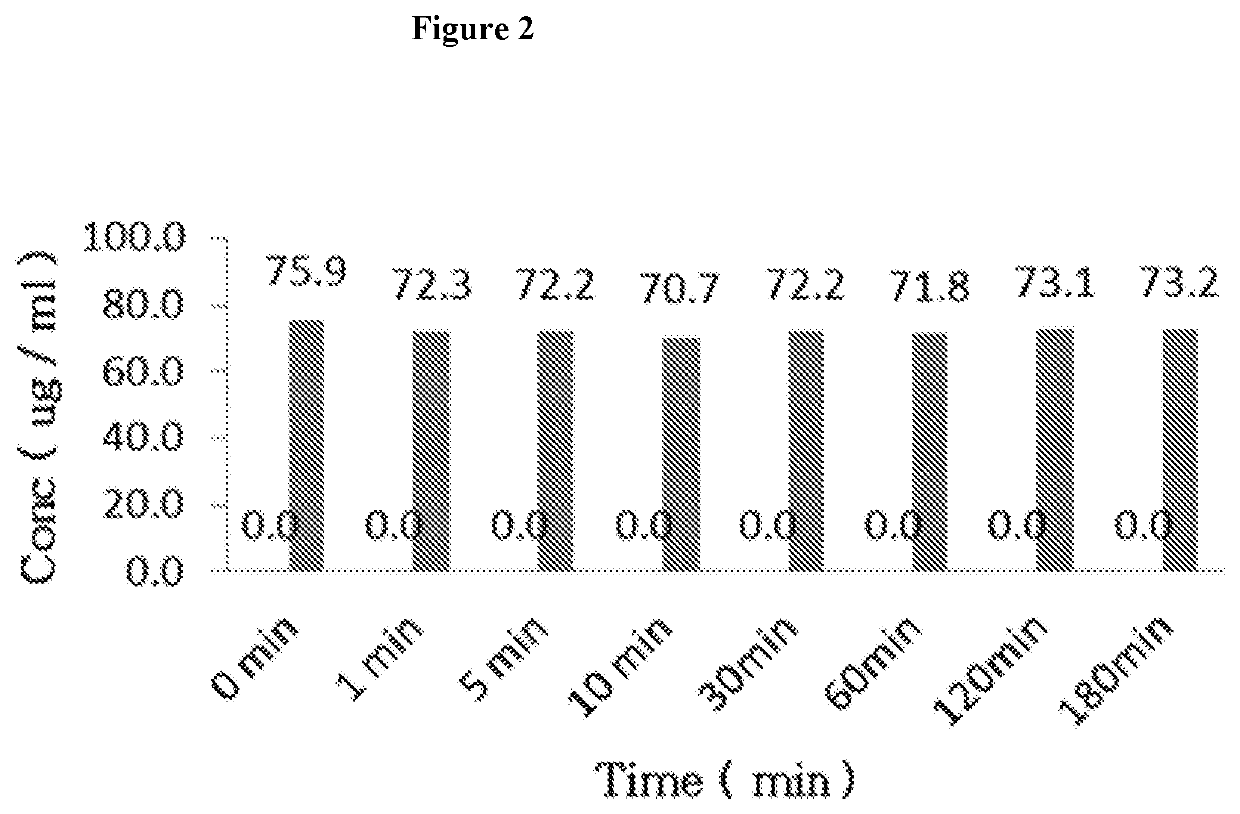
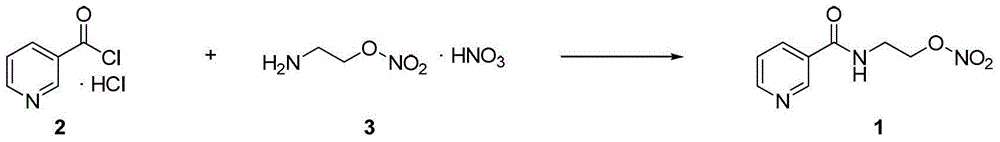Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Nicorandil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
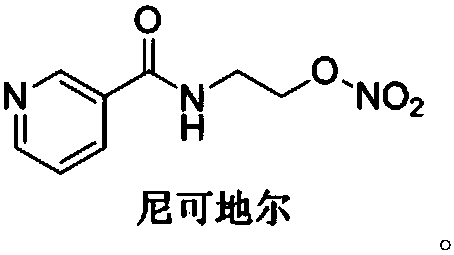
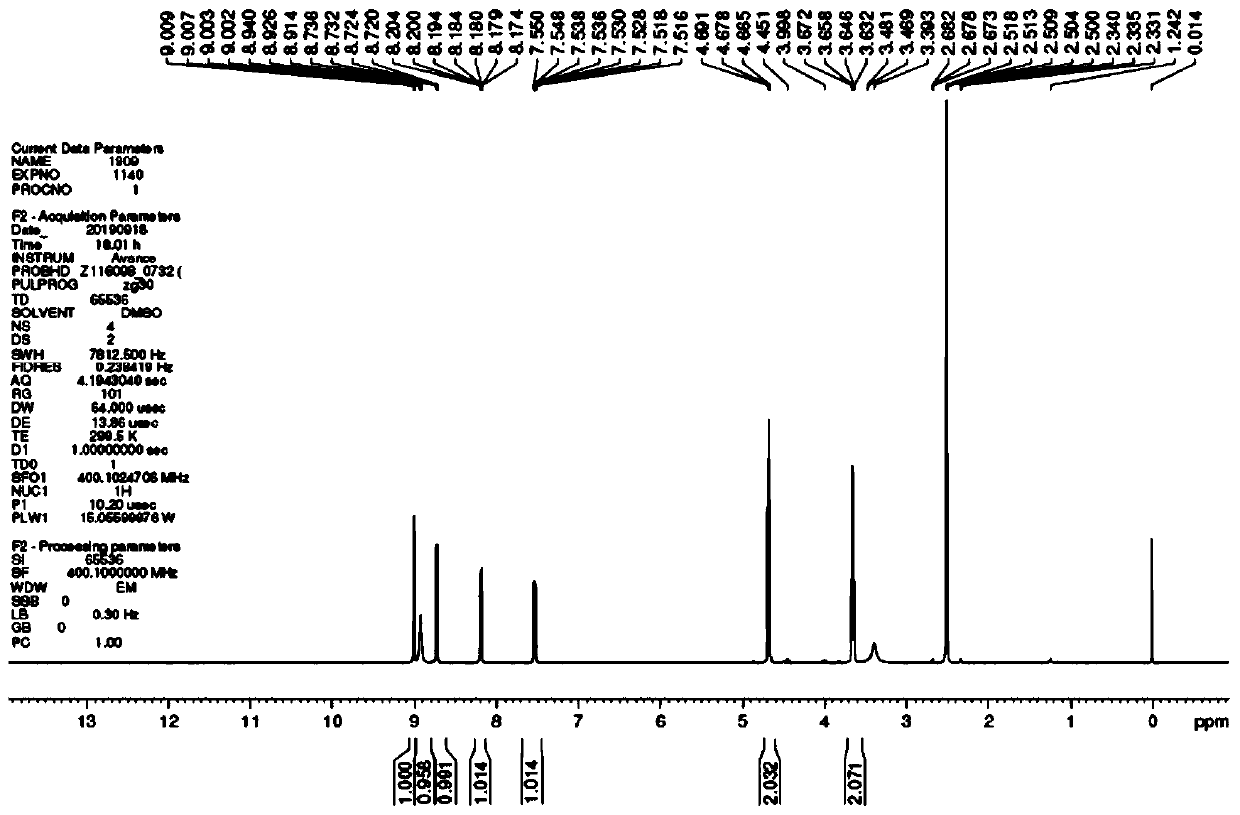
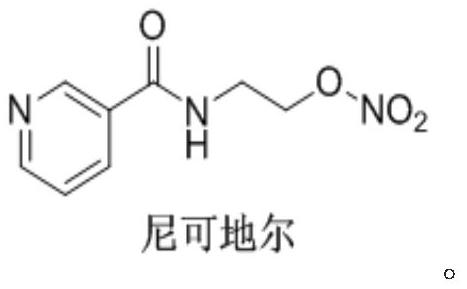
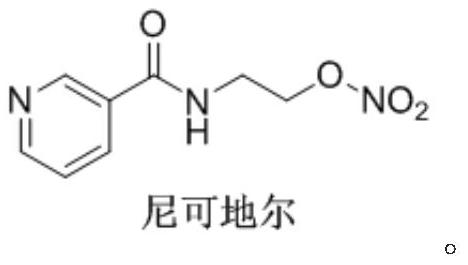
Nicorandil is a vasodilatory drug used to treat angina. Angina is chest pain that results from episodes of transient myocardial ischemia. This can be caused by diseases such as atherosclerosis, coronary artery disease and aortic stenosis. Angina commonly arises from vasospasm of the coronary arteries. There are multiple mechanisms causing the increased smooth muscle contraction involved in coronary vasospasm, including increased Rho-kinase activity. Increased levels of Rho-kinase inhibit myosin phosphatase activity, leading to increased calcium sensitivity and hypercontraction. Rho-kinase also decreases nitric oxide synthase activity, which reduces nitric oxide concentrations. Lower levels of nitric oxide are present in spastic coronary arteries. L-type calcium channel expression increases in spastic vascular smooth muscle cells, which could result in excessive calcium influx, and hypercontraction.
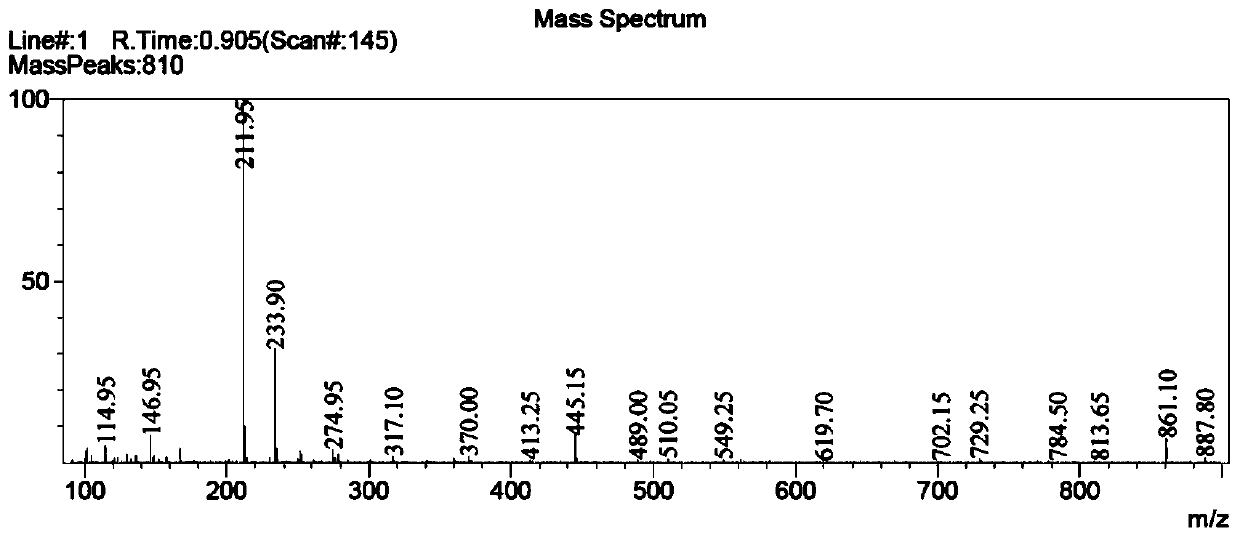
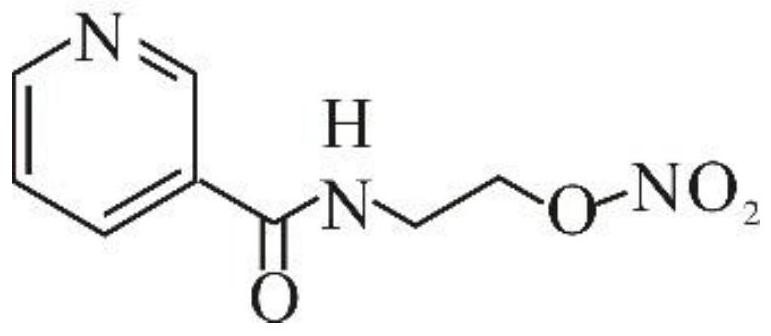
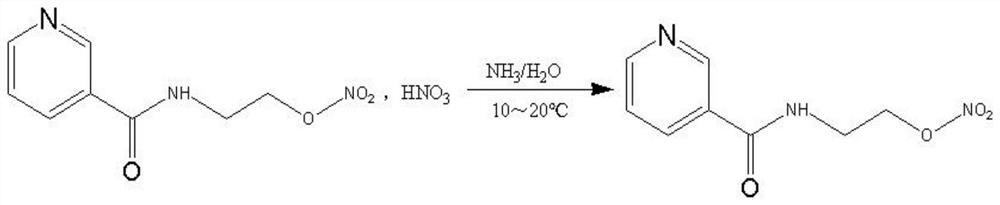
Process for the manufacture of nicorandil
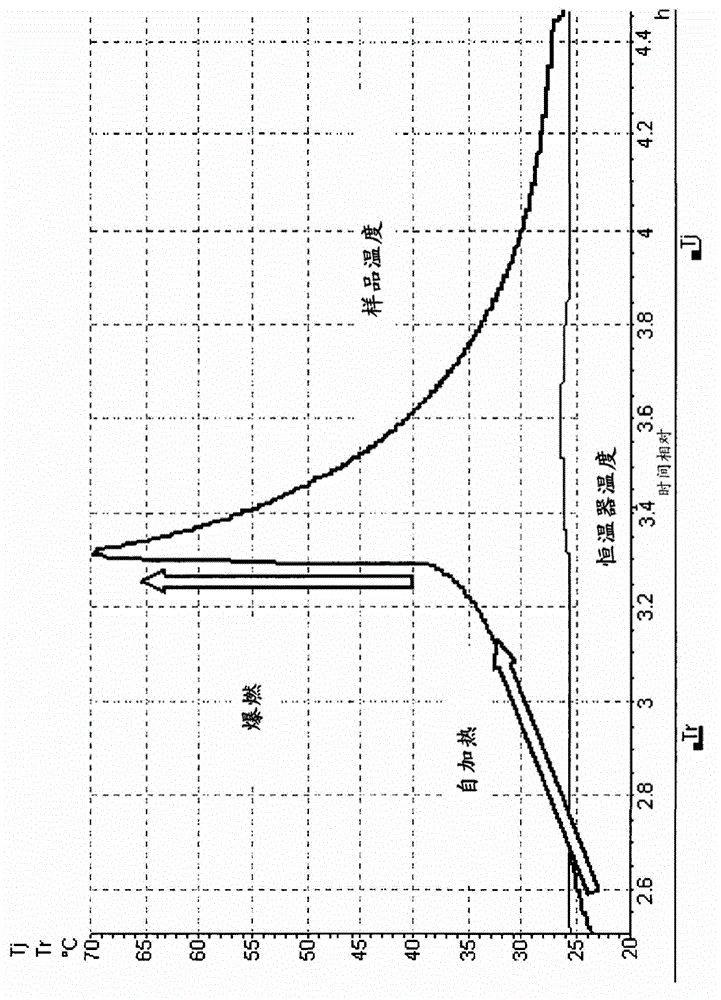
Disclosed is a process for the synthesis of Nicorandil (1), 2-(nicotinamide)ethyl nitrate, starting from N-(2-hydroxyethyl)nicotinamide (15), using nitration with nitric acid in the presence of acetic anhydride Said synthesis method is particularly advantageous because it solves the safety problems involved in the use of nitric acid as nitrating agent, and allows a product with excellent yields and quality to be isolated.
Owner:PROCOS
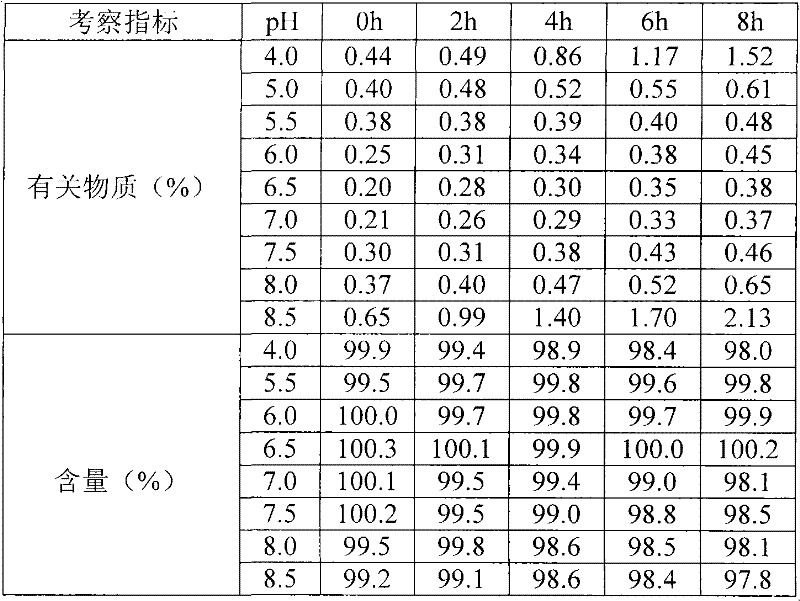
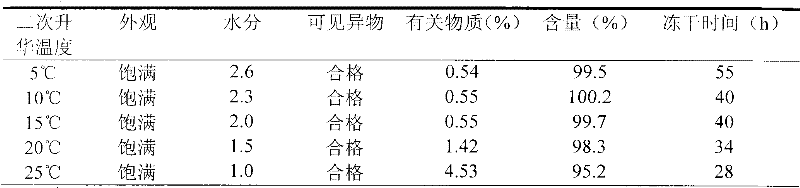
Nicorandil freeze-dried injection and preparation method thereof
ActiveCN101474161AQuality improvementImprove stabilityOrganic active ingredientsPowder deliveryClinical efficacyFreeze-drying
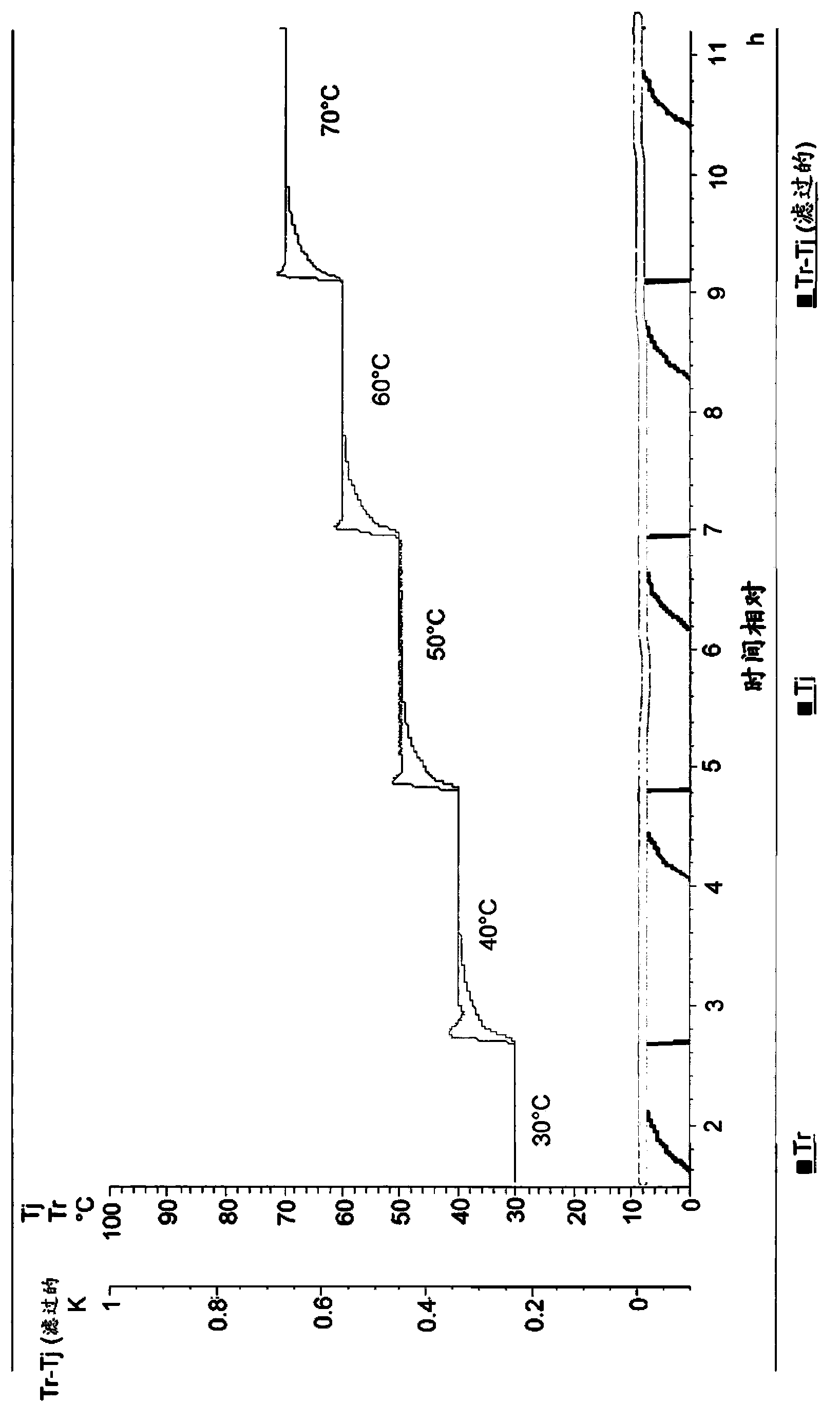
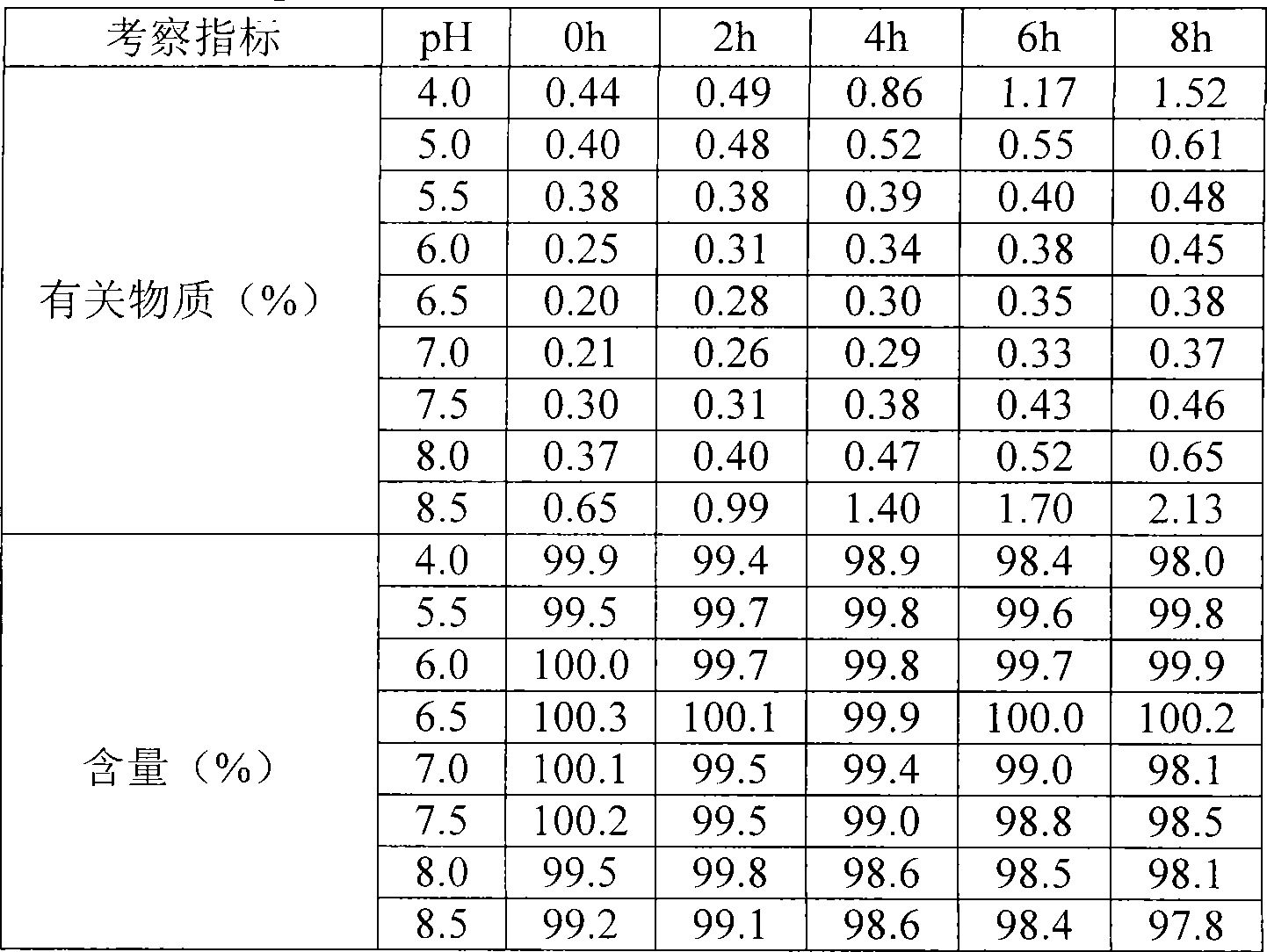
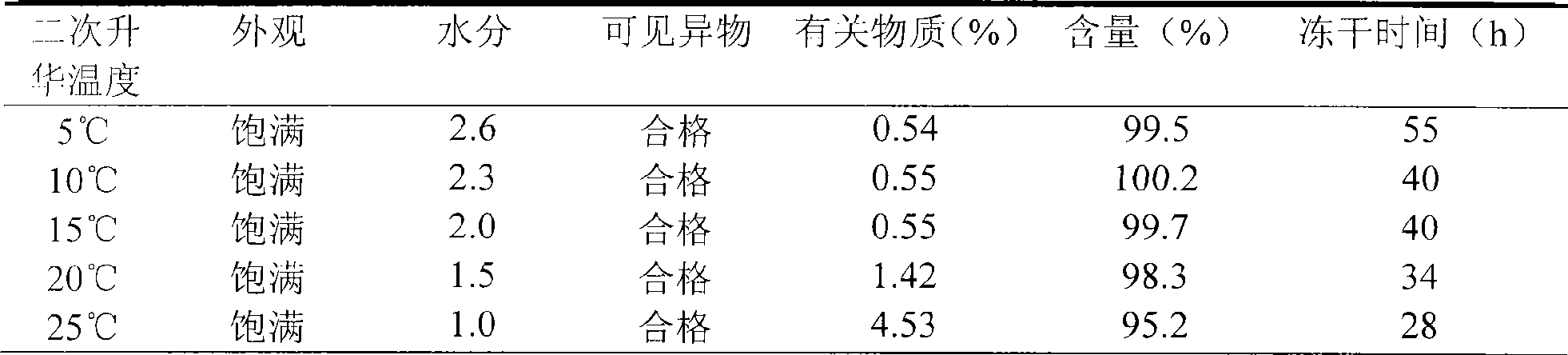
The invention relates to a stable freeze-drying nicorandil powder injection and a preparation method thereof. In the process of preparing the freeze-drying nicorandil powder injection, acidifying or alkalizing agent is adopted to regulate the pH value of intermediate solution for the pH value to range from 5.0 to 8.0, and secondary sublimation temperature is controlled in the process of freeze drying for the temperature to range from 5 DEG C to 20 DEG C, and thereby, the medicament content and the stability of related substances are improved. The invention overcomes the deficiencies in the prior art; and a stable freeze-drying nicorandil powder is obtained, and the freeze-drying powder injection has good clinical efficacy.
Owner:BEIJING SIHUAN KEBAO PHARM CO LTD
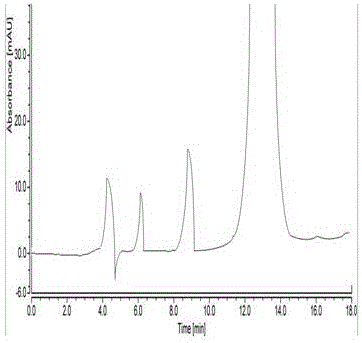
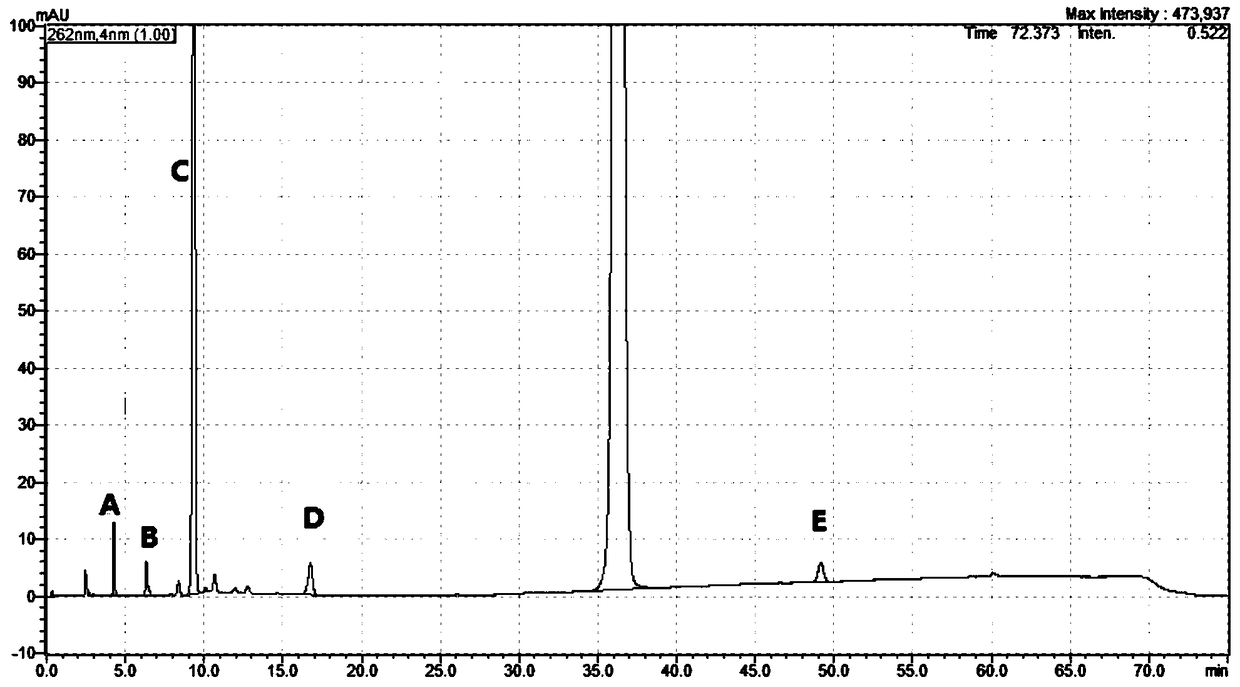
HPLC analytic method for Nicorandil-related substances
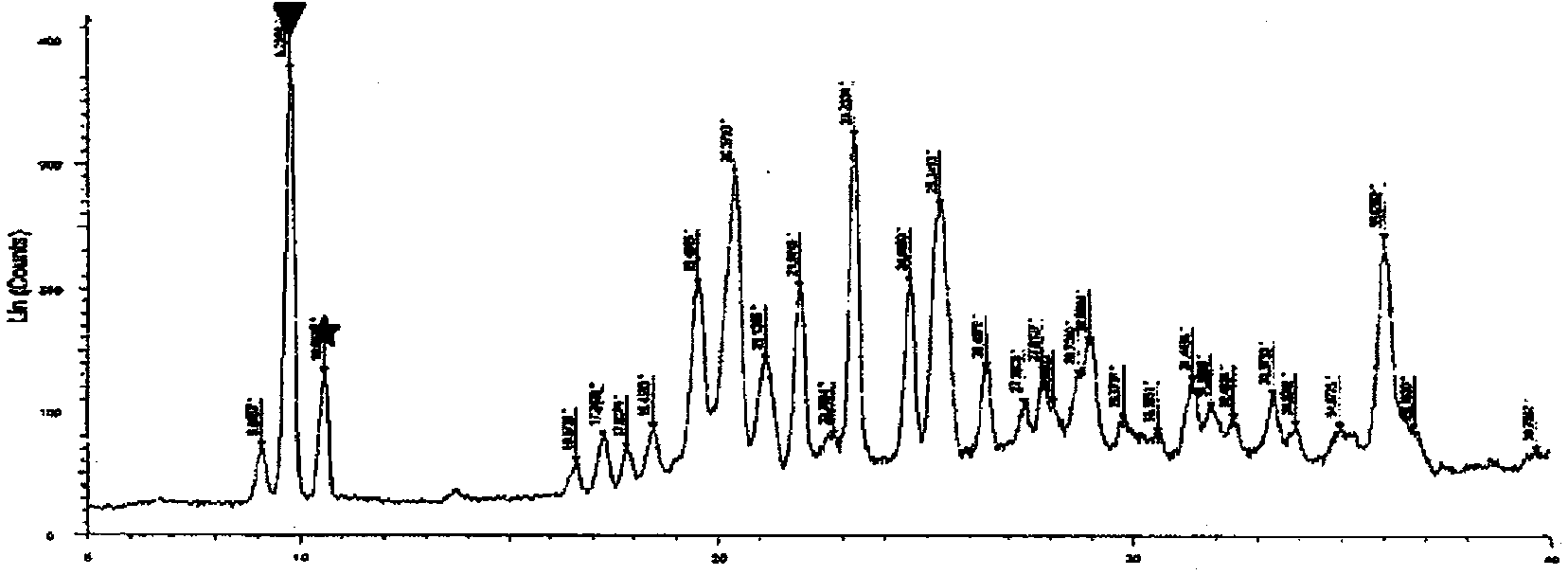
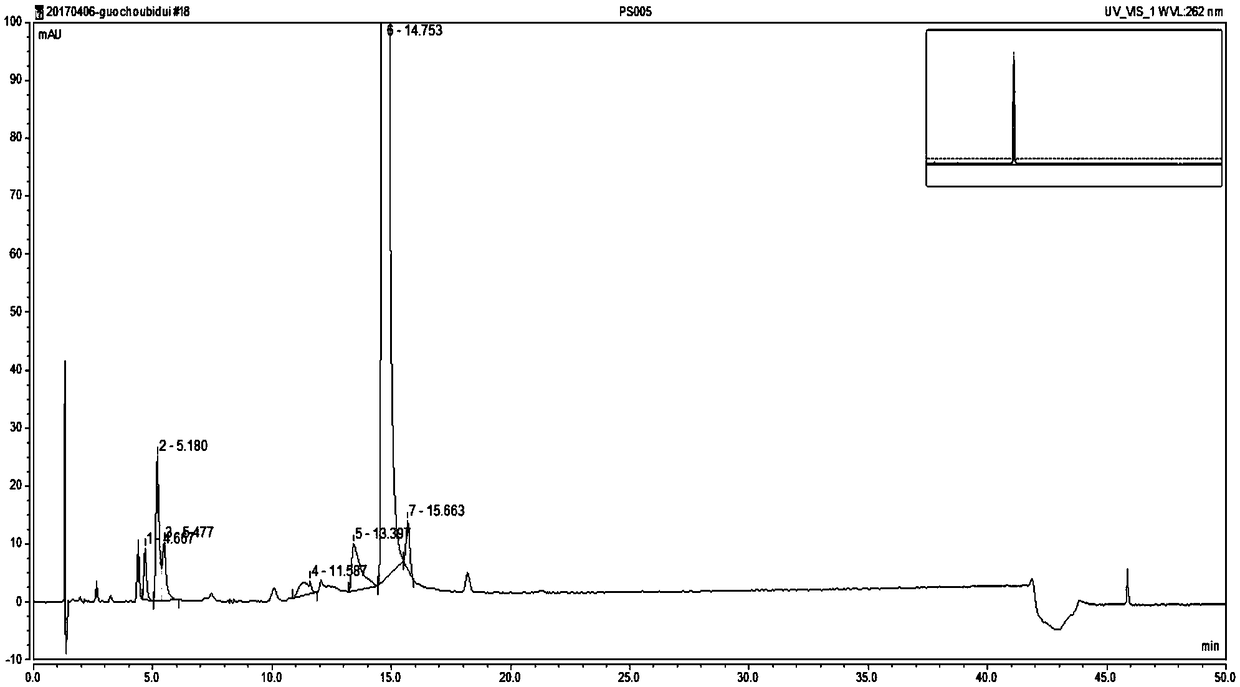
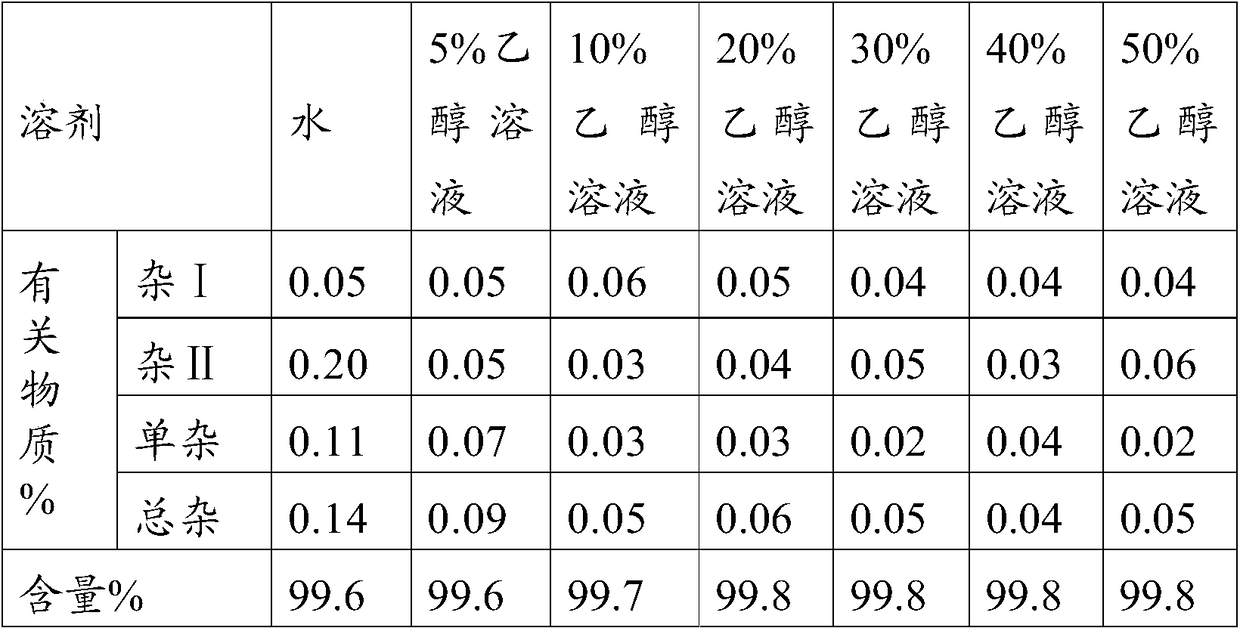
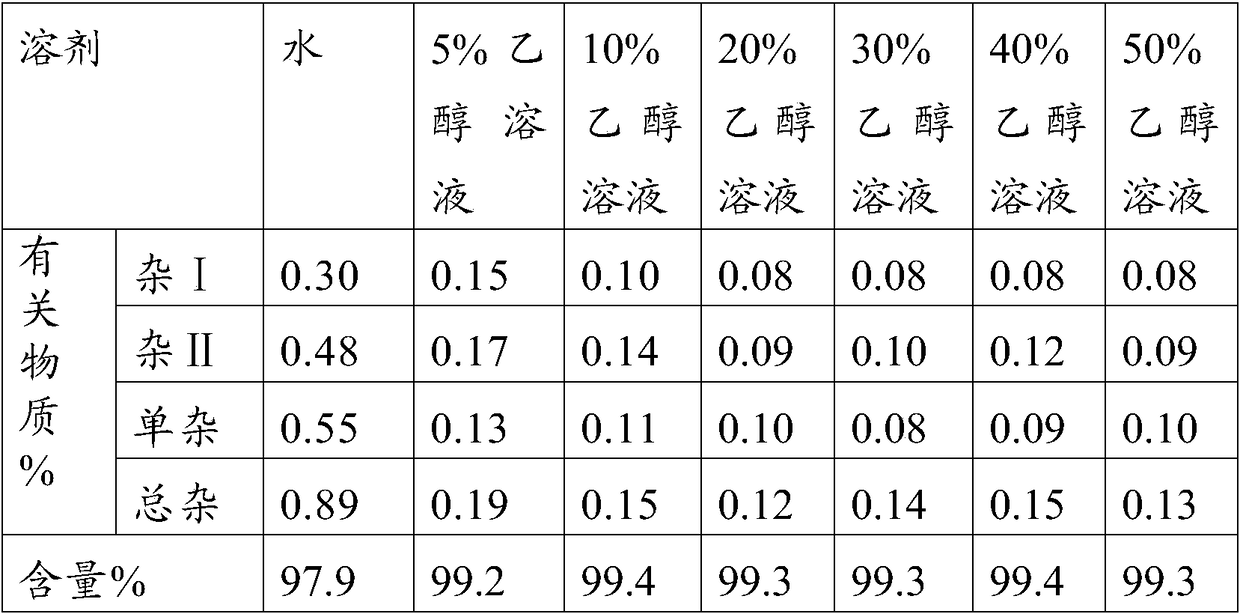
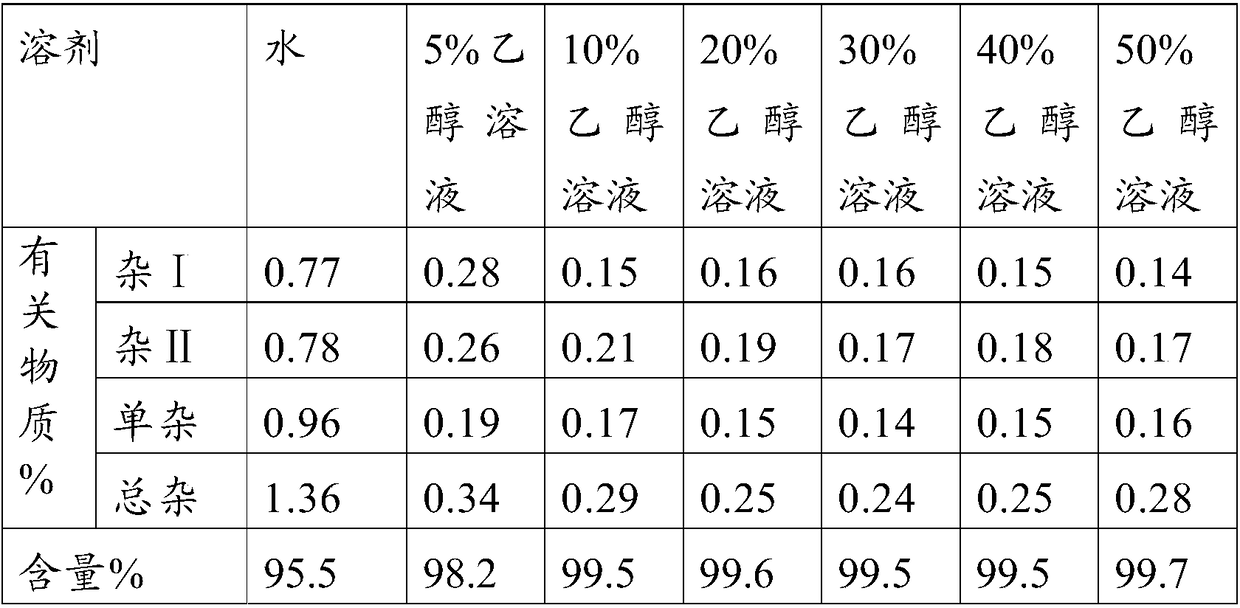
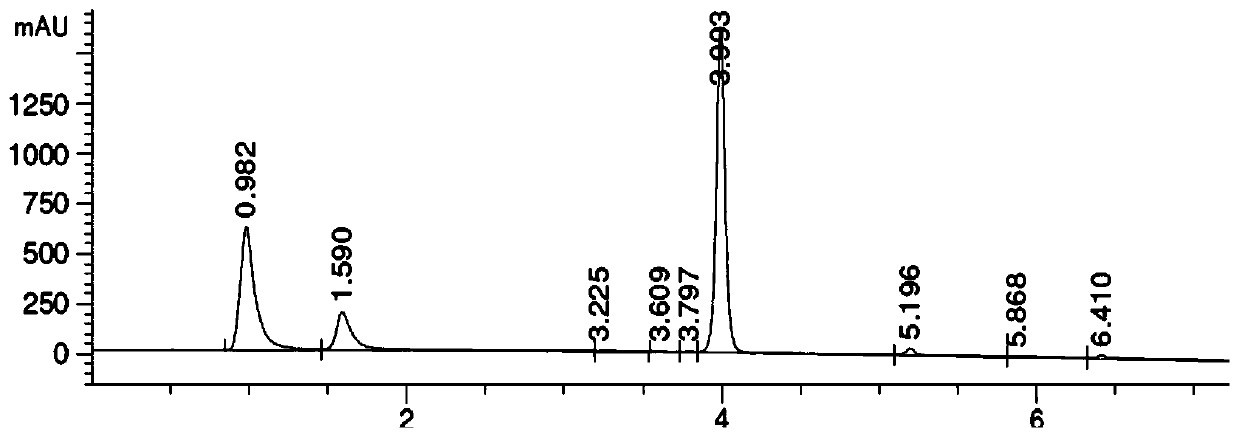
The invention belongs to the technical field of pharmaceutical analysis, and particularly relates to an HPLC analytic method for Nicorandil-related substances. The HPLC analytic method is characterized in that a stationary phase is a reversed-phase column of carbon octadecyl silane, and a moving phase is a mixed solution of a buffer solution, methyl alcohol and tetrahydrofuran. The HPLC analytic method is quick, simple and accurate, is good in repeatability and suitable for control and stability study of the related substances, lays a foundation for formulating quality standards of the related substances, and makes drug quality control and medication safety possible. Through scaling down organic solvents and volatile salt, which have high toxicity, the acquisition time is shortened, the economic cost is lowered, the HPLC analytic method is suitable for large-scale production, and meanwhile, harms to experimenters and environments are reduced.
Owner:XIAN KAIBEI NAITE INTELLIGENT ENG
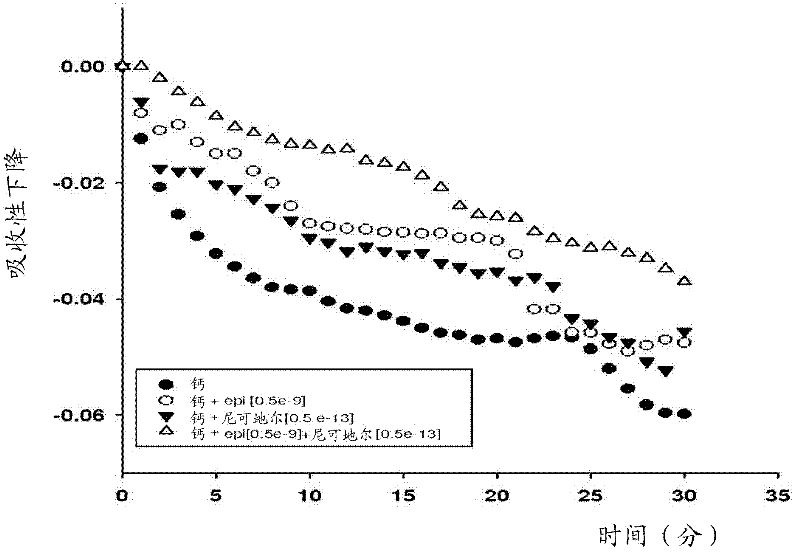
Methods and compositions for treatment of ischemic conditions and conditions related to mitochondrial function
InactiveCN102480951ASimple structureFunction increaseBiocideNervous disorderDiseaseMitochondrial depletion
The present invention relates to compositions and methods for prophylactic and / or therapeutic treatment of conditions related to mitochondrial function. In various aspects, the present invention comprises administering one or more compounds selected from the group consisting of epicatechin, an epicatechin derivative, catechin, a catechin derivative, nicorandil, and a nicorandil derivative in an amount effective to stimulate mitochondrial function in cells. The methods and compositions described herein provide for reducing infarct size in the heart following permanent ischemia or ischemia / reperfusion (IR) event or method for delaying, attenuating or preventing adverse cardiac remodeling, and can assist in prevention of impaired mitochondria biogenesis and thus prevention of the consequences of impaired mitochondrial biogenesis in various diseases and conditions, as well as provide for the active therapy of mitochondrial depletion that may have already occurred.
Owner:CARDERO THERAPEUTICS
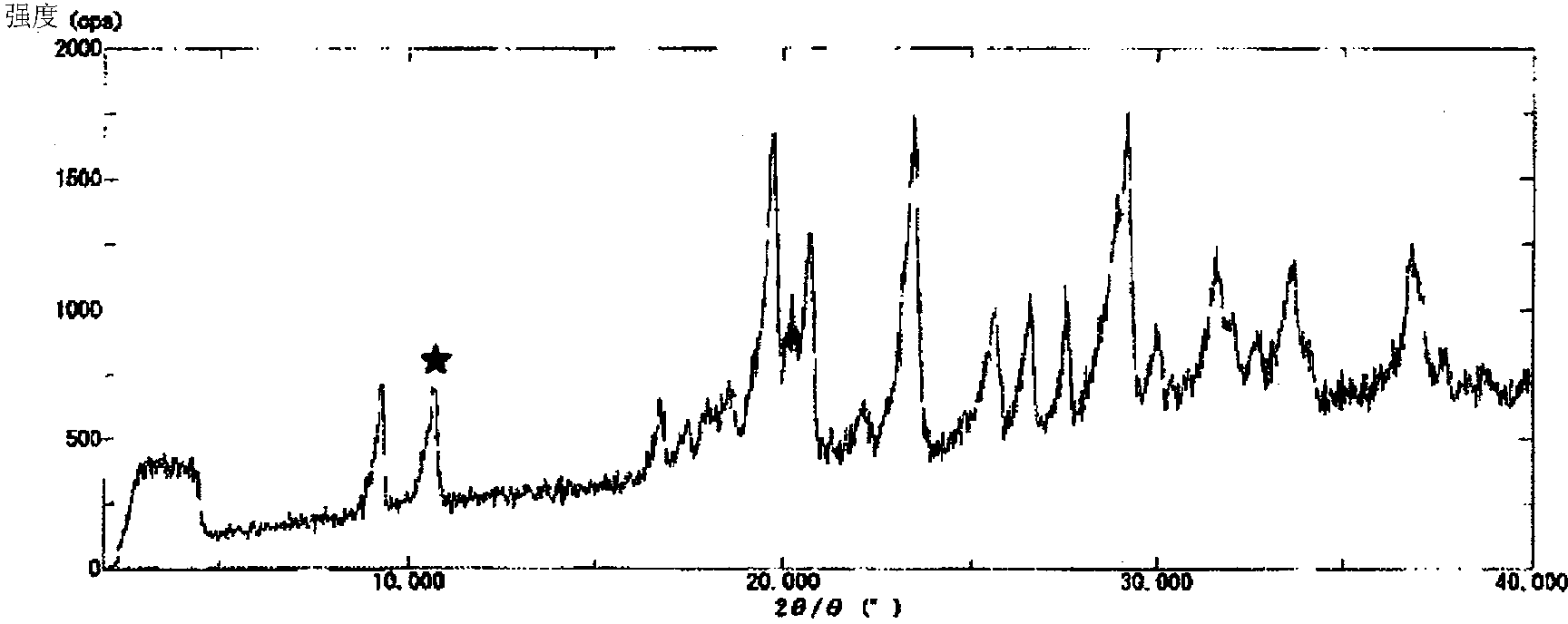
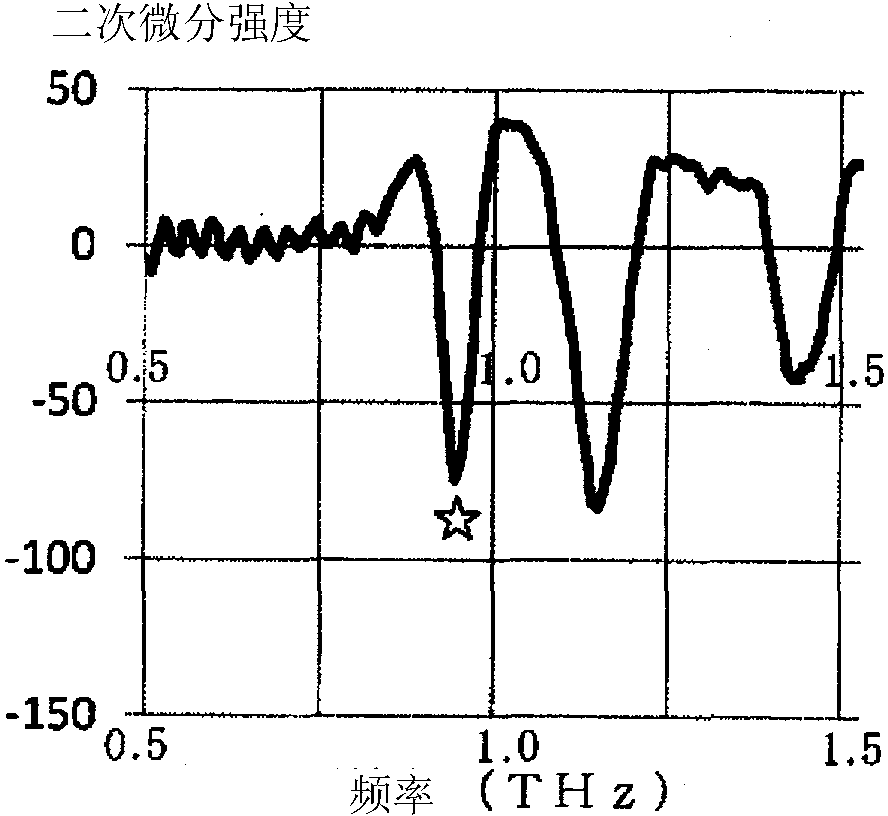
Nicorandil-containing pharmaceutical composition
InactiveCN103764146AGood storage stabilityGood effectPowder deliveryOrganic active ingredientsPharmaceutical drugCarboxylic acid
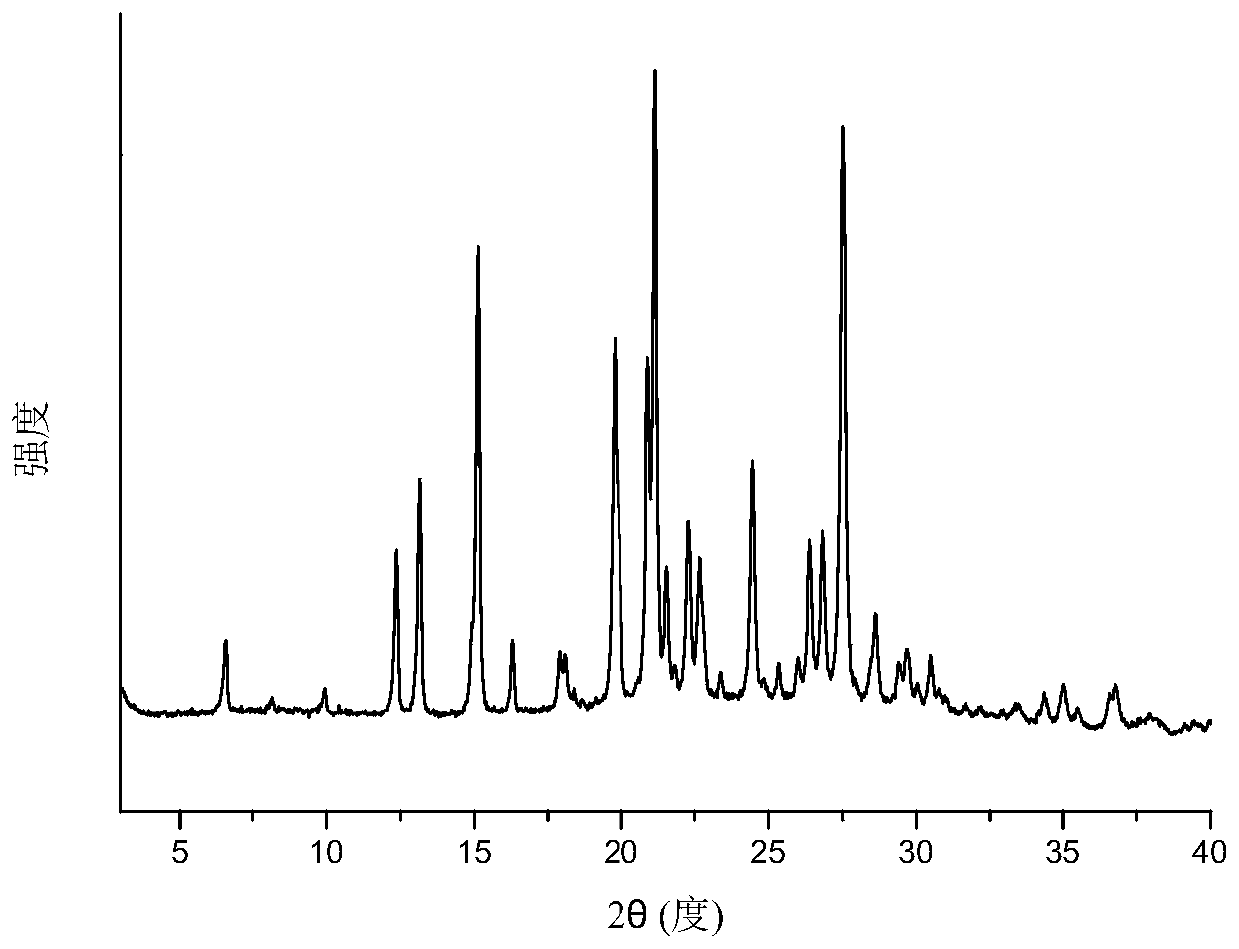
A pharmaceutical composition containing nicorandil, mannitol, and the alkali metal salt of an organic carboxylic acid, wherein (1) "the peak value of the delta crystals of mannitol at 9.8 DEG +-0.3 DEG (2[theta]) in X-ray diffraction " / "the peak value of nicorandil crystals at 10.6 DEG +-0.3 DEG (2[theta]) in X-ray diffraction" is 2.0 or less or (2) "the peak intensity at 1.01 THz +-0.2 THz of the secondary derivative spectrum in the absorption spectrum of the delta crystals of mannitol measured by means of terahertz transmission spectroscopy" / " the peak intensity at 0.95 THz +-0.2 THz of the secondary derivative spectrum in the absorption spectrum of nicorandil crystals measured by means of terahertz transmission spectroscopy" is 1.5 or less. This pharmaceutical composition exhibits excellent storage stability.
Owner:MOCHIDA PHARM CO LTD
Nicorandil freeze-drying powder preparation method
ActiveCN1839837AReduce the rate of degradationImprove stabilityPowder deliveryOrganic active ingredientsAntioxidantFreeze-drying
The invention discloses a process for preparing Nicorandil freeze-dried powder, which consists of charging 0.1-50.0 wt% of Nicorandil into water for injection, dissolving excipient simultaneously, degerming and filtering, loading, and finally freeze drying.
Owner:XIAN LIBANG PHARMA TECH
Method for preparing nicorandil tablet
InactiveCN1839836AAvoid degradationImprove stabilityOrganic active ingredientsPill deliveryAdjuvantMedicine
The invention discloses a process for preparing Nicorandil tablets through charging diluent, binding agent, crumbling agent, lubricating agent, flow adjuvant and anti-oxidizing agent into 0.5-25.0 wt% of Nicorandil. The tablets can be prepared through direct powder pelleting method, dry method granulation pelleting or blank granulation method.
Owner:XIAN LIBANG PHARMA TECH
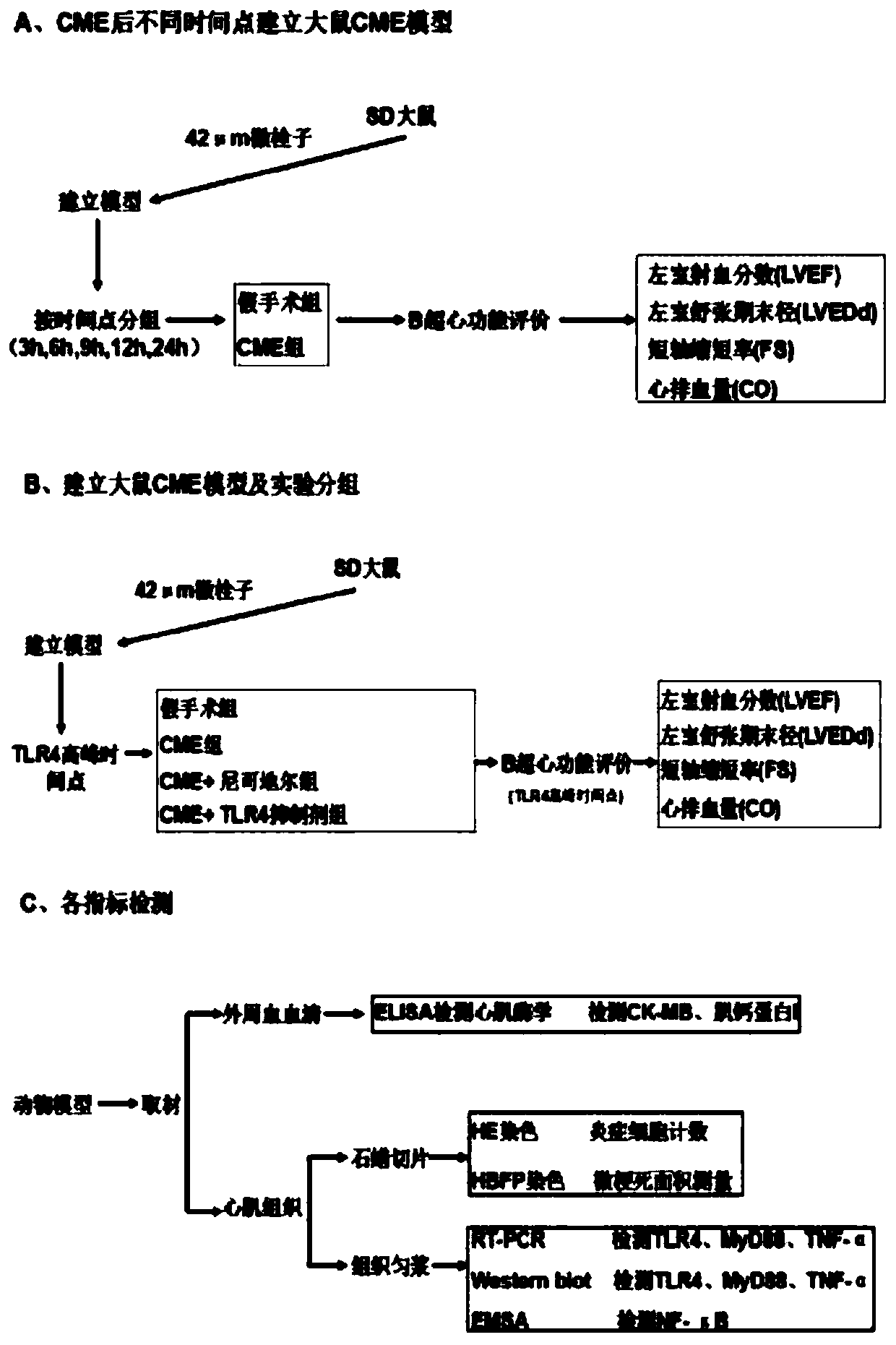
Drug for preventing and treating myocardial injury caused by coronary microembolism and animal model thereof
InactiveCN109700809AMyocardial injury protectionOrganic active ingredientsSurgical veterinaryTLR4Coronary-myocardial
The present invention belongs to the technical field of preventing and treating coronary myocardial injury and discloses a drug for preventing and treating the myocardial injury caused by coronary microembolism and an animal model thereof; SD rats are used and 42 [mu]m microemboli are used to prepare the coronary microembolism CME animal model; after selecting CME, 3 h, 6 h, 9 h, 12 h and 24 h areused as observation time points, observation groups at each time points are divided into a sham operation group and a CME group, and expression levels and cardiac function changes of TLR4 at the eachtime point are detected to find out peak time of activity of the TLR4; according to the peak time point of the activity of the TLR4, then the sham operation group, the CME group, a CME+nicorandil group and a CME+TLR4 inhibitor group. The animal model determines dynamic changes of TLR4-mediated cell signal transduction pathways of "TLR4 / MyD88 / NF-kappa-B / TNF-alpha" after the CME occurrence and determines whether nicorandil blocks the "TLR4 / MyD88 / NF-kappa-B / TNF-alpha signaling pathways to protect against myocardial damages caused by the CME.
Owner:AFFILIATED HOSPITAL OF GUILIN MEDICAL UNIV
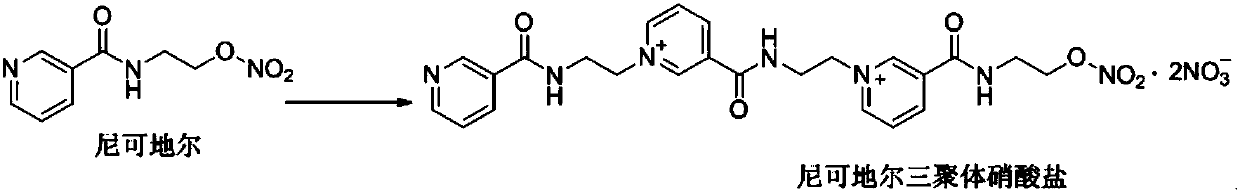
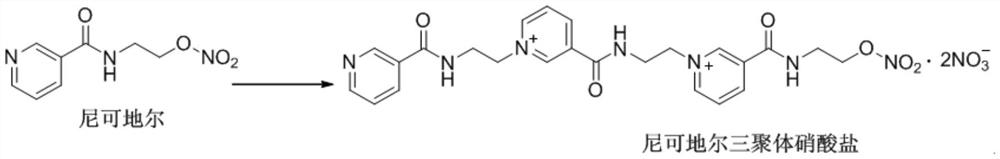
Method for preparing nicorandil tripolymer
The invention provides a method for preparing a nicorandil tripolymer. The method comprises the following steps: (1) dissolving nicorandil into an organic solvent, stirring and reacting for 4-7 hours;(2) slowly dropping a crystallization solvent into the reaction solution after the reaction is ended, slowly cooling to a room temperature, stirring and crystallizing, filtering to obtain a crude product, and washing with an organic solvent; and (3) adding the crude product into a re-crystallization solvent, and re-crystallizing, thereby obtaining the solid nicorandil tripolymer nitrate. According to the method for preparing the nicorandil tripolymer disclosed by the invention, the nicorandil tripolymer can be conveniently and rapidly prepared, and the prepared nicorandil tripolymer is high in yield and excellent in purity.
Owner:燃点(南京)生物医药科技有限公司
Preparation method of nicorandil
The invention discloses a preparation method of nicorandil. According to the method, N-(2-hydroxyethyl)niacinamide is used as a raw material and is subjected to nitrifying under the actions of dilutenitric acid, propionic acid and propionic anhydride to obtain nicorandil. The method has the advantages of mild conditions, simple operation, stable process, cheap and easily available raw materials,high product yield, easy treatment of three wastes, small environmental pollution, and suitability for industrial large-scale production.
Owner:BEIJING VOBAN PHARMA TECH CO LTD
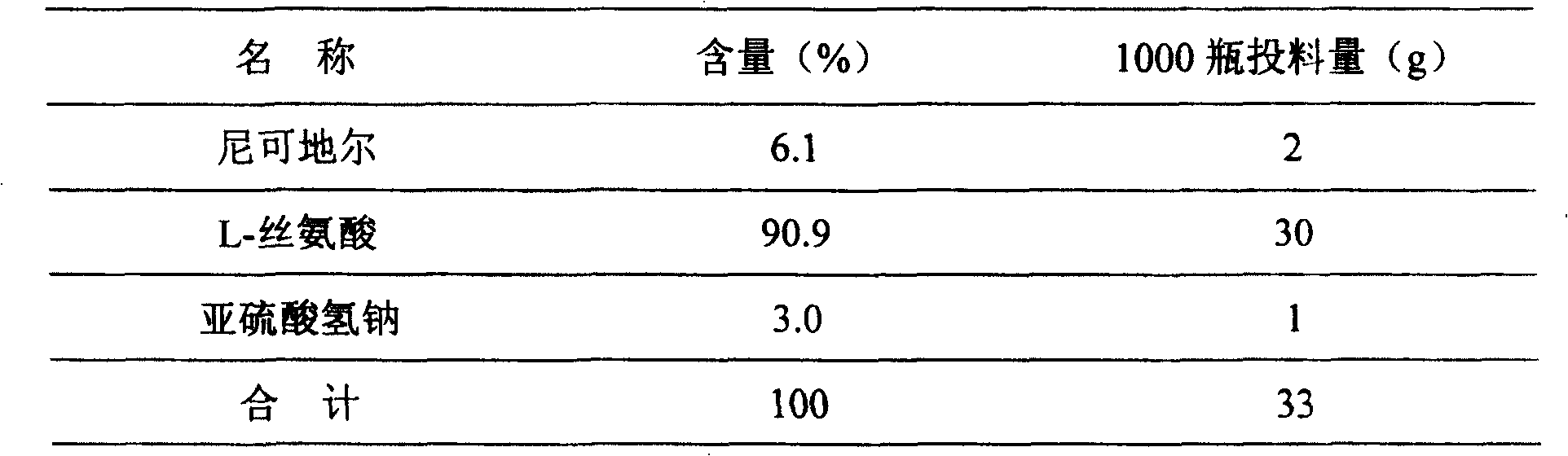
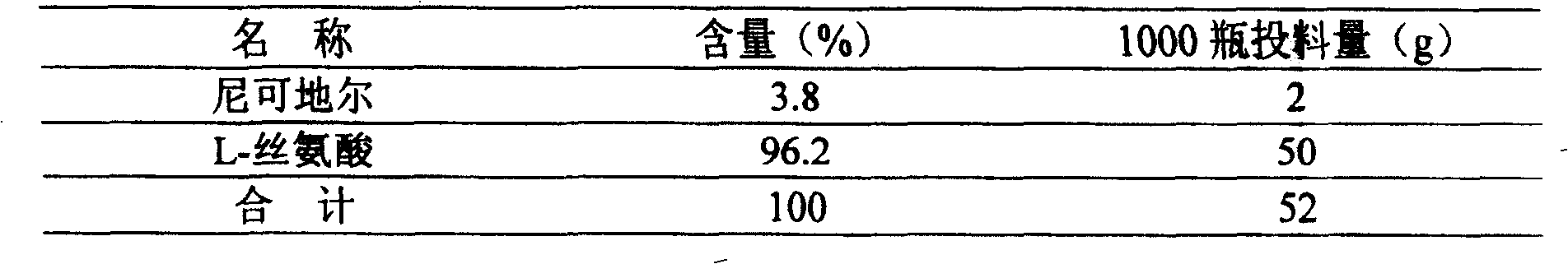
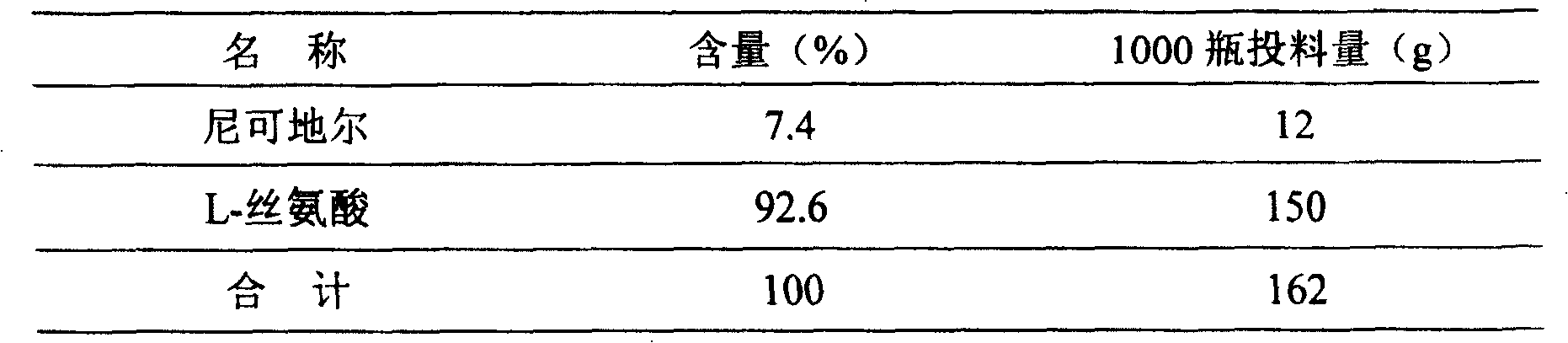
Method of measuring related substances in nicorandil tablets by HPLC (high performance liquid chromatography) correction factor process
The invention relates to a method of measuring related substances in nicorandil tablets by HPLC (high performance liquid chromatography) correction factor process. The method performs measuring according to specifications in high performance liquid chromatography set forth in the general principle 0512 of Chinese Pharmacopoeia 2015 edition volume IV; C18 chromatographic column is used, trifluoroacetic acid-triethylamine-tetrahydrofuran-water mixed solution is used as a mobile phase to carry out measuring; impurities that may be quantitatively measured include impurity A, impurity B, impurity C, impurity D, impurity E and related known substances; the method has good methodological properties, such as wide linear range of test, good linearity, small detection limit and quantification, and good precision.
Owner:BEIJING INST FOR DRUG CONTROL
Process for the manufacture of nicorandil
Disclosed is a process for the synthesis of Nicorandil (1), 2-(nicotinamide)ethyl nitrate, starting from N-(2-hydroxyethyl)nicotinamide (15), using nitration with nitric acid in the presence of acetic anhydride Said synthesis method is particularly advantageous because it solves the safety problems involved in the use of nitric acid as nitrating agent, and allows a product with excellent yields and quality to be isolated.
Owner:PROCOS SPA
A kind of preparation method of nicorandil freeze-dried preparation with good stability
Owner:BEIJING SIHUAN KEBAO PHARM CO LTD
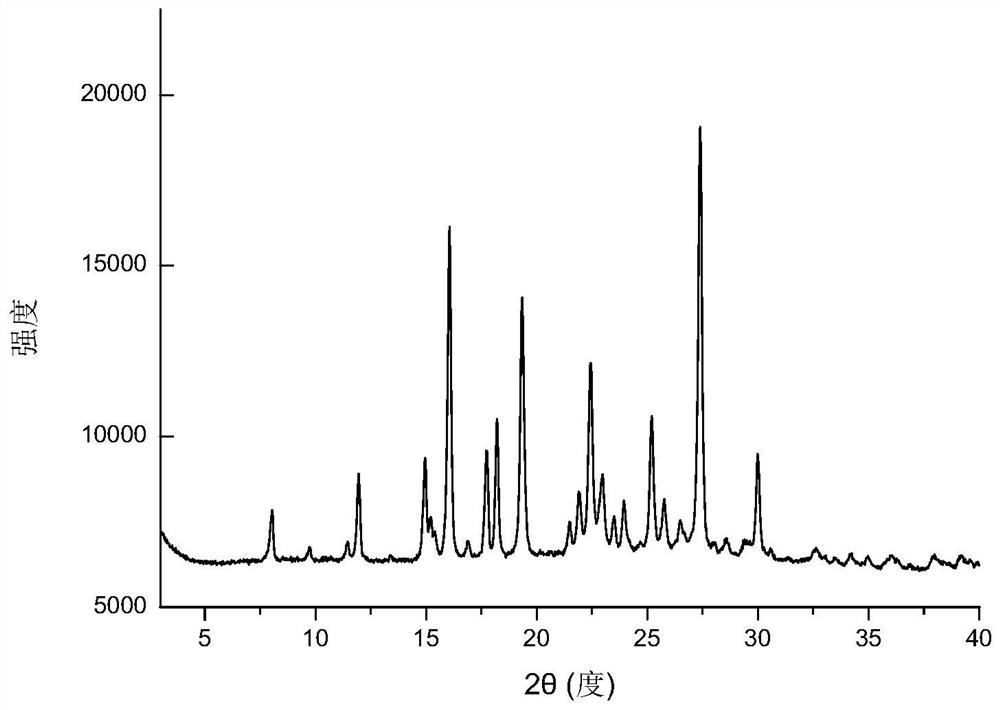
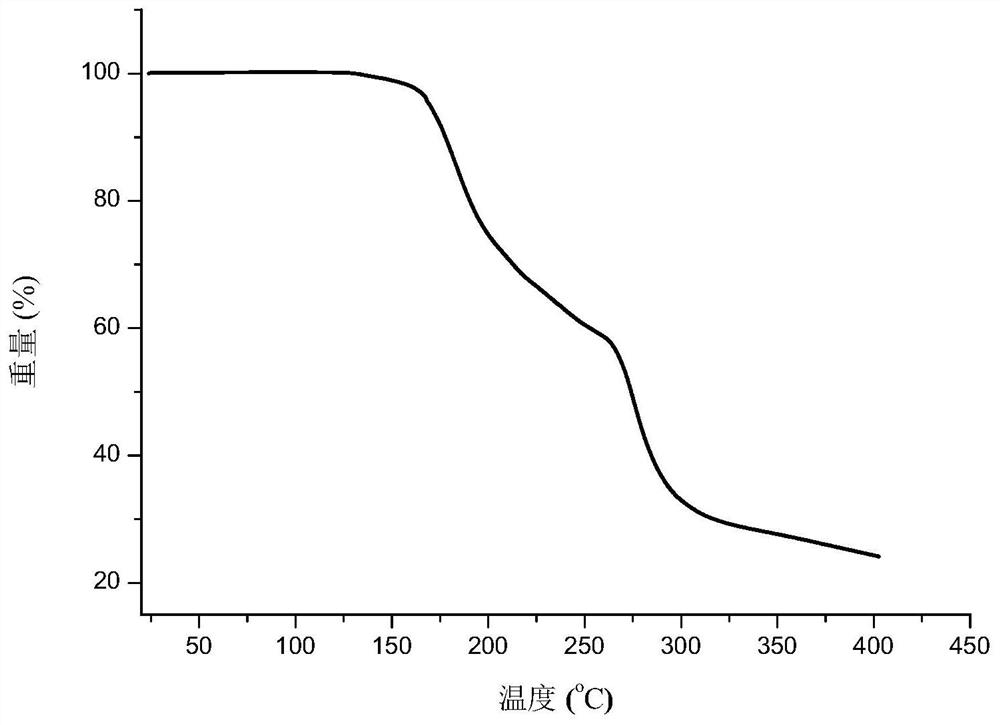
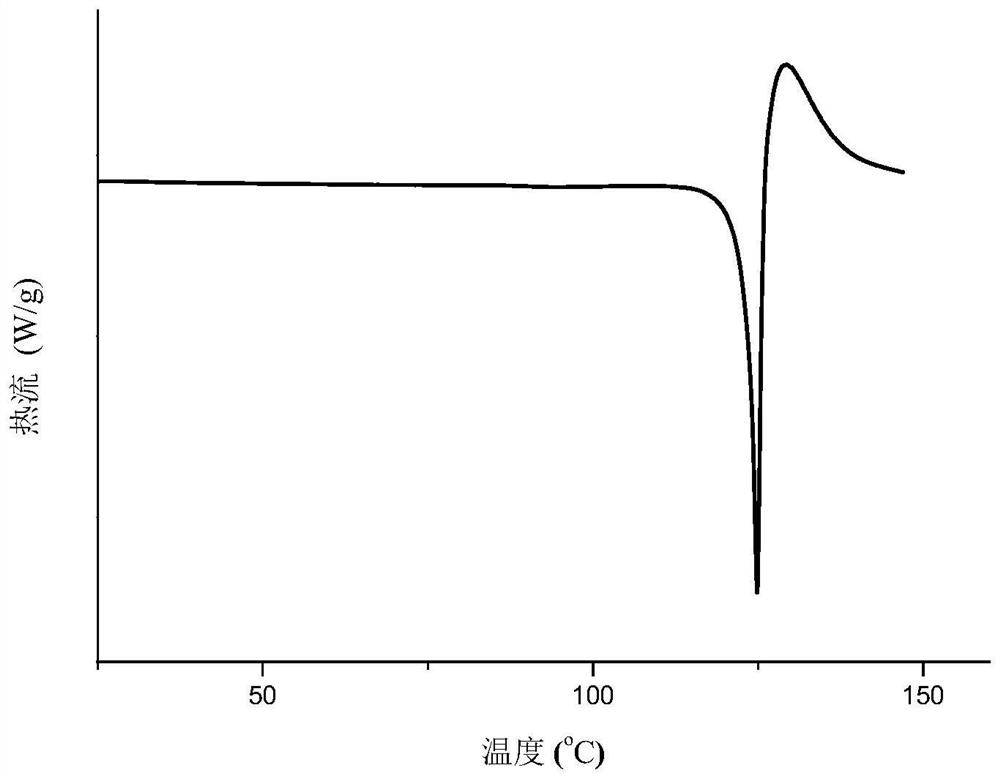
Eutectic crystal of nicorandil and salicylic acid as well as preparation method and application of eutectic crystal
ActiveCN111574441AEasy to prepareGood reproducibilityOrganic active ingredientsOrganic chemistry methodsPhysical chemistrySalicylic acid
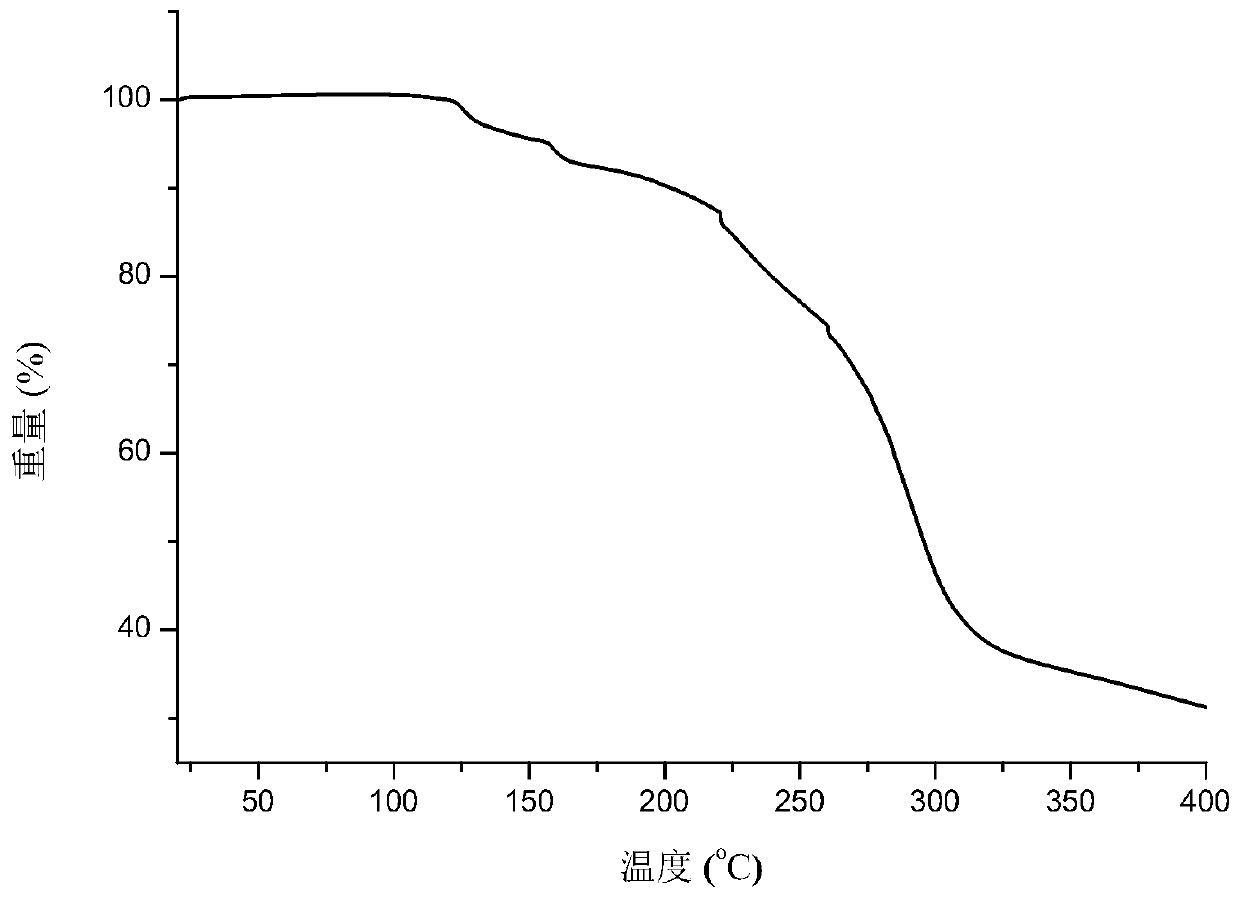
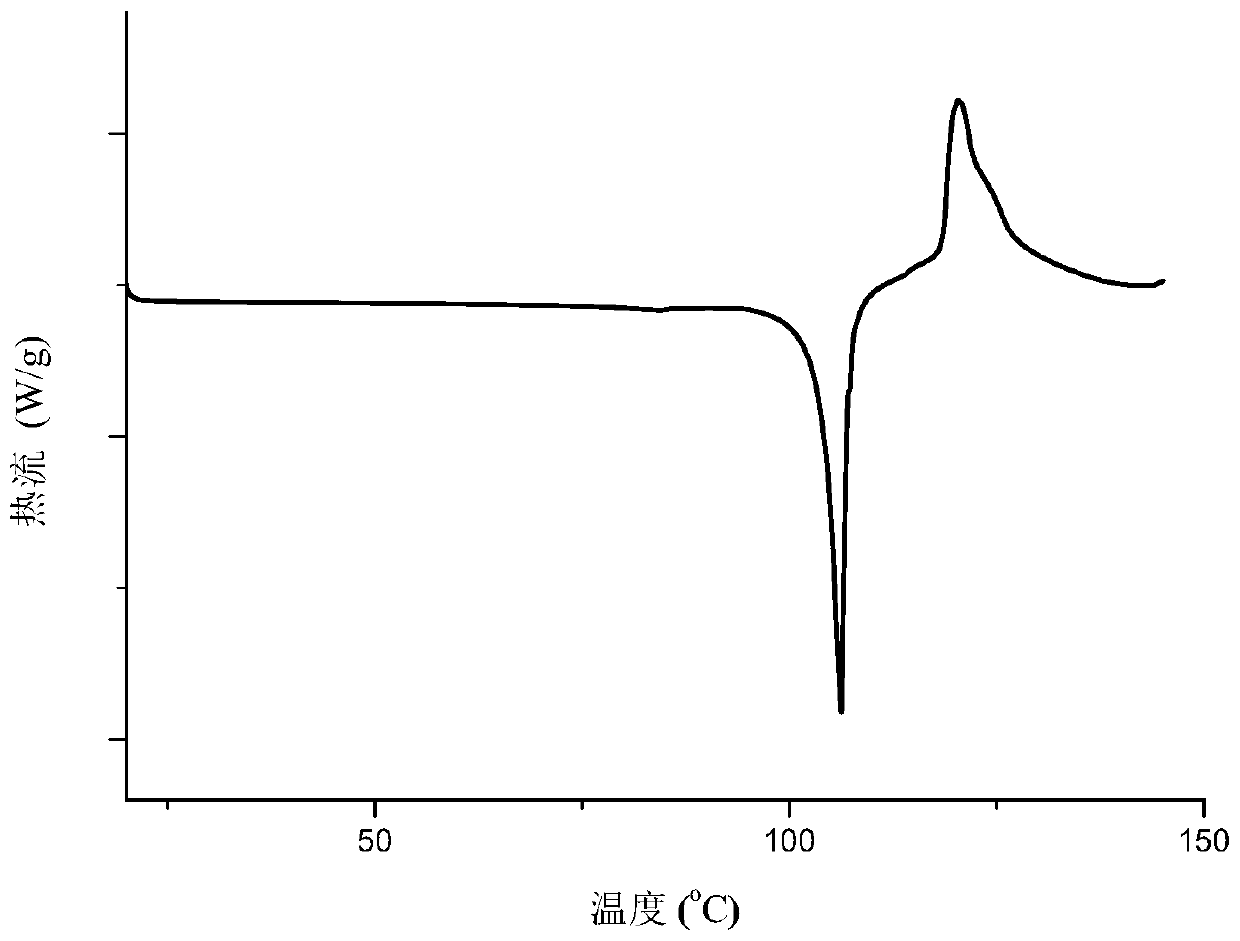
The invention provides a eutectic crystal of nicorandil and salicylic acid as well as a preparation method and application of the eutectic crystal. According to the invention, the eutectic crystal ofnicorandil and salicylic acid is comprehensively characterized by using analysis means such as X-ray diffraction analysis, thermogravimetric analysis, differential scanning calorimetry analysis, infrared spectroscopy and the like. Through stability test and comparison, the eutectic crystal has more excellent damp-heat stability compared with a nicorandil active pharmaceutical ingredient, and the safety, effectiveness and quality controllability of the medicine are improved.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Preparation method of nikkodil
The invention belongs to the technical field of medicine synthesis, and particularly relates to a preparation method of nikkodil. The preparation method includes: performing a reaction on nitric acidwith N-(2-hydroxyethyl) nicotinamide under the action of a dehydrating agent and a diluent to prepare N-(2-hydroxyethyl) nicotinamide nitrate, wherein the molar ratio of the N-(2-hydroxyethyl) nicotinamide to the nitric acid to the dehydrating agent is 1 : (2-3) : (1-2), and the volume-weight ratio of the diluent to the N-(2-hydroxyethyl) nicotinamide is (5-20) : 1. According to the preparation method of the nikkodil, the reaction process is mild and controllable, the preparation risk is low, the heat release is not obvious, a large amount of acid water is not generated, the safety problem issolved, and the environmental protection pressure is reduced; and the prepared product is high in purity and excellent in quality.
Owner:ZHANGJIAGANG JIULI NEW MATERIAL TECH CO LTD
Nicorandil freeze-drying powder preparation method
ActiveCN100417381CReduce the rate of degradationImprove stabilityOrganic active ingredientsPowder deliveryAntioxidantFreeze-drying
The invention discloses a process for preparing Nicorandil freeze-dried powder, which consists of charging 0.1-50.0 wt% of Nicorandil into water for injection, dissolving excipient simultaneously, degerming and filtering, loading, and finally freeze drying.
Owner:XIAN LIBANG PHARMA TECH
Compositions containing nicorandil, preparation method and use
The present invention provides a composition comprising (i) Nicorandil, and (ii) a lubricant selected from a saturated higher aliphatic acid and its salts and / or a saturated higher alcohol, which is solid at ambient temperature, wherein the lubricant is not micronized; processes for preparing such composition; processes for preparing tablets from such composition; and tablets prepared by such processes.
Owner:AVENTIS PHARMA SA (US)
Anti-angina pharmaceutical composition containing nicorandil
InactiveCN105106163AGood synergyGood anti-anginal effectOrganic active ingredientsDrageesSide effectAngina
The invention discloses an anti-angina pharmaceutical composition containing nicorandil. The anti-angina pharmaceutical composition comprises the nicorandil, beta receptor blocker and pharmaceutically acceptable excipients. The pharmaceutical composition has the advantages that the pharmaceutical composition has good angina and myocardial ischemia resisting effects, drug resistance cannot be caused easily, various adverse reactions of single medicine use can be overcome, toxic and side effects are lowered, and the pharmaceutical composition shows good synergistic effect in angina resisting and the treatment of myocardial ischemia.
Owner:XIAN HANFENG PHARMA
Preparation method of nicorandil for injection
ActiveCN113599353AReduce moistureReduce water of crystallizationOrganic active ingredientsPowder deliveryCitrate sodiumNicorandil
The invention relates to a preparation method of nicorandil for injection. The nicorandil for injection, disclosed by the invention, is prepared from nicorandil, mannitol, sodium citrate and water for injection through the procedures of liquid preparation and freeze drying, and the weight ratio of all the components is as follows: nicorandil: mannitol: sodium citrate: water for injection is 2: 3: 1: (300-600). The preparation method of the nicorandil for injection, disclosed by the invention, comprises the following steps: step 1, liquid preparation, and step 2, freeze-drying, wherein the freeze-drying step further comprises the following steps: step A, pre-freezing, freeze-thawing, crystallizing and annealing, and step B, sublimation stage.
Owner:TIANJIN TASLY ZHIJIAO PHARMA
Nicorandil lipid microsphere preparation and preparation method thereof
ActiveCN110123749AAvoid direct exposureAvoid contactOrganic active ingredientsEmulsion deliveryMedicineMicrosphere
An embodiment of the invention provides a Nicorandil lipid microsphere preparation and a preparation method thereof and relates to the field of drug dosage forms, aiming to solve the technical problemthat an existing Nicorandil dosage form is not high in stability. The Nicorandil lipid microsphere preparation comprises Nicorandil, oil for injection, an emulsifier and water for injection, whereinthe Nicorandi is wrapped in the oil for injection in the size of 10-900nm, and the oil for injection is dispersed in the water for injection.
Owner:太阳升(亳州)生物医药科技有限公司
Nicorandil lipid microsphere preparation and preparation method thereof
ActiveCN110123749BAvoid direct exposureAvoid contactOrganic active ingredientsEmulsion deliveryMicrospherePharmaceutical drug
Owner:太阳升(亳州)生物医药科技有限公司
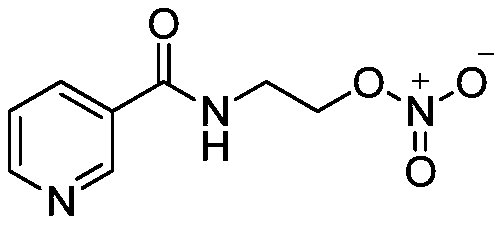
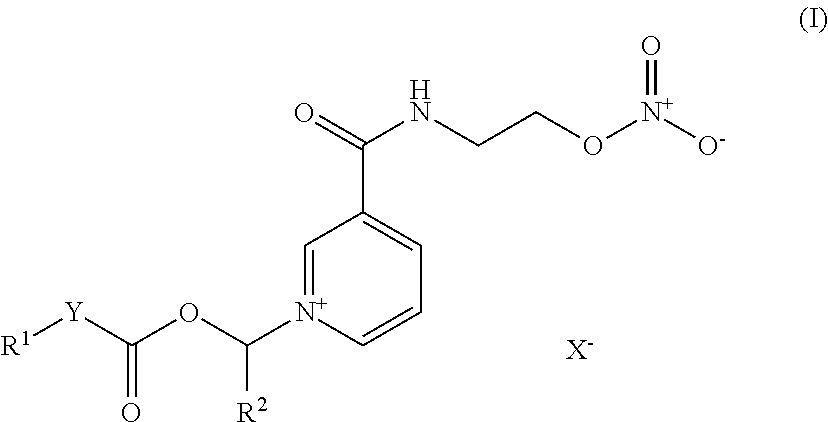
Nicorandil derivatives
Disclosed herein are pyridyl compounds. Also described are specific conjugated nicorandil compounds. Also disclosed are pharmaceutical compositions that include the compounds. Methods of using the pyridyl compounds are disclosed for the treatment of diseases or conditions related to kidney or kidney functions.
Owner:UNICYCIVE THERAPEUTICS INC
Sustained-release preparation of nicorandil
InactiveCN1994283AImprove securityImprove effectivenessOrganic active ingredientsInorganic non-active ingredientsMedicineCurative effect
The invention relates to a slow-release agent of Nicotie and relative production, wherein it uses Nicotie as material, adds slow-release skeleton material and drug to obtain solid disperser; releases the drug to hold blood density. The invention can reduce feeding time, reduce blood peak-valley condition, improve safety and reduce drug consumption.
Owner:刘凤鸣
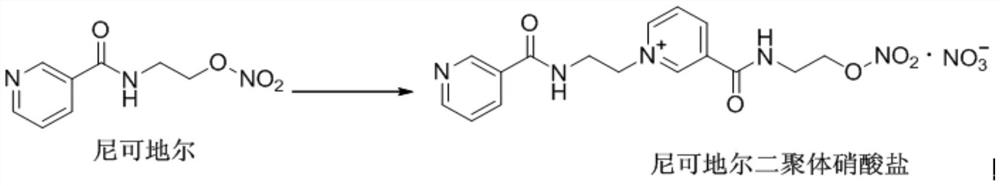
A kind of preparation method of nicorandil dimer
The invention provides a preparation method of nicorandil dimer, comprising the following steps: (1) dissolving nicorandil in an organic solvent, stirring and reacting for 2‑6 h; (2) cooling down slowly to Slowly add a crystallization solvent to the reaction solution at room temperature, stir to crystallize, filter to obtain the crude product, and wash with an organic solvent; (3) Add the above crude product to the recrystallization solvent, and after recrystallization, solid Nicorandil is obtained Dimeric nitrate. The preparation method of the nicorandil dimer disclosed by the invention can conveniently and rapidly obtain the nicorandil dimer, and the obtained nicorandil dimer has a high yield and good purity.
Owner:BURNING POINT (NANJING) BIOPHARMACEUTICAL TECH CO LTD
A kind of preparation method of nicorandil trimer
The invention provides a preparation method of a nicorandil trimer, which comprises the following steps: (1) dissolving nicorandil in an organic solvent, stirring and reacting for 4‑7 h; Add the crystallization solvent dropwise to the liquid, slowly cool to room temperature, stir and crystallize, filter to obtain the crude product, and wash with organic solvent; (3) Add the above crude product into the recrystallization solvent, and after recrystallization, solid Nicorandil is obtained Trimeric nitrate. The preparation method of the nicorandil trimer disclosed by the invention can conveniently and quickly obtain the nicorandil trimer, and the obtained nicorandil trimer has a high yield and good purity.
Owner:BURNING POINT (NANJING) BIOPHARMACEUTICAL TECH CO LTD
Nicorandic drop pill and preparing method thereof
InactiveCN1528301ADisintegration and dissolution fastHigh dissolution rateOrganic active ingredientsPill deliveryDissolutionTherapeutic effect
The present invention utilizes ultramicropulverization and dripping pill preparation production process to make nicorandil dripping pills, and can attain the goal of raising disintegration and dissolution speed, quickly obtaining therapeutic effect, raising production cost and convenient administration. Said pill not only can be sucked, but also can be swallowed, and its compliance property is good.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Nicorandil freeze-dried injection and preparation method thereof
The invention relates to a stable freeze-drying nicorandil powder injection and a preparation method thereof. In the process of preparing the freeze-drying nicorandil powder injection, acidifying or alkalizing agent is adopted to regulate the PH value of intermediate solution for the pH value to range from 5.0 to 8.0, and secondary sublimation temperature is controlled in the process of freeze drying for the temperature to range from 5 DEG C to 20 DEG C, and thereby, the medicament content and the stability of related substances are improved. The invention overcomes the deficiencies in the prior art; and a stable freeze-drying nicorandil powder is obtained, and the freeze-drying powder injection has good clinical efficacy.
Owner:BEIJING SIHUAN KEBAO PHARM CO LTD
Preparation method of Nicodil
InactiveCN113501784AEase of industrial productionRapid responseOrganic chemistryAcetic anhydrideBiochemical engineering
The invention discloses a preparation method of Nicorandil, the preparation method comprises the following steps: directly synthesizing N-(2-ethoxyl) nicotinamide by taking nicotinamide as an initial raw material through a transamidation method, and nitrifying by using a mixture of non-fuming nitric acid, acetic anhydride and concentrated sulfuric acid to obtain Nicorandil. The method simplifies the existing synthesis process, is easy and convenient to operate and is more suitable for large-scale production; meanwhile, the yield of products obtained in each step of reaction is increased, the types and the dosage of reaction reagents are greatly reduced, and the production cost is lower. The technical problems of more reaction steps, high reagent consumption, low production efficiency, serious environmental pollution, poor quality of the prepared product and low yield of the existing preparation process are solved.
Owner:HAIKOU TIANXINGJIAN PHARMA RES CO LTD
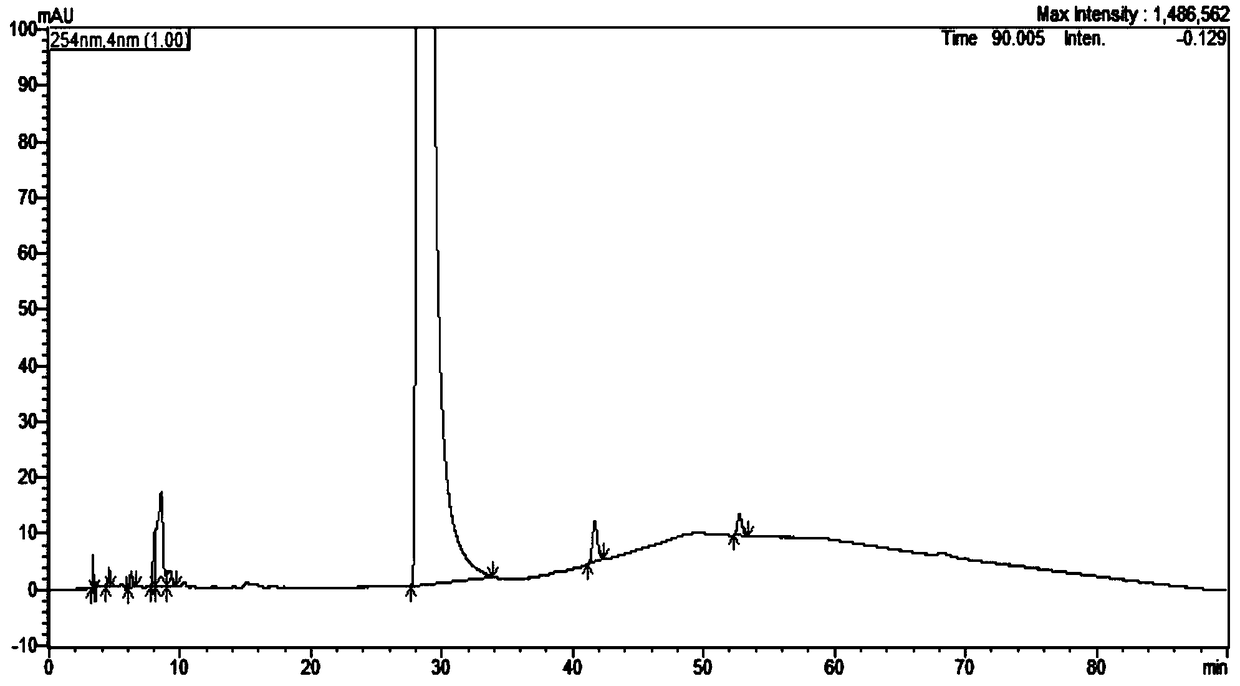
Method for determining content of phorone in Nicodil by gas chromatography-mass spectrometry
PendingCN113552233AHigh penetration rateReduce use costComponent separationMass Spectrometry-Mass SpectrometryVolumetric flask
The invention discloses a method for determining the content of phorone in Nicodil by gas chromatography-mass spectrometry, which comprises the following steps of: 1, preparing a reference substance solution, precisely weighing a phorone reference substance, putting the phorone reference substance into a volumetric flask, dissolving the phorone reference substance to a scale by using a diluent, and uniformly shaking; 2, preparing a test solution, precisely weighing a dinitrandil raw material medicine, putting the dinitrandil raw material medicine into a volumetric flask, dissolving the dinitrandil raw material medicine to a scale by using a diluent, and uniformly shaking; 3, taking the diluent as a blank sample, and placing the diluent in a volumetric flask; and 4, precisely measuring the test solution and the reference solution, performing sample introduction, performing separation analysis by adopting a gas chromatograph-mass spectrometer, and recording a spectrogram. The detection method provided by the invention is verified by a test, and a result shows that the method is strong in specificity, good in precision and high in accuracy, and can be used for rapidly determining the content of the phorone in the Nicodil.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
Eutectic crystal of nicorandil and 1-hydroxy-2-naphthoic acid, and preparation method and application thereof
ActiveCN111606847AGood chemical stabilityEasy to prepareOrganic active ingredientsOrganic chemistry methodsHeat stabilityPharmaceutical Substances
The invention relates to a eutectic crystal of nicorandil and 1-hydroxy-2-naphthoic acid, and a preparation method and application thereof. The eutectic crystal of the nicorandil and the 1-hydroxy-2-naphthoic acid is comprehensively characterized by applying analysis means such as X-ray diffraction analysis, thermogravimetric analysis, differential scanning calorimetry analysis, infrared spectroscopy and the like. Through stability test and comparison, the eutectic crystal has more excellent damp-heat stability compared with a nicorandil raw material medicine, and the safety, effectiveness andquality controllability of the medicine are improved.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com