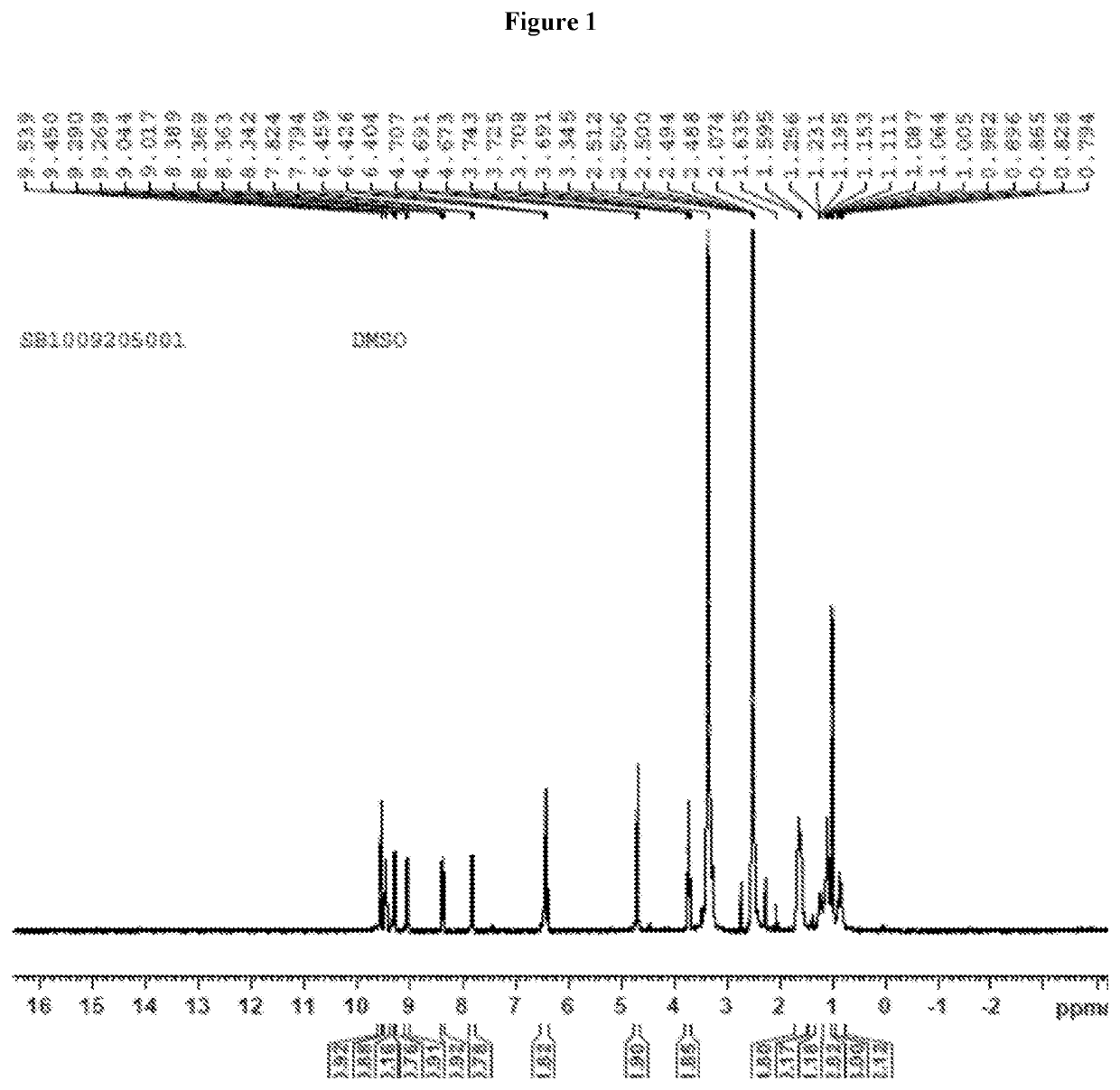
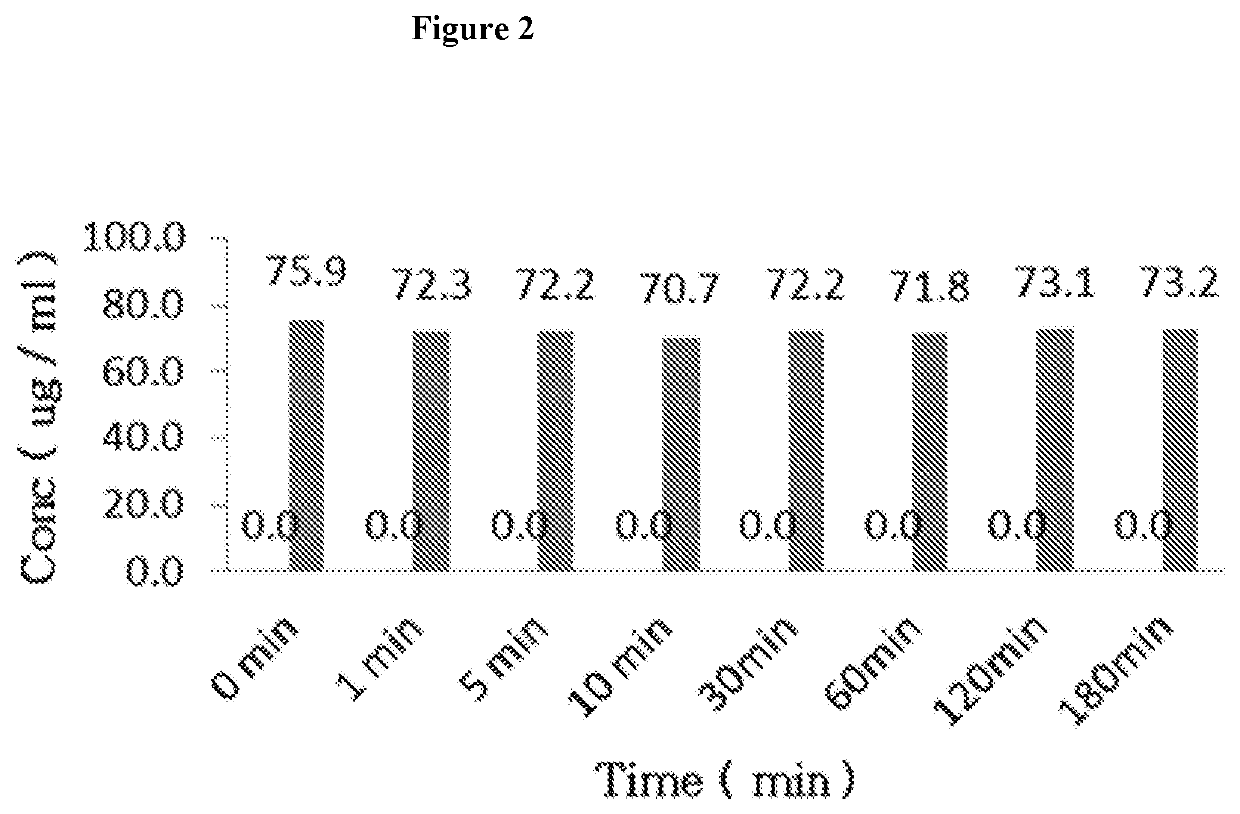
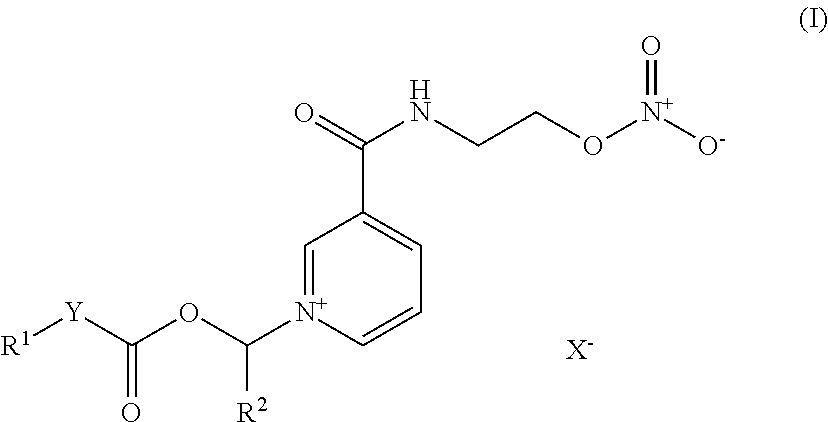
Nicorandil derivatives
a technology of nicorandil and derivatives, applied in the field of compound, pharmaceutical composition and medicaments, can solve the problems of increased mortality, hypertension and cardiovascular disease, and death of patients with ckd
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 1-(((3,3-dimethylbutanoyl)oxy)methyl)-3-((2-(nitrooxy)ethyl)carbamoyl)pyridin-1-ium iodide (Compound 1)
[0434]
[0435]To a solution of nicorandil, (2-(nicotinamido)-ethylnitrate) (0.23 mmol) in acetonitrile (3 mL) was added iodomethyl isopropyl carbamate (0.28 mmol) dropwise. The resulting mixture was stirred overnight at RT. The progress of the reaction was monitored by TLC. The excess of acetonitrile was removed under vacuum and the resulting residue was dissolved in MeOH and washed with an excess of diethyl ether. This process was repeated twice and the solvent was evaporated under vacuum to get titled compound 1 as a yellow sticky solid.
[0436]m / z 340.1 (M+).
example 2
Preparation of 3-((2-(nitrooxy)ethyl)carbamoyl)-1-(((piperidine-1-carbonyl)oxy)methyl)pyridin-1-ium iodide (Compound 2) (Dugar et al. U.S. Pat. No. 9,359,376)
[0437]
[0438]To a solution of nicorandil, (2-(nicotinamido)-ethylnitrate) (0.56 mmol) in acetonitrile (5 mL) was added iodomethyl 1-piperidinyl carbamate (0.68 mmol) dropwise. The resulting mixture was stirred overnight at RT. The progress of the reaction was monitored by TLC. The excess of acetonitrile was removed under vacuum and the resulting residue was dissolved in MeOH and washed with an excess of diethyl ether. This process was repeated twice and the solvent was evaporated under vacuum to get titled compound 2 as a yellow solid (0.230 g, 84%). (mp 117-120° C.)
[0439]m / z 353.2 (M+)
example 3
Preparation of 1-(((diisopropylcarbamoyl)oxy)methyl)-3-((2-(nitrooxy)ethyl)carbamoyl)pyridin-1-ium iodide (Compound 3)
[0440]
[0441]To a solution of nicorandil, (2-(nicotinamido)-ethylnitrate) (0.71 mmol) in acetonitrile (5 mL) was added iodomethyl diisopropylcarbamate (0.71 mmol) dropwise. The resulting mixture was stirred overnight at RT. The progress of the reaction was monitored by TLC. The excess of acetonitrile was removed under vacuum and the resulting residue was dissolved in MeOH and washed with an excess of diethyl ether. This process was repeated twice and the solvent was evaporated under vacuum to get titled compound 3 as a yellow solid (0.220 g, 85%). (mp 122-125° C.).
[0442]m / z 369.2 (M+)
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com