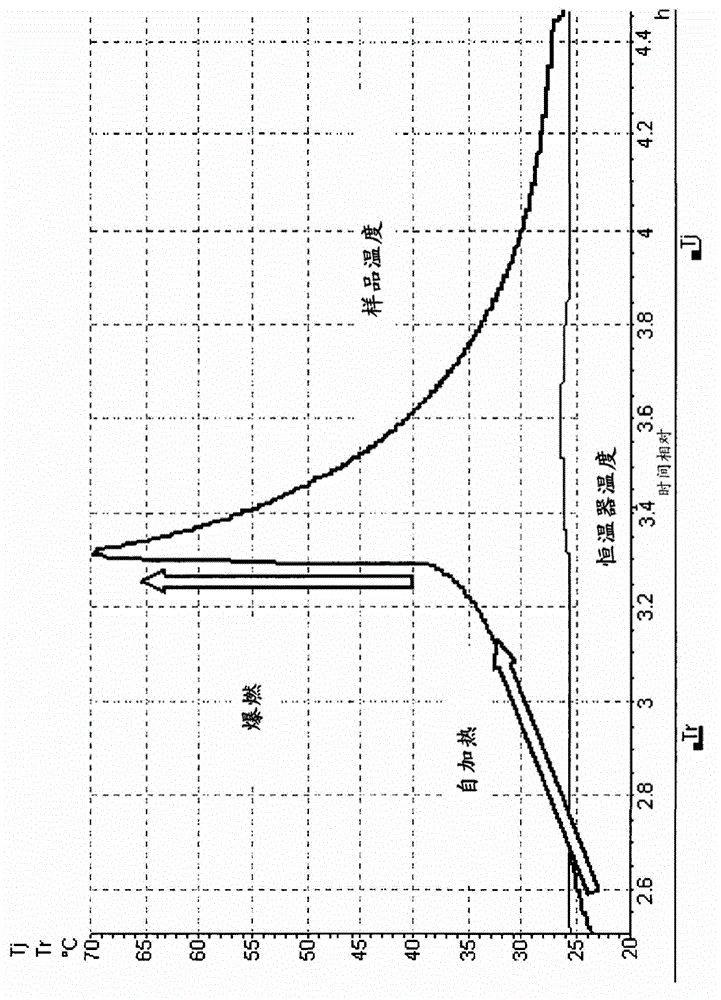
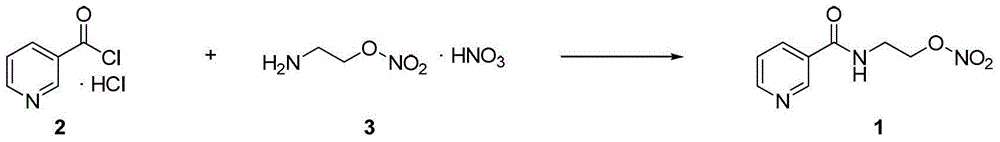Process for the manufacture of nicorandil
A nicorandil and molar ratio technology, applied in the field of preparing nicorandil, can solve the problems of large processing volume, unfavorable economic and industrial safety, safety hazards and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Synthesis of Nicorandil
[0058] 6.00 g of glacial acetic acid was charged into a 100 mL three-necked reaction flask equipped with an anchor-shaped magnetic stirrer, a temperature probe, and a dropping funnel, and then 1.35 g of 68% nitric acid was added. The system was kept constant at a temperature of 22°C, and 4.00 g of acetic anhydride was added in about 15 minutes. Stir the system for about 15 minutes; then add 1.00 g of N-(2-hydroxyethyl)nicotinamide in ≈15 minutes at a temperature not exceeding 22°C. The system was stirred at 22°C for 30 minutes, and then the progress of the reaction was checked by HPLC. N-(2-hydroxyethyl)nicotinamide content <1%. Pour the contents of the flask slowly into 24% ammonia water (14.0g), being careful not to exceed 35°C. Reduce the temperature to 5°C and keep it for about 30 minutes. The solid was separated by filtration and washed with 1 mL of water.
[0059] The solid was dried at 40°C for 48 hours to obtain 1.13 g of crude nicorand...
Embodiment 2
[0061] Initial zoom
[0062] 300 g of glacial acetic acid was charged into a 1000 mL three-necked reaction flask equipped with an anchor-shaped magnetic stirrer, a temperature probe, and a dropping funnel, and then 67.5 g of 68% nitric acid was added. Keep the system at a constant temperature of 18°C, and add 200g of acetic anhydride within about 45 minutes, being careful not to exceed 25°C, and stop adding if it exceeds the threshold. The reaction is very fast, so the reaction can be controlled by adding reagents. Stir the system for about 30 minutes; then cool to 15°C, and add 50.0g of N-(2-hydroxyethyl)nicotinic acid amide in five portions of the same 10.0g each at 15-minute intervals. Take care to restore the temperature to 15°C before. The system was stirred at 22°C for 90 minutes, and then the progress of the reaction was checked by HPLC. N-(2-hydroxyethyl)nicotinamide content <1%. Pour the contents of the flask slowly into 24% ammonia (700g), being careful not to excee...
Embodiment 3
[0065] Synthesis from sulfuric acid / nitric acid mixture (reaction time 90 minutes; temperature 10℃)
[0066] 18.4 g of concentrated sulfuric acid was charged into a 100 mL three-necked reaction flask filled with nitrogen and equipped with an anchor-shaped magnetic stirrer, a temperature probe, and a dropping funnel. The mixture was cooled to 0°C, and then 16.9 g of 68% nitric acid was added dropwise thereto. In view of the strong exothermic reaction, proceed with care. The system was kept at a constant temperature of 10°C, and 10.0 g of N-(2-hydroxyethyl)nicotinamide was added in about 5 minutes to maintain the temperature. The system was stirred for 30 minutes, and then the conversion was checked by HPLC. The residual N-(2-hydroxyethyl)nicotinamide content is 33.5%. The reaction mixture is colorless. The mixture was left at 10°C with stirring for another 1 hour, and then the conversion was checked again. The residual N-(2-hydroxyethyl)nicotinamide content is 25.3%. The reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com