Method of measuring related substances in nicorandil tablets by HPLC (high performance liquid chromatography) correction factor process
A technology of Nicorandil tablets and related substances, which is applied in the field of medicine and can solve the problems of poor understanding of Nicorandil tablets, poor specificity, and inability to effectively separate and analyze them.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
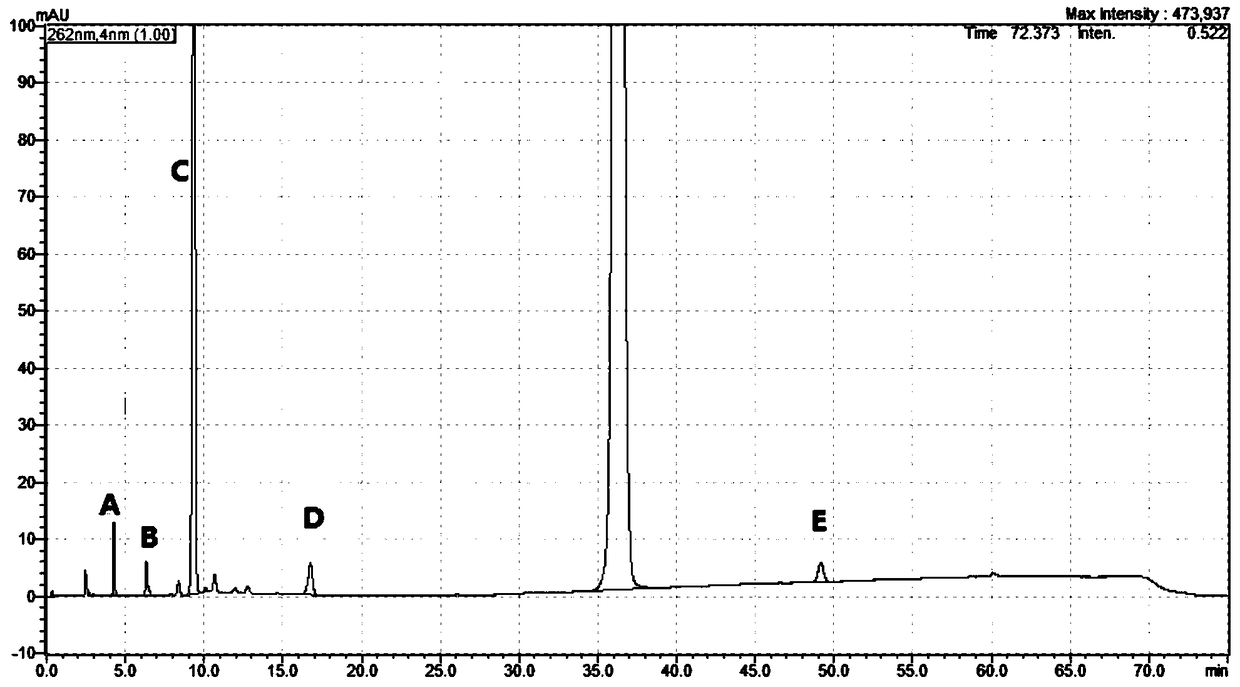
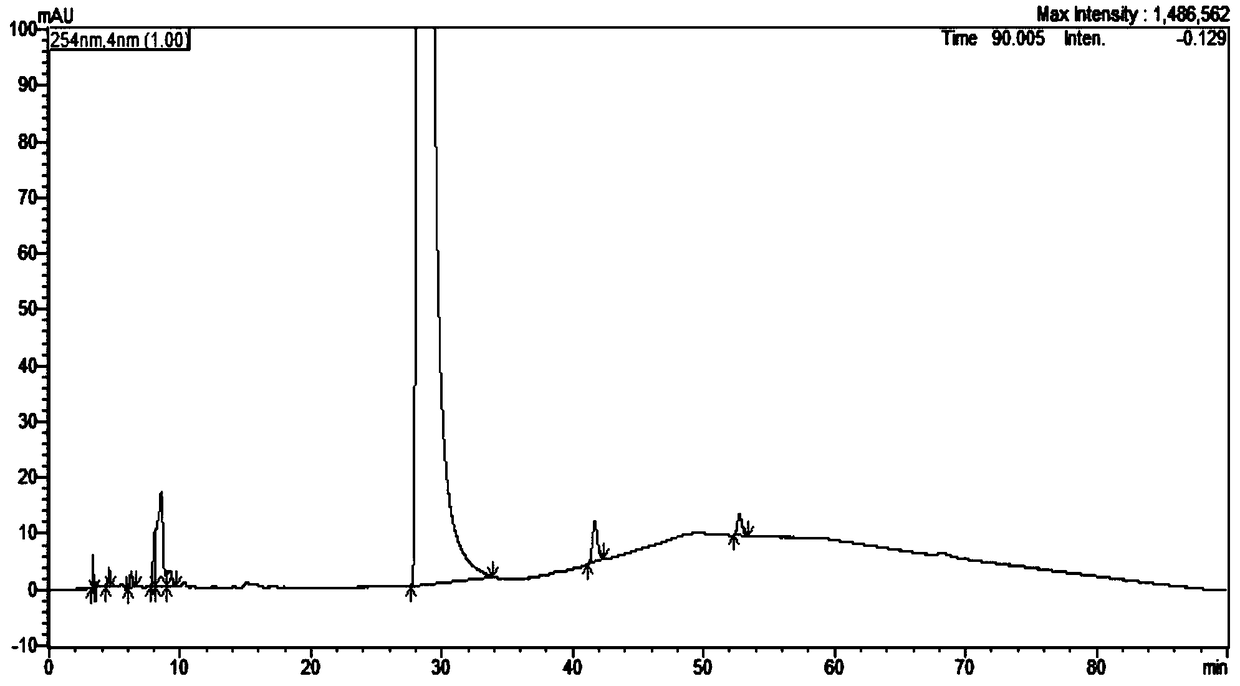
[0058] Example 1: Methodological research on the determination of related substances in Nicorandil tablets using the HPLC correction factor method
[0059] 1. Methods and Results
[0060] Nicorandil reference substance, impurity A reference substance (China Institute for Food and Drug Control), impurity B, C, D working reference substance (self-made), impurity E working reference substance (Canada TLC company), Nicorandil tablet samples All are derived from market sampling.
[0061] The chromatographic conditions adopt Atlantis T3C18 chromatographic column, (4.6×250mm, 5μm), with trifluoroacetic acid-triethylamine-tetrahydrofuran-water (3:5:3:989) as mobile phase A, with water: tetrahydrofuran: triethylamine : Trifluoroacetic acid (972:20:5:3) is the mobile phase B, the gradient elution is carried out in the following table, the flow rate is 1.2ml / min, the column temperature is 25°C, and the detection wavelength is 262nm.
[0062] time (minutes)
Mobile phase A...
Embodiment 2
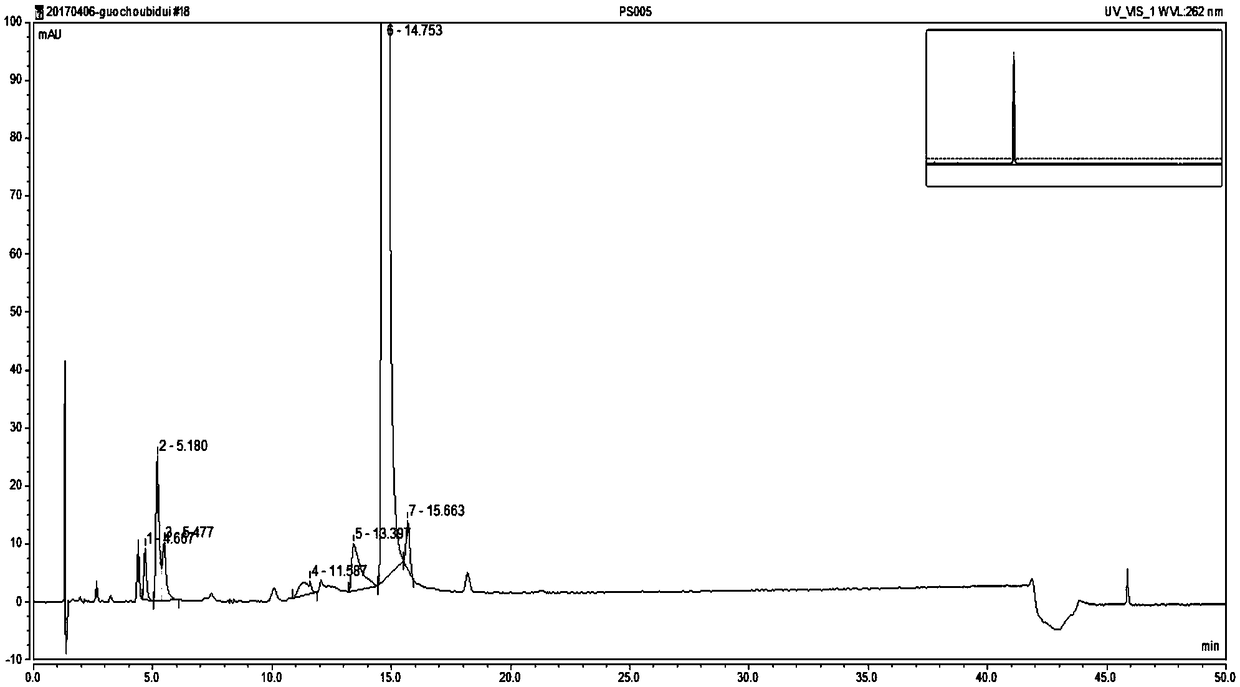
[0083] Embodiment 2: Determination of Nicorandil Tablet Related Substances Using HPLC Calibration Factor Method
[0084] Use high-performance liquid chromatography to detect the related substances in the Nicorandil tablet, and the operation steps are as follows:
[0085] (1) measure according to the specification in the high-performance liquid chromatography that Chinese Pharmacopoeia 2015 edition four general rules 0512 carries;
[0086] (2) Chromatographic conditions and system suitability test: use octadecylsilane bonded silica gel as a chromatographic column (this example uses WatersAtlantis T3 column, 250 × 4.6mm, 5μm), with trifluoroacetic acid-triethylamine - Tetrahydrofuran-water (3:5:3:989) is mobile phase A, water: tetrahydrofuran: triethylamine: trifluoroacetic acid (972:20:5:3) is mobile phase B, and the gradient is shown in the table below The elution program is gradient elution, the flow rate is 1.2ml / min, the column temperature is 25°C, and the detection wave...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com