Nicorandil lipid microsphere preparation and preparation method thereof
A technology of nicorandil grease and nicorandil, which is applied in the field of nicorandil lipid microsphere preparation and its preparation, can solve the problem of low dosage form stability and achieve the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] Corresponding to the aforementioned nicorandil lipid microsphere preparation, an embodiment of the present invention also provides a preparation method of the nicorandil lipid microsphere preparation, and the method includes the following steps.
[0032] Step 1: Add emulsifier and stabilizer to the oil for injection, and mix evenly to obtain an oil phase.
[0033] In this step, the specific process parameters are not limited, and it is better to be able to dissolve and mix uniformly. For example, the formulated amount of oil for injection can be preheated to 60-70°C, and the formulated amount of emulsifier and stabilizer can be added in sequence under stirring, and mixed evenly to form a homogeneous oil phase.
[0034] Step 2: Add Nicorandil into the oil phase and mix evenly to obtain the medicated oil phase.
[0035] This step does not limit the specific process parameters, it is better to be able to mix uniformly. For example, at a temperature of 60-70°C, under high...
Embodiment 1
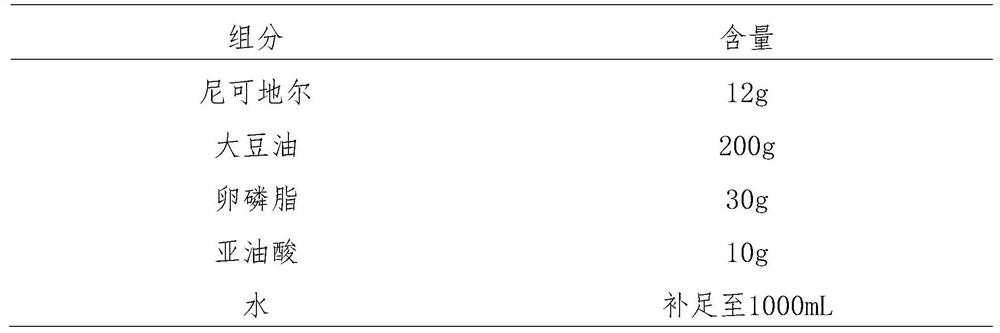
[0042] Nicorandil formulations were prepared in accordance with the following formula quantities.
[0043]
[0044]In a water bath, preheat the formulated amount of soybean oil to 65°C, then add the formulated amount of lecithin under nitrogen protection and stirring, dissolve and add the formulated amount of linoleic acid, and mix evenly to obtain a homogeneous oil phase. Continue to use a water bath to keep the temperature of the homogeneous oil phase at 65°C. Under high-speed stirring at 8000 rpm, add the prescribed amount of Nicorandil raw material into the homogeneous oil phase, and stir for 3 minutes to make it evenly dissolve in the soybean oil , to obtain the drug-containing oil phase. Continue to use a water bath to keep the temperature of the medicated oil phase at 65° C., and add the medicated oil phase to 800 mL of water for injection under high-speed stirring at 8000 rpm. Stir for 10 minutes to obtain evenly dispersed colostrum. Add water for injection to the...
Embodiment 2
[0046] Nicorandil formulations were prepared in accordance with the following formula quantities.
[0047]
[0048] In a water bath, preheat the formulated amount of soybean oil to 65°C, then add the formulated amount of lecithin under nitrogen protection and stirring, dissolve and add the formulated amount of linoleic acid, and mix evenly to obtain a homogeneous oil phase. Continue to use a water bath to keep the temperature of the homogeneous oil phase at 65°C. Under high-speed stirring at 8000 rpm, add the prescribed amount of Nicorandil raw material into the homogeneous oil phase, and stir for 3 minutes to make it evenly dissolve in the soybean oil , to obtain the drug-containing oil phase. Continue to use a water bath to keep the temperature of the medicated oil phase at 65° C., and add the medicated oil phase to 800 mL of water for injection under high-speed stirring at 8000 rpm. Stir for 10 minutes to obtain evenly dispersed colostrum. Add water for injection to th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com