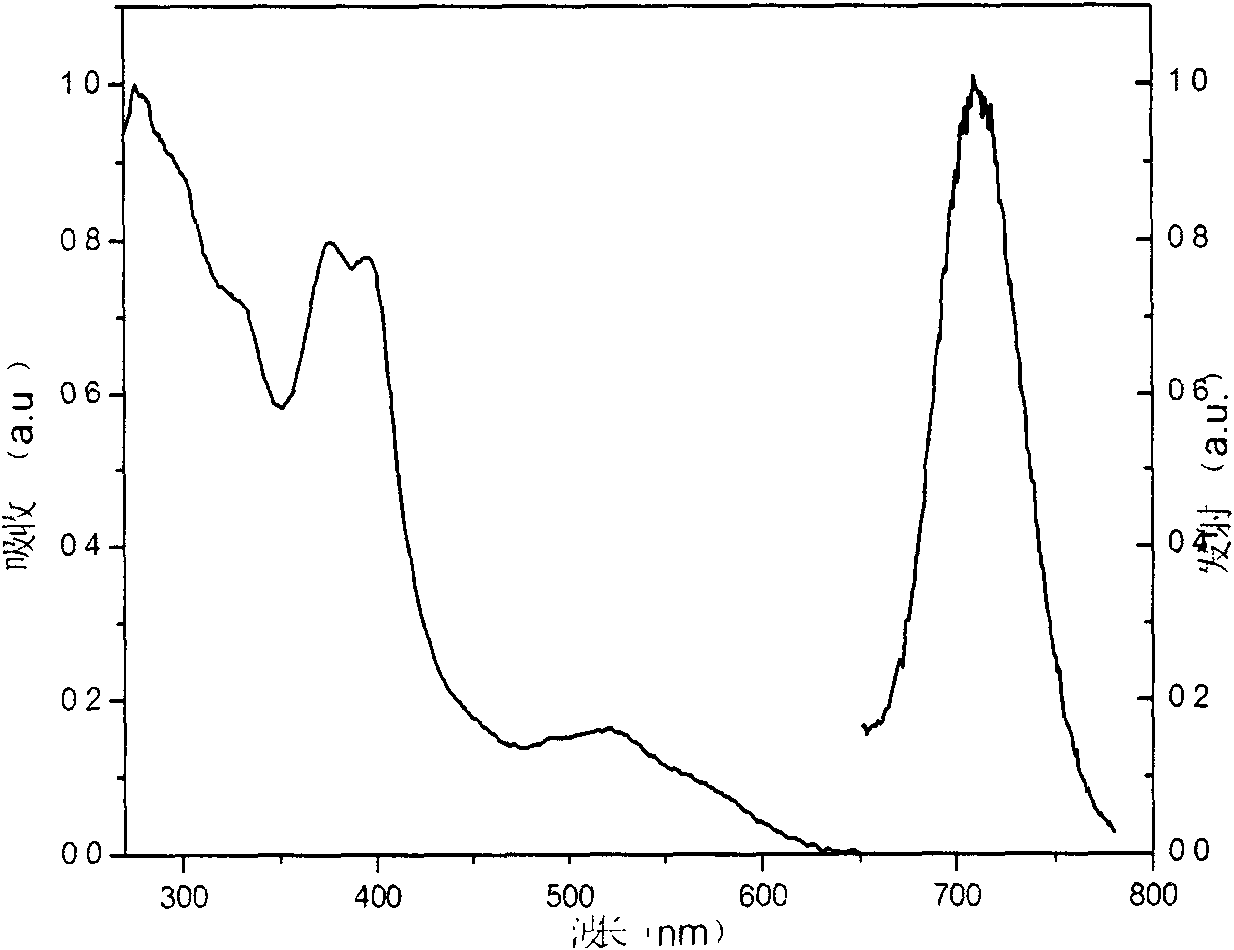
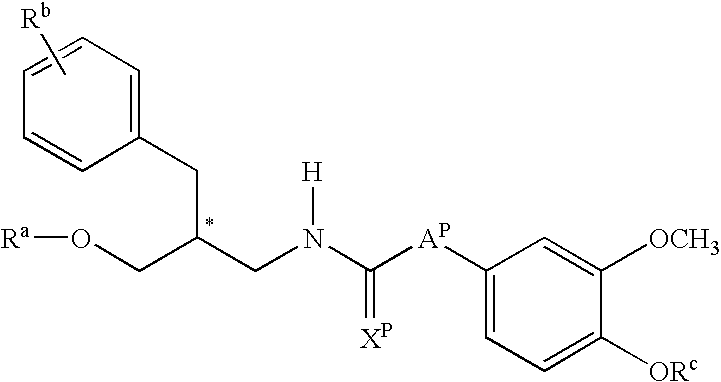
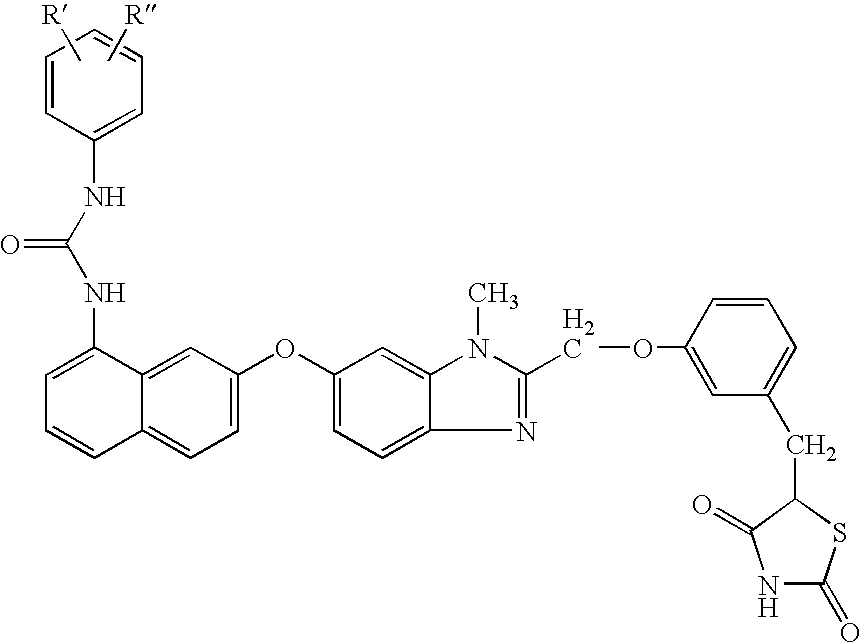
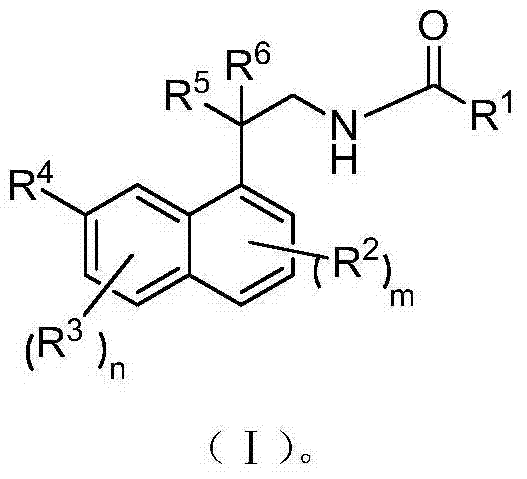
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
171 results about "Naphthalene Derivative" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
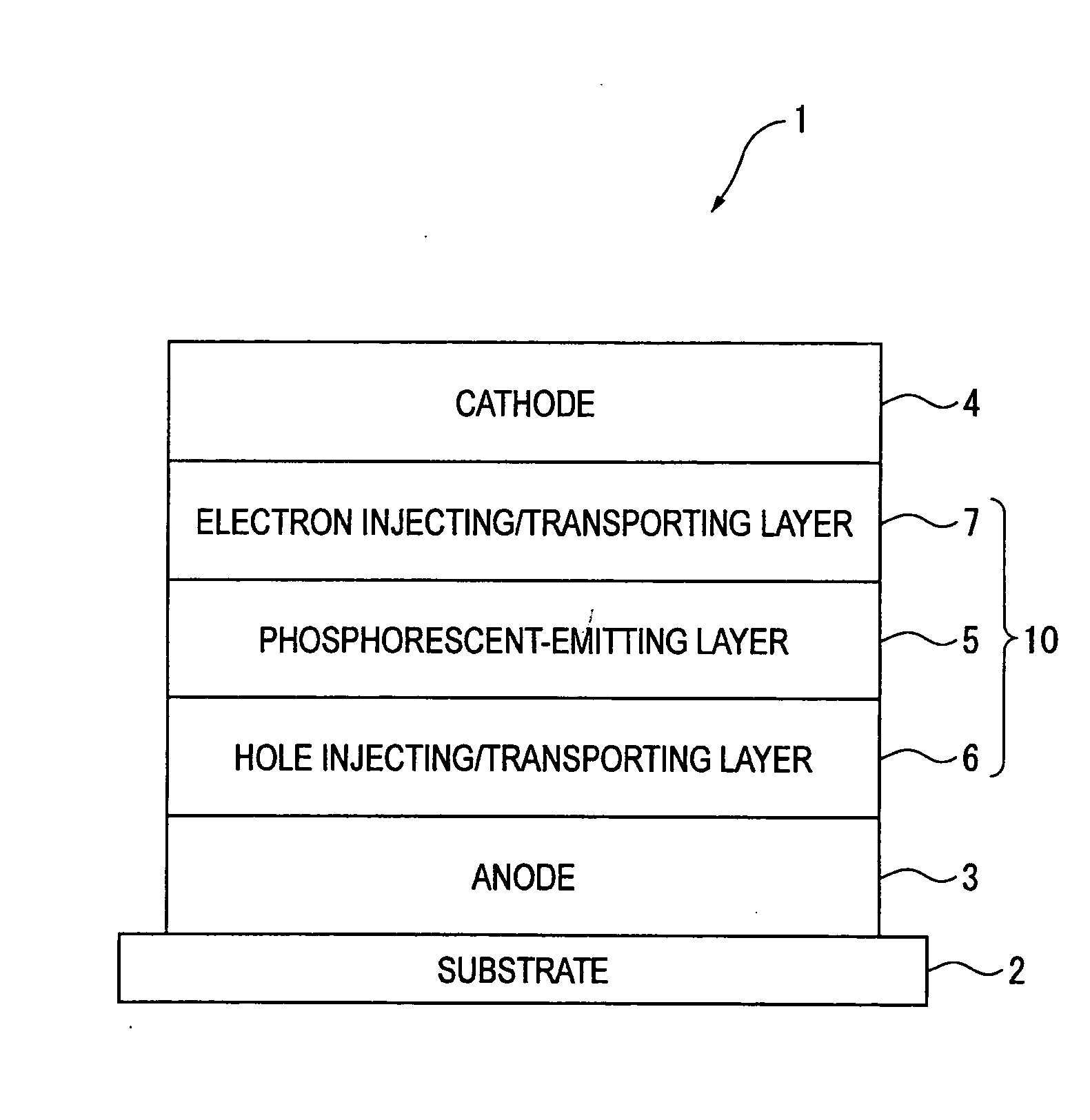
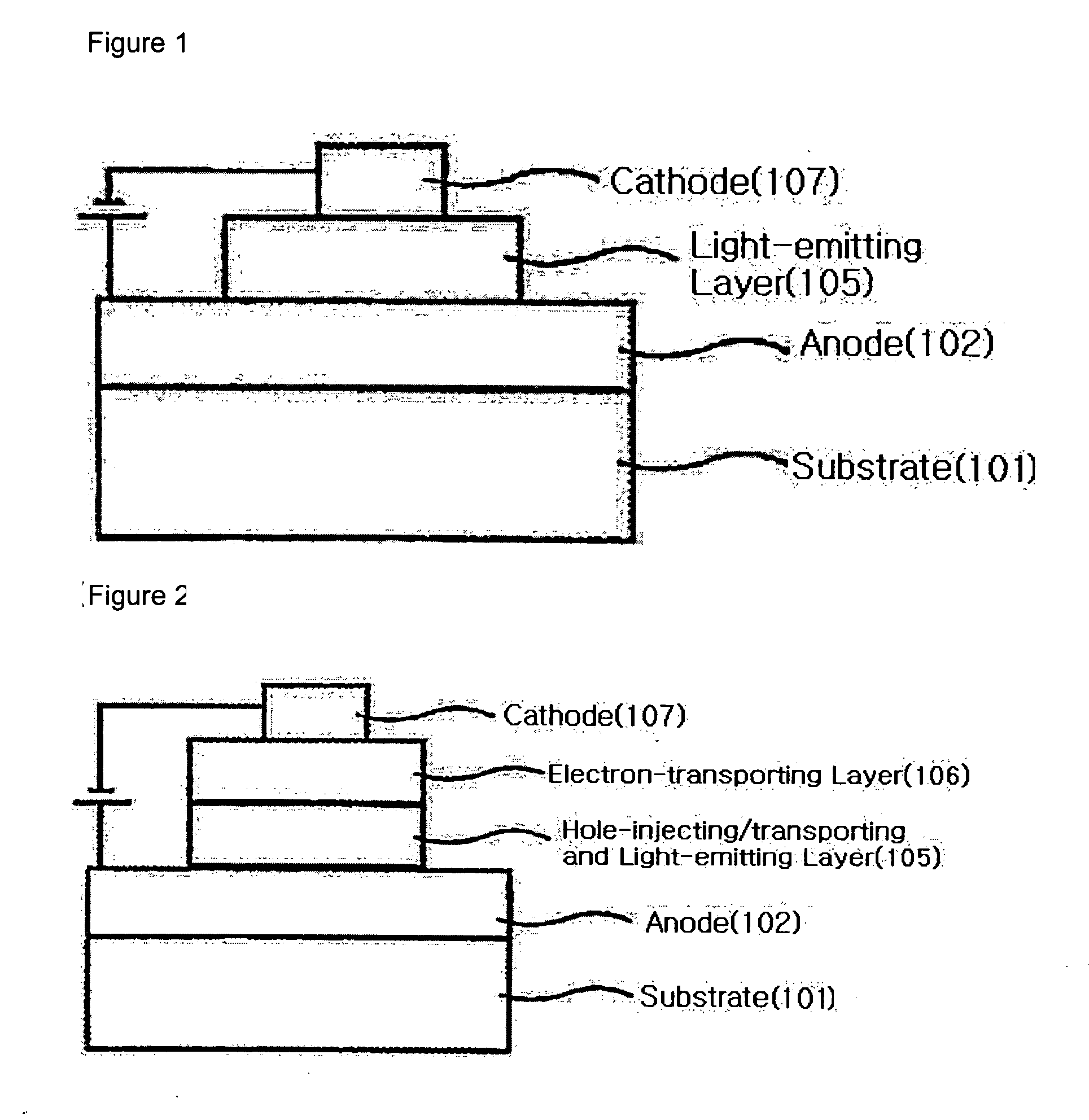
Naphthalene derivative, material for organic electroluminescence device, and organic electroluminescence device using the same
InactiveUS20090008607A1Excellent in luminous efficiency and heat resistance and lifetimeImprove efficiencyOrganic chemistryElectrical apparatusAlkoxy groupOrganic electroluminescence
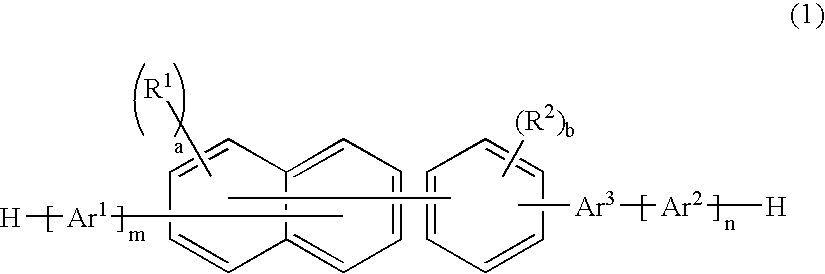
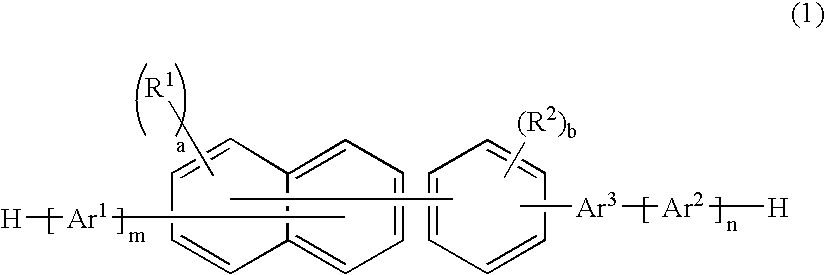
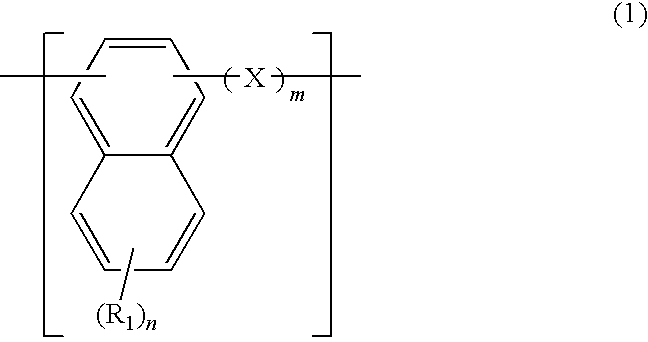
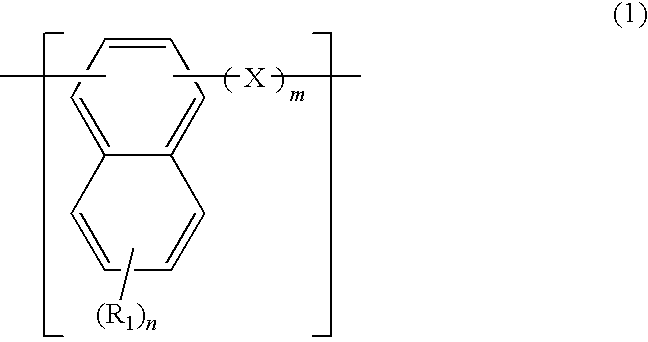
A naphthalene derivative represented by the following formula (1) is provided. In the formula (1), Ar1 and Ar2 each represent an aromatic hydrocarbon cyclic group having 6 to 18 carbon atoms forming a ring. The aromatic hydrocarbon cyclic group has none of anthracene skeleton, pyrene skeleton, aceanthrylene skeleton and naphthacene skeleton. Ar3 represents a benzene skeleton or a naphthalene skeleton. R1 and R2 each represent an alkyl group, a cycloalkyl group, an alkoxy group, a cyano group, a silyl group or a halogen atom. a and b each represent an integer in a range of 0 to 4. m and n each represent an integer in a range of 1 to 4.
Owner:IDEMITSU KOSAN CO LTD
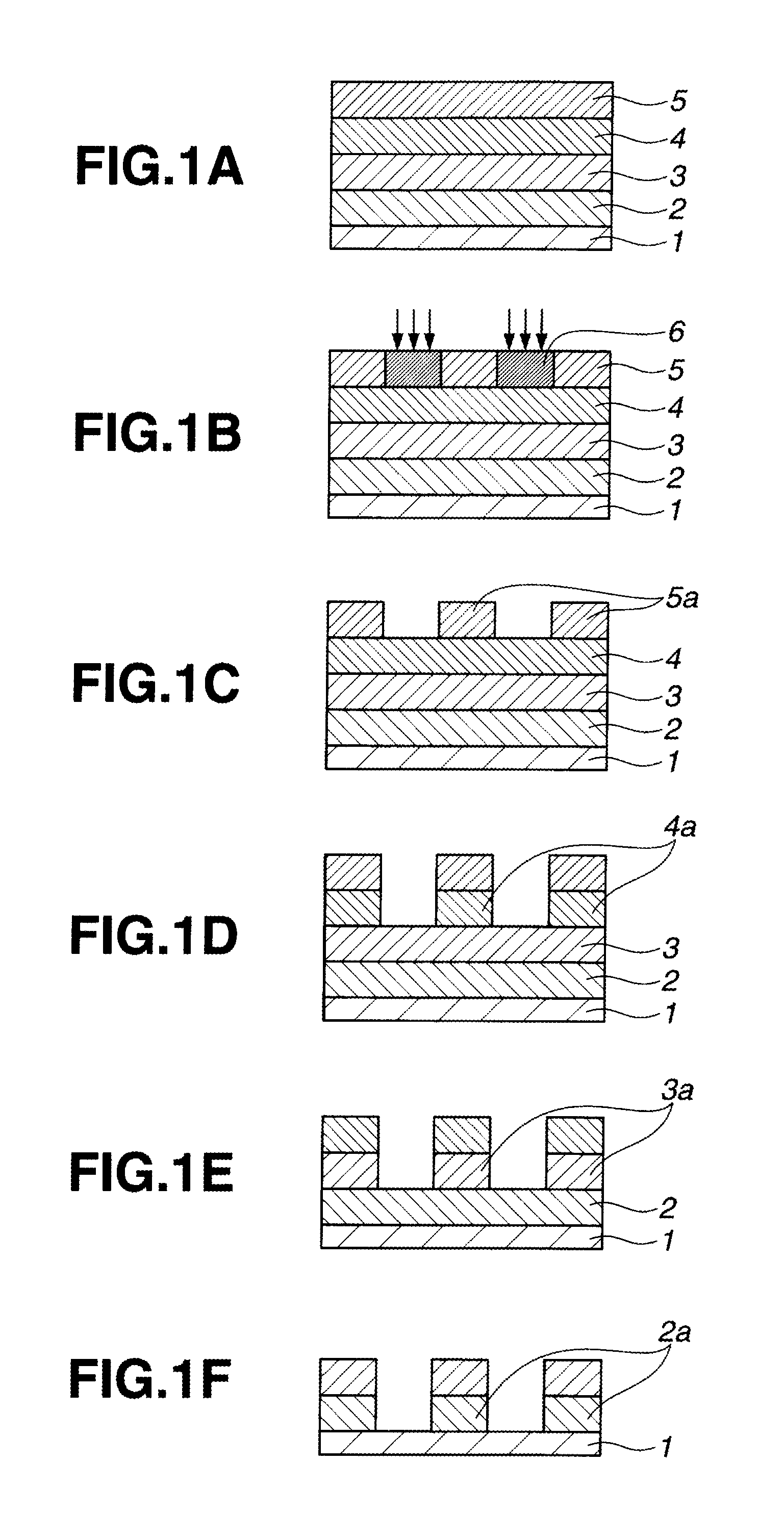
Naphthalene derivative, resist bottom layer material, and patterning process
ActiveUS20120064725A1Excellent etch resistanceMinimizing outgassingOrganic chemistryOrganic compound preparationBenzeneResist
A naphthalene derivative having formula (1) is provided wherein An and Art denote a benzene or naphthalene ring, and n is such a natural number as to provide a weight average molecular weight of up to 100,000. A material comprising the naphthalene derivative or a polymer comprising the naphthalene derivative is spin coated to form a resist bottom layer having improved properties. A pattern forming process in which a resist bottom layer formed by spin coating is combined with an inorganic hard mask formed by CVD is available.
Owner:SHIN ETSU CHEM IND CO LTD
Alkyl or aryl substituted dihydronaphthalene derivatives having retinoid and/or retinoid antagonist-like biological activity
Compounds of the formulawhere the symbols have the meaning described in the application, have retinoid-like or retinoid antagonist-like biological activity.
Owner:ALLERGAN INC
Electroluminescence polymer, organic el device, and display
InactiveUS20070032632A1Stable EL characteristicLess susceptibleDischarge tube luminescnet screensElectroluminescent light sourcesArylPolymer science
A novel electroluminescence polymer offers stable EL characteristics: it forms little aggregates and is less susceptible to morphological changes during and after film formation. The EL polymer comprises a binaphthyl derivative structural unit represented by the following formula (1a) and an aryl structural unit represented by the following formula (1b): wherein Ar is an aryl structural unit that can form an electroluminescent π-conjugated polymer; R1, R2, R3, and R4 are each independently a different functional group; the double bonds of the binaphthyl structural unit indicated by dashed lines and solid lines are each an unsaturated double bond or a saturated single bond; m and p are each independently an integer of 0 to 2; n and o are each independently an integer of 0 to 8; x is the molar fraction of the binaphthyl derivative structural units; and y is the molar fraction of the aryl structural units.
Owner:DEXERIALS CORP
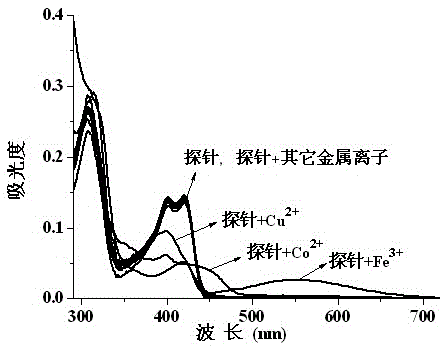
Fluorescent probe reagent for concurrent selection and determination of multiple metal ions, and preparation and appliance
InactiveCN105482812APreparation conditions are easy to controlReduce distractionsOrganic chemistryColor/spectral properties measurementsOrganic synthesisPhotochemistry
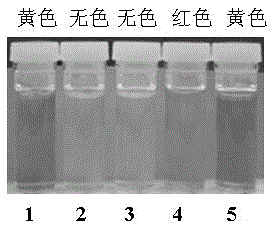
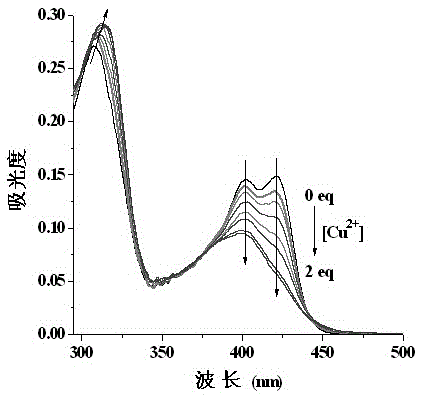
The invention discloses a probe reagent for concurrent selection and determination of multiple metal ions, and preparation and appliance, and belongs to the field of organic synthesis and analytical chemistry. Tri (2-aminoethyl) amine serves as a parent, wherein rhodamine B is connected to an amino chain, 2-hydroxy-1-naphthaldehyde groups are connected to other two amino chains respectively, and thus a tripod structured rhodamine-hydroxyl naphthalene derivative probe is prepared. In 1,4-dioxane / water (19 / 1, v / v, pH=7) solution, the probe respectively detects Cu2+, Co2+ and Fe3+ by utilizing rate absorption of different wavelengths, and the detection does not interfere with each other; in acetonitrile / water (19 / 1, v / v) solution, fluorescence emission of different wavelengths under different pHs is utilized, the probe respectively detects Zn2+, Al3+, Hg2+ and Cu2+, and the detection does not interfere with each other; under an ultraviolet lamp of 365 nm, Zn2+, Al3+ and Hg2+ are detected to show blue, pink and orange red fluorescence respectively, and through -Zn2+ mixture detection by the probe, Cu2+ shows blue fluorescence vanishing. The probe structure is as follows.
Owner:GUIZHOU UNIV
Novel titanium dioxide-graphene nano-composite material as well as manufacturing method and application thereof
The invention relates to a method for preparing graphene, a method for carrying out graphene surface modification by taking one or more than two of pyrene, pyrene derivatives, naphthalene and naphthalene derivatives as a modifying agent as well as a method for manufacturing TiO2-graphene nano-composite material by utilizing the reaction of the surface-modified graphene and a titanium-containing compound and a product thereof. The method for preparing graphene comprises the steps of (a1) reducing dispersive graphite oxide in inert solvent by using a reducing agent to form a graphene-containing reaction mixture, wherein the reducing agent comprises sodium borohydride, vitamin C or combination thereof; and (a2) isolating graphene from the reaction mixture. The obtained graphene is flocculent and the TiO2-graphene nano-composite material has efficient photocatalytic performance.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Binaphthalene derivatives, preparation method thereof and organic electronic device using the same
ActiveUS20070108892A1Increase the driving voltageImprove stabilityOrganic chemistryDischarge tube luminescnet screensElectron injectionOrganic light emitting device
The present invention relates to a new binaphthalene derivative, a preparation method thereof, and an organic electronic device using the same. The binaphthalene derivative according to the present invention can perform functions of hole injection and transportation, electron injection and transportation, or light emission in an organic electronic device including an organic light-emitting device, and the device according to the present invention has excellent characteristics in terms of efficiency, drive voltage and stability, and in particular excellent effects such as a low voltage and a long life time.
Owner:LG CHEM LTD
Naphthalene derivative, resist bottom layer material, resist bottom layer forming method, and patterning process
ActiveUS20110311920A1Excellent etch resistanceImprove heat resistanceOrganic chemistryOrganic compound preparationResistBenzene
A naphthalene derivative having formula (1) is provided wherein cyclic structures Ar1 and Ar2 denote a benzene or naphthalene ring, X is a single bond or C1-C10 alkylene, m is 0 or 1, and n is such a natural number as to provide a molecular weight of up to 100,000. A material comprising the naphthalene derivative or a polymer comprising the naphthalene derivative is spin coated to form a resist bottom layer having improved properties. A pattern forming process in which a resist bottom layer formed by spin coating is combined with an inorganic hard mask formed by CVD is available.
Owner:SHIN ETSU CHEM IND CO LTD
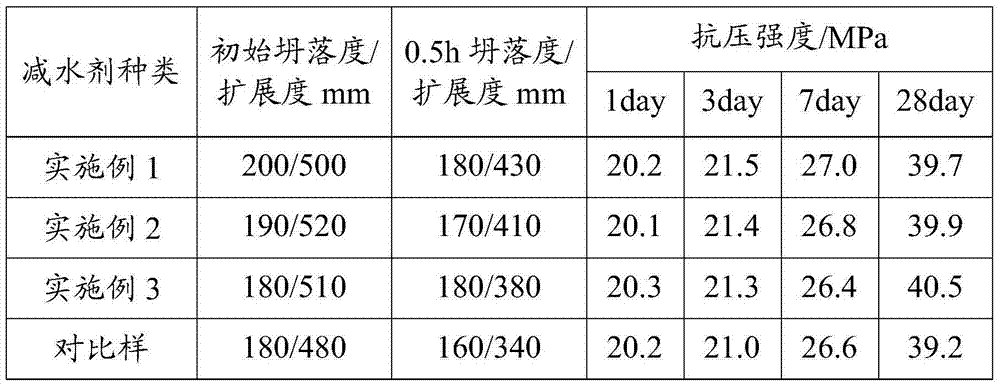
Preparation method of slump loss resistant naphthalene water reducer
ActiveCN103864332AEnhanced controllability of molecular structureSave resourcesSodium metasilicateOrganic solvent
The invention discloses a preparation method of a slump loss resistant naphthalene water reducer. The naphthalene water reducer is prepared by sulfonating, hydrolyzing, condensing and neutralizing raw materials including crude naphthalene, naphthalene derivatives such as methylnaphthalene, concentrated sulfuric acid, formaldehyde, caustic soda liquid, water and the like. According to the production process, monomer substituents, such as methylnaphthalene and acenaphthene are introduced, an organic solvent azeotropic method is adopted for synthesizing, and finally sodium metasilicate is adopted for neutralizing to adjust pH to 7-10 to obtain the final naphthalene water reducer. The slump loss resistant naphthalene water reducer is prepared by the implementation mode disclosed by the invention, the crude naphthalene is replaced by partial naphthalene derivatives, an effective path of reducing the cost is proposed, naphthalene steam and a solvent are condensed to form a naphthalene solution to be recycled, and thus the product yield is improved and the environmental pollution is reduced.
Owner:SHAANXI KZJ NEW MATERIALS
Method for the production of coated particles
InactiveUS6358562B1Easy to wearIncrease resistancePigmenting treatmentPretreated surfacesMicrowaveFerrocene
A method for producing coated particles includes the steps of converting particles consisting of a compound of one of (a) a metal with a non-metal or (b) a semi-metal with a non-metal to an aerosol form; contacting the particles in aerosol form with a gas including at least one aromatic compound; and guiding the particles in aerosol form together with the gas through a plasma zone of a microwave plasma. The at least one aromatic compound is preferably selected from the group consisting of benzol, benzol derivatives, naphthalene, and naphthalene derivatives. The gas preferable further includes at least one metallocene which is preferably selected from the group consisting of ferrocene or magnesocene.
Owner:KERNFORSCHUNGSZENTRUM KARLSRUHE GMBH
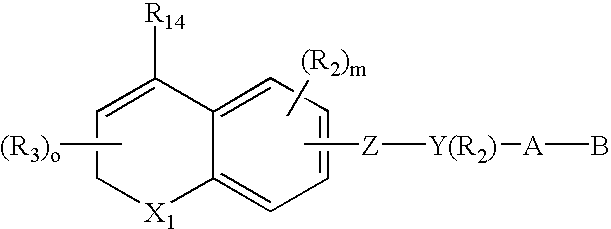
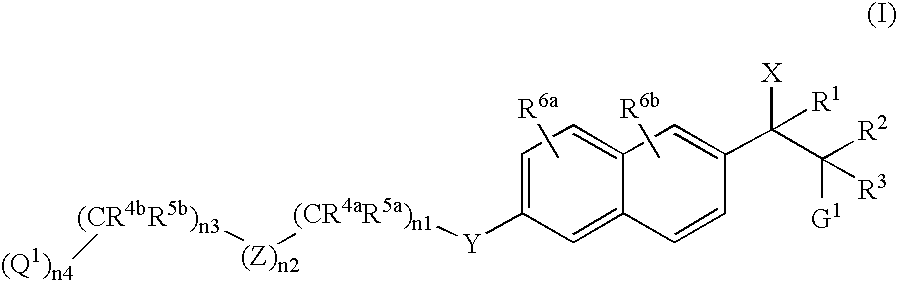
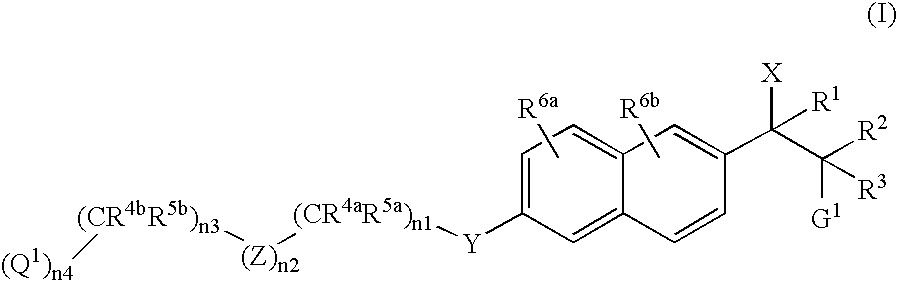
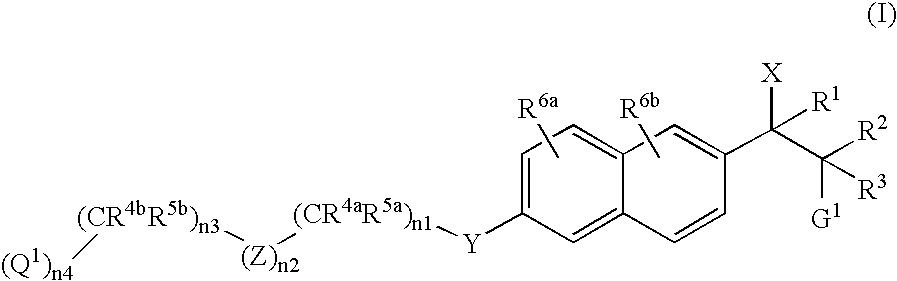
Naphthylene derivatives as cytochrome P450 inhibitors
Compounds of the formula and pharmaceutically acceptable salts thereof, wherein n1, n2, n3, n4, G1, Q1, Z, R1, R2, R3, R4a, R4b, R5a, and R5b are defined herein, inhibit the cytochrome P450RAI enzyme and are useful for the treatment and / or prevention of various diseases and conditions which respond to treatment by retinoids and by naturally occurring retinoic acid.
Owner:OSI PHARMA INC
Optically active quaternary ammonium salt having axial asymmetry and process for producing alpha-amino acid and derivative thereof with the same
ActiveUS20070161624A1Simple structureEasy to provideBiocideOrganic compound preparationMedicinal chemistryAlpha amino acid
The present invention provides a compound of the following formula (I) below. This compound (I) can be produced by reacting a 2,2′-dimethylene bromide-1,1′-binaphthyl derivative, which can be produced by a relatively small number of processes, with an easily available secondary amine. This compound (I) is useful as a chiral phase-transfer catalyst.
Owner:KISHIDA CHEM
O- or S- substituted tetrahydronaphthalene derivatives having retinoid and/or retinoid antagonist-like biological activity
InactiveUS6344561B2Avoid infectionReduce inflammationOrganic active ingredientsBiocideRetinoidDrug biological activity
Compounds of the formulawhere the symbols have the meaning described in the application, have retinoid-like or retinoid antagonist-like biological activity.
Owner:ALLERGAN INC
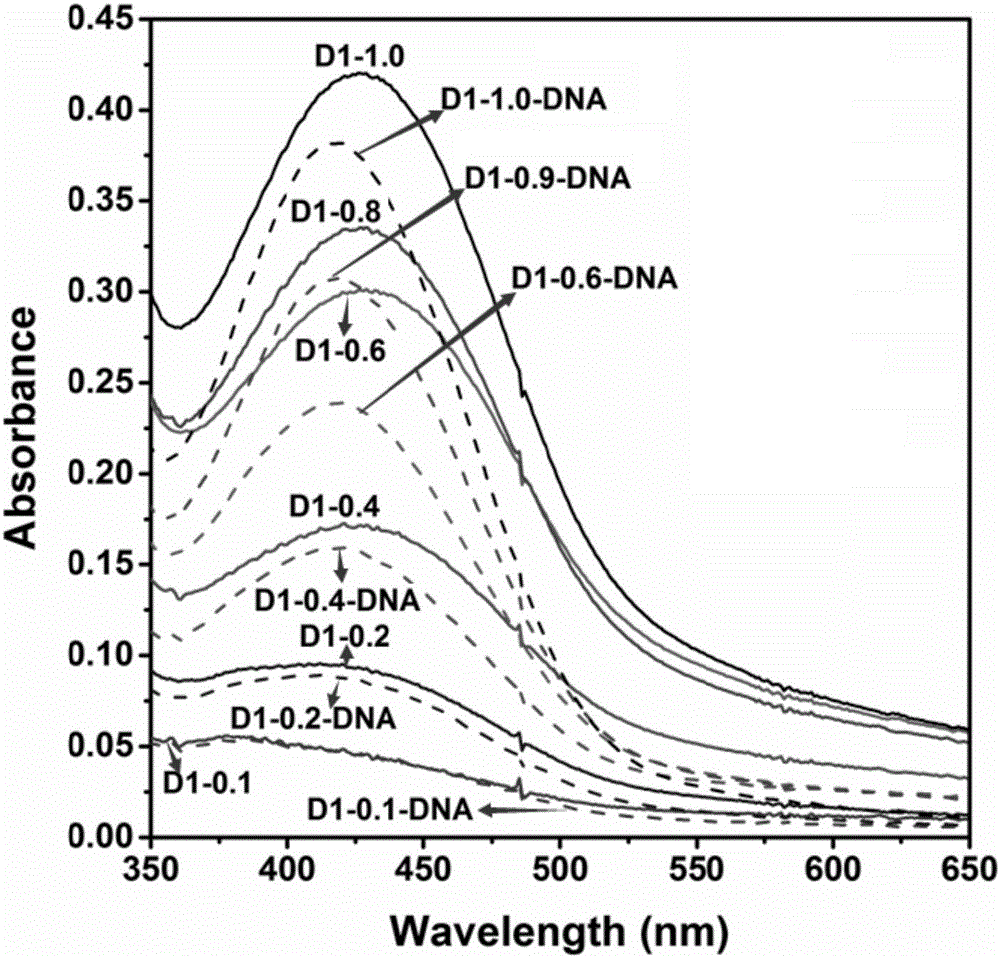
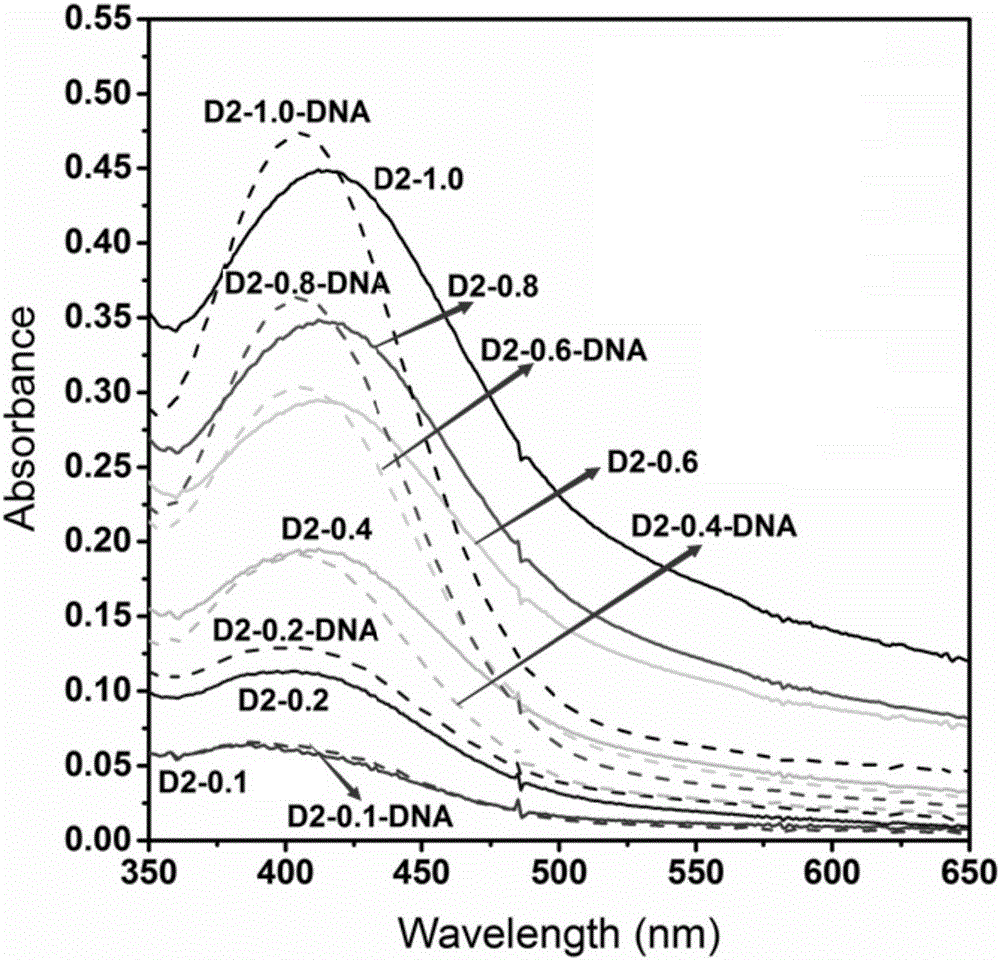
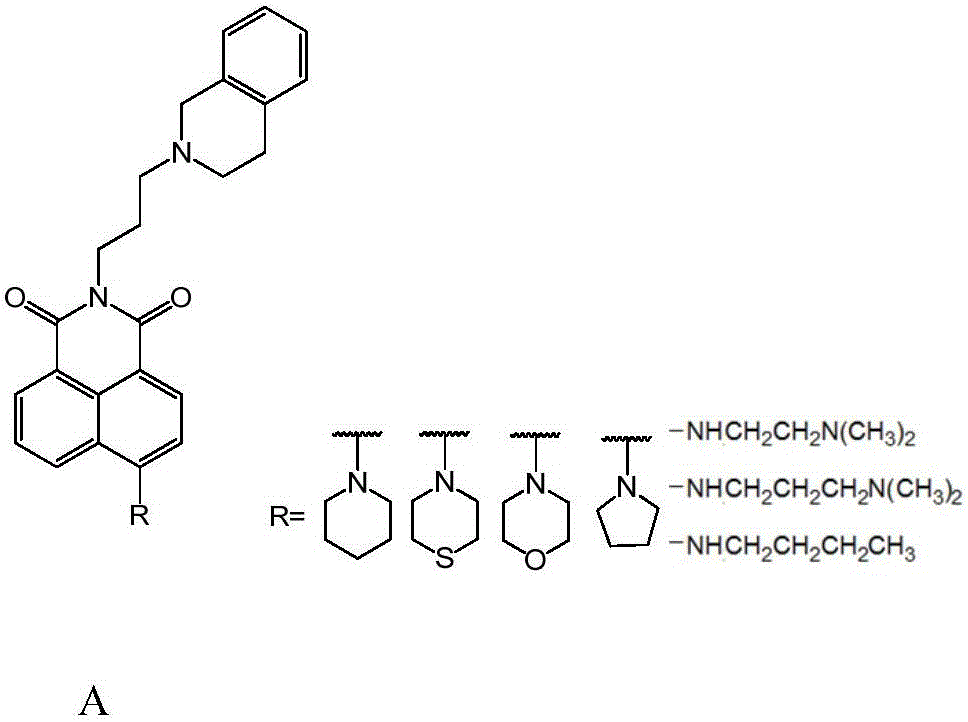
1,8-anhydride naphthalene derivative with side chain containing isoquinoline and synthesis and application thereof
The invention discloses a 1,8-anhydride naphthalene derivative with the side chain containing isoquinoline and synthesis and application thereof, and belongs to the field of biological organic synthesis. According to the 1,8-anhydride naphthalene derivative with the side chain containing isoquinoline, 1,2,3,4-tetrahydro naphthalene is introduced into one end of naphthalimide through an alkyl chain to serve as pharmacophore, a different cyclammonium side chain is introduced into the other end of naphthalimide, the purpose is to introduce isoquinoline pharmacophore with the antitumor activity to increase the conjugate area and improve biological activity of molecules, and therefore the antitumor effect is improved.
Owner:DALIAN UNIV OF TECH
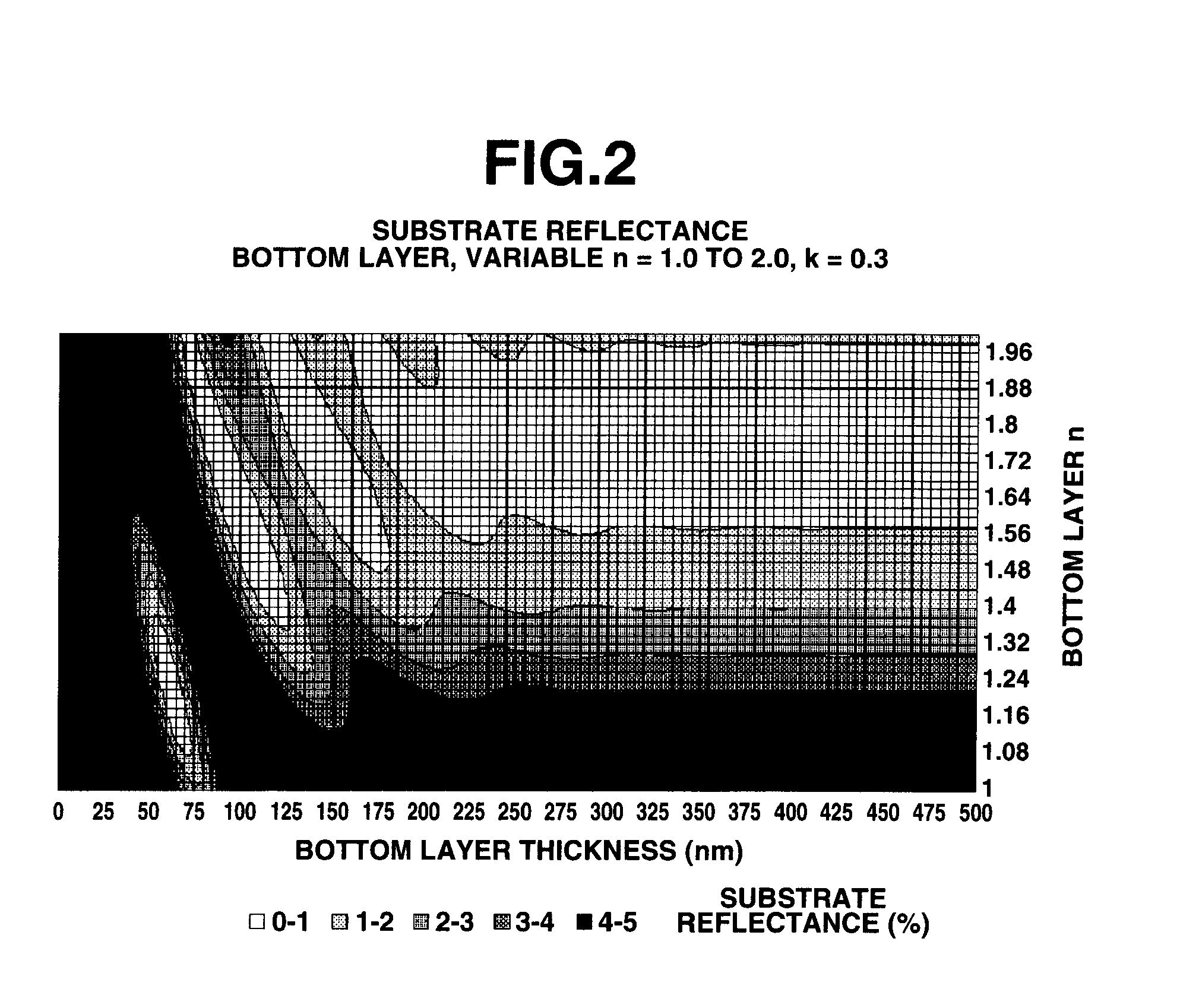
Composition for forming lower layer film and pattern forming method
ActiveUS7749681B2The effect is accurateAccurate performancePhotosensitive materialsRadiation applicationsStructural unitPolymer chemistry
Owner:JSR CORPORATIOON
Perylene derivative synthesis process, perylene derivative and organic EL device
InactiveUS20050054852A1Satisfactory yieldImprove production efficiencyOrganic compound preparationSolid-state devicesCyano radicalSulfuryl
The invention aims to provide a perylene derivative preparation process featuring satisfactory yields and improved preparation efficiency, a perylene derivative obtained by the process, and an organic EL device using the same. The object is achieved by a perylene derivative preparation process comprising subjecting to coupling reaction a 1,8-dihalogenated naphthalene derivative of the formula (1): wherein X is Cl, Br or I, R1 to R4, R11 and R12 each are hydrogen, alkyl, alkoxy, alkylthio, alkenyl, alkenyloxy, alkenylthio, aralkyl, aralkyloxy, aralkylthio, aryl, aryloxy, and arylthio radicals which may be substituted, amino radical, cyano radical, hydroxyl radical, —COOM1 radical (wherein M1 is hydrogen, alkyl, alkenyl, aralkyl or aryl), —COM2 radical (wherein M2 is hydrogen, alkyl, alkenyl, aralkyl, aryl or amino), or —OCOM3 radical (wherein M3 is alkyl, alkenyl, aralkyl or aryl), and at least two adjoining radicals selected from among R1 to R4, R11 and R12 may bond or fuse together to form a substituted or unsubstituted carbocyclic aliphatic ring, aromatic ring or fused aromatic ring with the carbon atoms on which they substitute, with the proviso that when the carbocyclic aliphatic ring, aromatic ring or fused aromatic ring has substituent radicals, the substituent radicals are the same as R1 to R4, R11 and R12, to thereby synthesize a perylene derivative of the formula (2): wherein R1′ to R4′, R11′ and R12′ are as defined for R1 to R4, R11 and R12 in formula (1), and R1 to R4, R11 and R12 and R1′ to R4′, R11′ and R12′ may be the same or different.
Owner:FUTABA CORPORATION
Composition for forming lower layer film and pattern forming method
ActiveUS20090098486A1Excellent pattern transfer performanceImprove etch selectivityPhotosensitive materialsRadiation applicationsPolymer sciencePerylene derivatives
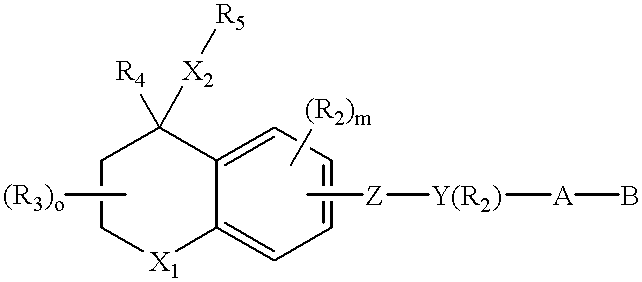
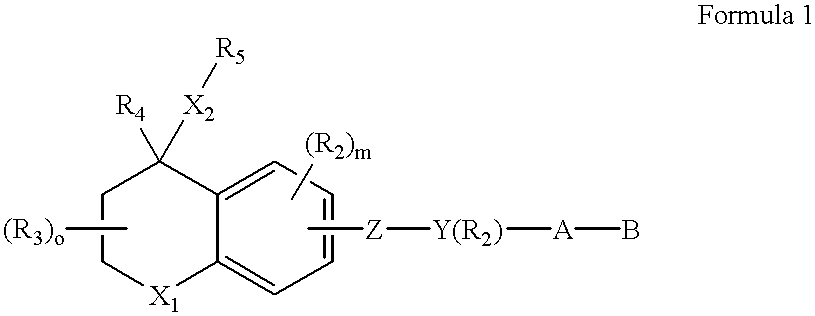
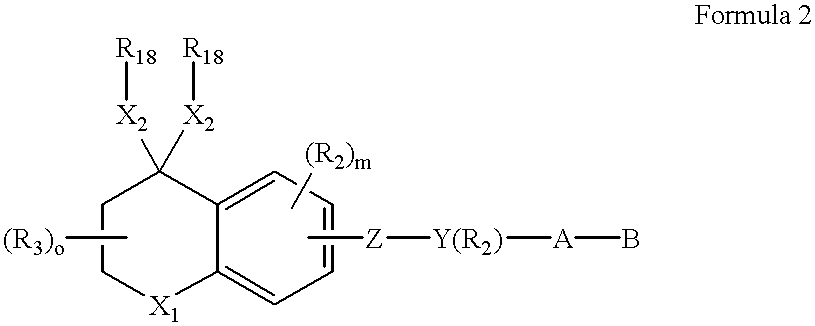
A composition for forming a lower layer film comprises a polymer (A) having a naphthalene derivative structural unit shown by the following formula (1),wherein R1 represents a hydroxyl group and the like, X represents a substitutable alkylene group having 1 to 20 carbon atoms and the like, n represents 0 to 6, m represents 1 to 8, and n+m represents an integer from 1 to 8, provided that two or more R1s may be the same or different and two or more Xs may be the same or different.
Owner:JSR CORPORATIOON
2,6-diphenyl naphthalene derivative and preparation method and application thereof
ActiveCN106083606AImprove external quantum efficiencyImprove luminous efficiencyAmino preparation from aminesOrganic compound preparationOrganic electroluminescenceOptoelectronic materials
The invention provides a 2,6-diphenyl naphthalene derivative and a preparation method and application thereof, and relates to the technical field of organic optoelectronic materials. The 2,6-diphenyl naphthalene derivative obtained by optimizing the molecular structure design has the higher optical extraction efficiency, can be used for preparing organic electroluminescence devices and especially can effectively improve the optical emitting efficiency of OLED devices by serving as an optical extraction material of the organic electroluminescence devices, and the OLED devices are superior to existing commonly-used OLED devices. The invention further provides the preparation method of the 2,6-diphenyl naphthalene derivative. The preparation method is simple, and the raw materials are easy to obtain.
Owner:CHANGCHUN HYPERIONS TECH CO LTD
Naphthalene derivative, resist bottom layer material, and patterning process
ActiveUS8846846B2Excellent etch resistanceImprove heat resistanceOrganic chemistryOrganic compound preparationBenzeneResist
A naphthalene derivative having formula (1) is provided wherein Ar1 and Ar2 denote a benzene or naphthalene ring, and n is such a natural number as to provide a weight average molecular weight of up to 100,000. A material comprising the naphthalene derivative or a polymer comprising the naphthalene derivative is spin coated to form a resist bottom layer having improved properties. A pattern forming process in which a resist bottom layer formed by spin coating is combined with an inorganic hard mask formed by CVD is available.
Owner:SHIN ETSU CHEM IND CO LTD
Organometallic complex for organic light-emitting layer and organic light-emitting diode using the same
InactiveUS20070212569A1Improve efficiencyLong lastingOrganic chemistryDischarge tube luminescnet screensHost materialLight-emitting diode
An organometallic complex for an organic light-emitting layer and an organic light-emitting diode are provided. A naphthalene derivative as a ligand is introduced to the organometallic complex. The organic light-emitting diode uses the organometallic complex as a phosphorescent host material. The organic light-emitting diode exhibits high current efficiency, high power efficiency and long lifetime when compared to conventional devices using BAlq.
Owner:SFC CO LTD
Application of dihydroarylnaphthalene lignan derivatives and their compositions in the preparation of drugs for preventing and treating mammary gland hyperplasia
The invention relates to application of a dihydro arylnaphthalene lignan derivative and a composition thereof to preparing a medicament for preventing and treating hyperplasia of mammary glands. The dihydro arylnaphthalene lignan derivative has a structure which is shown in a formula I.
Owner:CENT SOUTH UNIV
Iridium complex phosphorescent materials with wavelengths from infrared to near-infrared range and preparation method thereof
InactiveCN102226083AThe synthesis process is simpleLow costGroup 8/9/10/18 element organic compoundsSolid-state devicesIridium8-Hydroxyquinoline
The invention relates to a class of phosphorescent iridium complexes with wavelengths from infrared to near-infrared range, particularly relates to a class of bicyclo metal iridium complexes (C^N)2Ir(L^Y) and ionic metal iridium complexes (C^N)2Ir(N^N)<+>Z<->, with structures represented by formula (I) and formula (II) respectively, wherein R1 and R2 are independently one of hydrogen atom, halogen atom, cyan group, nitro group, acyl group, C1-18 linear chain, branched chain or cyclic aliphatic alkyl group, substituted alkyl group, alkoxy group, aryloxy, alkylthio group, arylthio, aliphatic amine group, aromatic amine group, substituted siloxane group, substituted silicon group, aryl group, substituted aryl group, heterocyclic aryl group or substituted heterocyclic aryl group; C^N is a phenylquinoline substituted naphthalene derivative; L^Y is one of N-COOH, 8-hydroxyquinolines, beta-diketones, N^NH and other compounds; N^N is one of dipyridine, diquinoline, 1,10-o-phenanthroline and derivatives thereof and other compounds; and Z is hexafluorophosphate radical or perchlorate radical. The bicyclo metal iridium complexes (C^N)2Ir(L^Y) are shown in formula (I), and the ionic metal iridium complexes (C^N)2Ir(N^N)<+>Z<-> are shown in formula (II).
Owner:NANJING UNIV OF POSTS & TELECOMM
Quinoline-substituted anhydride naphthalene derivative, iridium complex thereof and application in pH (potential of hydrogen) sensing
InactiveCN102659766AEasy to manufactureLow costGroup 8/9/10/18 element organic compoundsColor/spectral properties measurementsChemical structureCarbon ion
The invention discloses a quinoline-substituted anhydride naphthalene derivative, an iridium complex thereof and application in pH (potential of hydrogen) sensing. A chemical structure of a fluorescence sensing chemistry device can be shown by a general formula (I) and a general formula (II), wherein each of R1 and R2 is one of hydrogen ion, halogen ion, nitryl, linear chains, branched chains or annular fat alkyl of 1 to 18 carbon ions, substituted alkyl, akloxy, alkyl sulphanyl, aryl, substituted aryl, heterocyclic aryl and substituted heterocyclic aryl. By a traditional method for preparing the iridium complex, the iridium complex based on the quinoline-substituted anhydride naphthalene derivative is obtained by means of coordination and showed in the formula (II). A pH sensor for fluorescence and phosphorescence detects pH values by color change of solution, change of ultraviolet visual absorption spectra and change of fluorescence transmitting spectra.
Owner:NANJING UNIV OF POSTS & TELECOMM
O-or S-substituted tetrahydronaphthalene derivatives having retinoid and/or retinoid antagonist-like biological activity
InactiveUS6051731AEliminate and reduce side effectAvoid infectionSilicon organic compoundsBiocideMedicinal chemistryRetinoid
Compounds of the formula ##STR1## where the symbols have the meaning described in the application, have retinoid-like or retinoid antagonist-like biological activity.
Owner:ALLERGAN INC
Tetrahydro-naphthalene derivatives
This invention relates to tetrahydro-naphthalene derivatives and salts thereof which are useful as active ingredients of pharmaceutical preparations. They have the general formula (I)in which R1 represents hydrogen or C1-6 alkyl, and X represents —N(H)Y1, —(H)—C1-6 alkyleneY1, biphenyl or C1-6 alkyl substituted by biphenyl, and the group Y1 is an optionally substituted biphenyl. The tetrahydro-naphthalene derivatives of the present invention have excellent activity as VR1 antagonists and are useful for the prophylaxis and treatment of diseases associated with VR1 activity, in particular for the treatment of urinary incontinence, overactive bladder, urge urinary incontinence, chronic pain, neuropathic pain, postoperative pain, rheumatoid arthritic pain, neuralgia, neuropathies, algesia, nerve injury, ischaemia, neurodegeneration, stroke, inflammatory disorders, asthma and COPD.
Owner:XENTION LTD
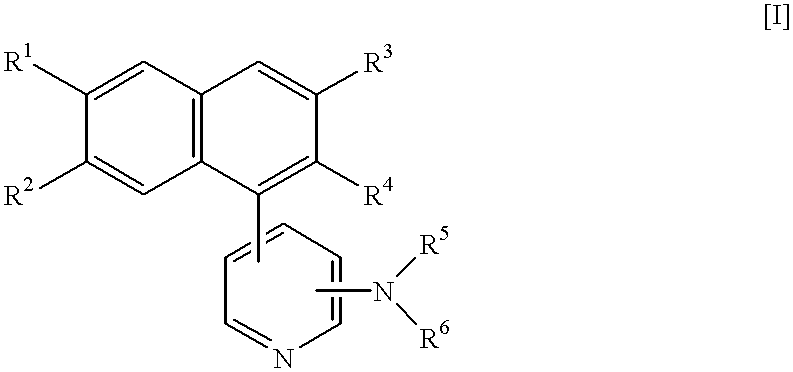
Naphthalene derivatives and application thereof in drugs
The invention provides a number of naphthalene derivatives or stereoisomers, tautomers, nitrogen oxides, metabolites, pharmaceutically acceptable salts or prodrugs thereof which are used for excitement of melatonin receptors. The invention also discloses pharmaceutical compositions containing such compounds and application thereof in treatment of central nervous system dysfunction of mammals, especially human.
Owner:SUNSHINE LAKE PHARM CO LTD
Naphthalene derivatives, process for the preparation thereof, and intermediates therefor
Naphthalene derivatives of the formula [I]:wherein R1 and R2 are the same or different and are each H, protected or unprotected OH, one of R3 and R4 is protected or unprotected hydroxymethyl, and the other is H, lower alkyl, or protected or unprotected hydroxymethyl, R5 and R6 are, the same or different and are each H, substituted or unsubstituted lower alkyl, substituted or unsubstituted phenyl or protected or unprotected NH2, or both combine together with the adjacent N to form substituted or unsubstituted heterocyclic group, and pharmaceutically acceptable salts thereof, these compounds showing excellent bronchoconstriction inhibitory activity, and hence, being useful in the prophylaxis or treatment of asthma.
Owner:MITSUBISHI TANABE PHARMA CORP
Room temperature phosphorescent compound and composition and application thereof
The invention relates to a room temperature phosphorescent compound and composition and application thereof based on phenothiazine or phenoxazine and derivatives substituting naphthalene or naphthalene derivatives, and belongs to the technical field of phosphorescent compounds. The room temperature phosphorescent compound is represented in a formula (1) or (2), and has room temperature phosphorescence characteristics. The invention further provides a room temperature phosphorescent composition with high luminous efficiency, and the room temperature phosphorescent composition is composed of thecompound represented by the formula (1) or (2) and a compound containing a 1,3,5-triazine group. The phosphorescent compound has the characteristics of room temperature phosphorescence emission in both the crystalline state and the amorphous state, and the compound and the composition are used as a luminescent material for preparing a luminescent layer of an organic electroluminescent device, andthe prepared organic electroluminescence device achieves an important breakthrough in pure organic electrophosphorescent devices.
Owner:JILIN UNIV
Preparation method for temperature-sensitive core-shell structured microspheres and application thereof in separation
InactiveCN102977242AIncrease profitEasy Coupling ReactionIon-exchange process apparatusOther chemical processesColloidal silicaMicrosphere
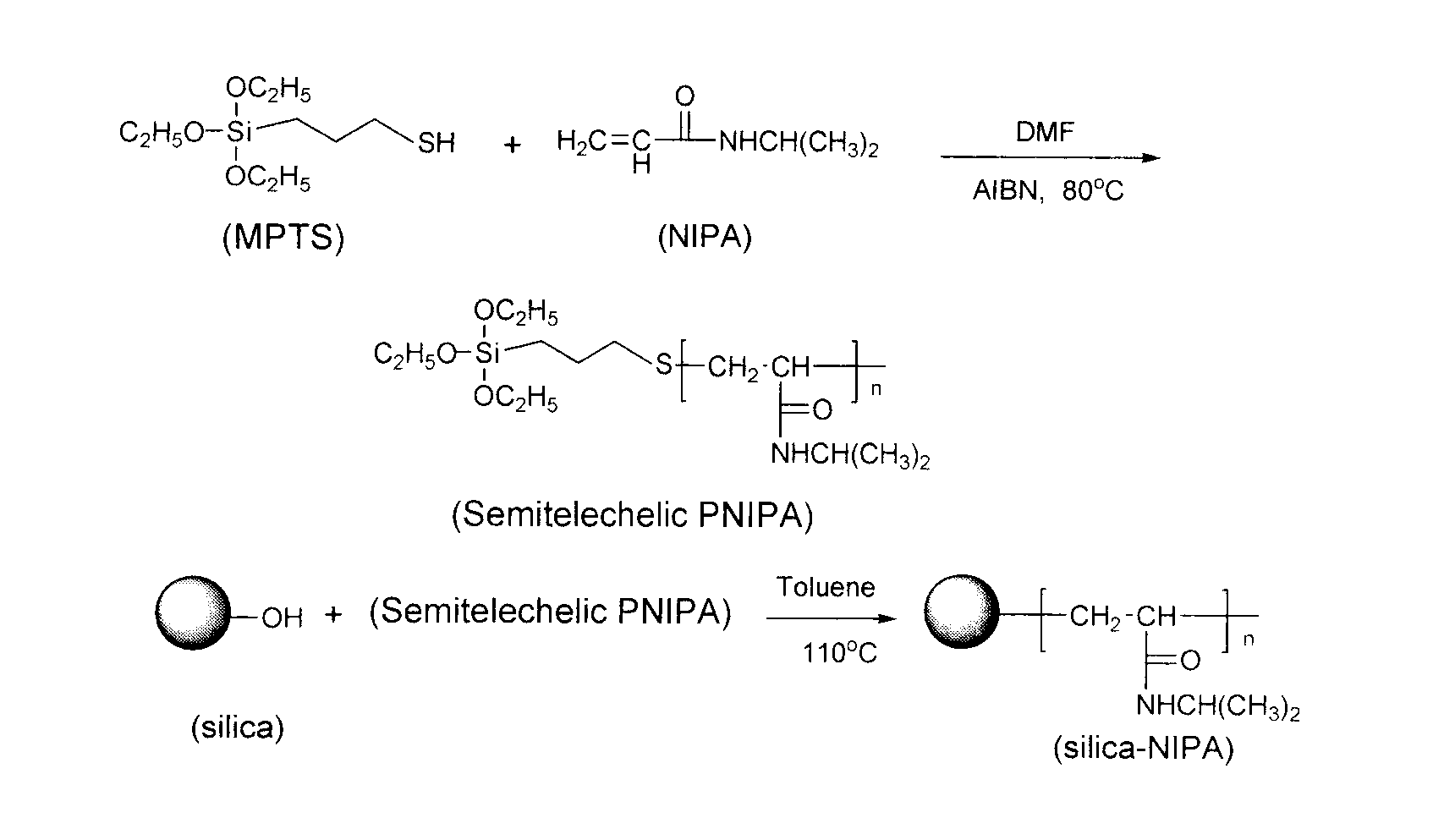
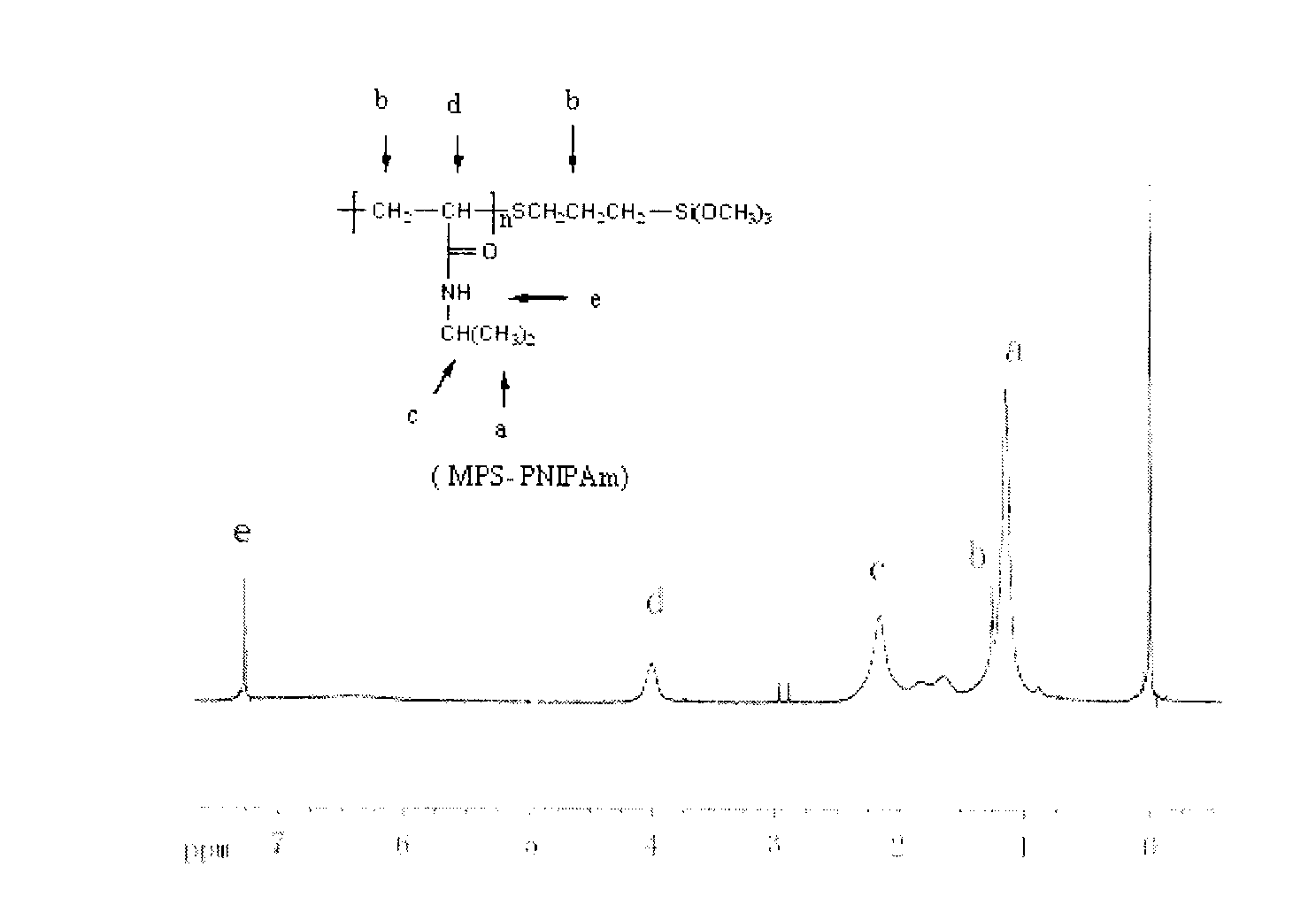
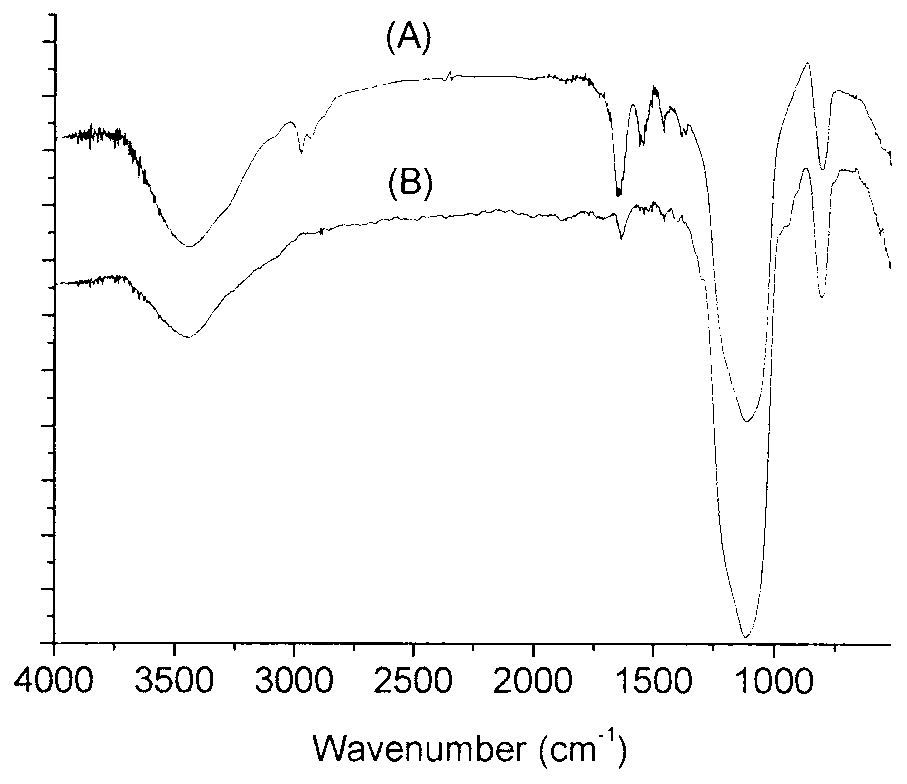
A preparation method for temperature-sensitive core-shell structured microspheres and an application thereof in separation. According to the present invention, first mercaptopropyl triethoxysilane (MPT) and N-isopropyl acrylamide are reacted to generate semi-telechelic polymers, then the semi-telechelic polymers are coupled with ultrafine colloidal silica particles, so composite particles with poly-N-isopropyl acrylamide as the shells and colloidal silica particles as the cores are prepared. Differential scanning calorimetry tests show that the composite particles are temperature-sensitive. The prepared composite particles are used as a stationary phase in high pressure liquid chromatography, and water is used as a main ingredient of a mobile phase, and naphthalene derivatives are separated successfully through the control of column temperatures, achieving good separation effect.
Owner:JIANGNAN UNIV
Tetrahydro-naphthalene derivatives as vanilloid receptor antagonists
Owner:XENTION LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com