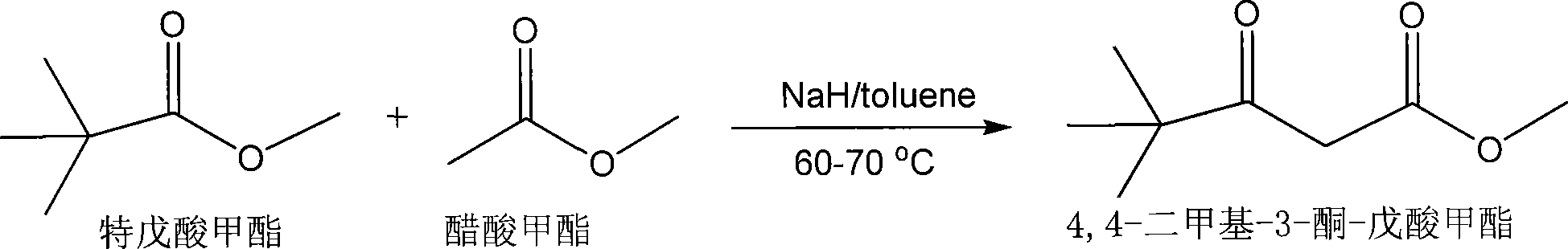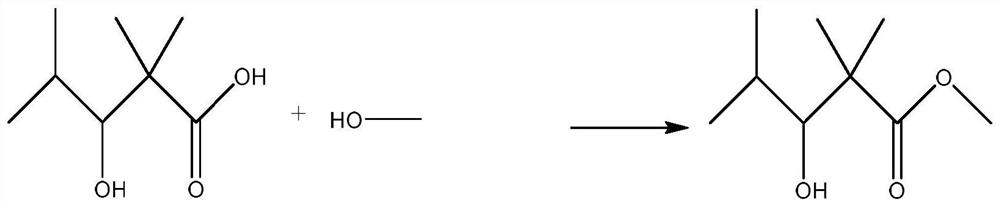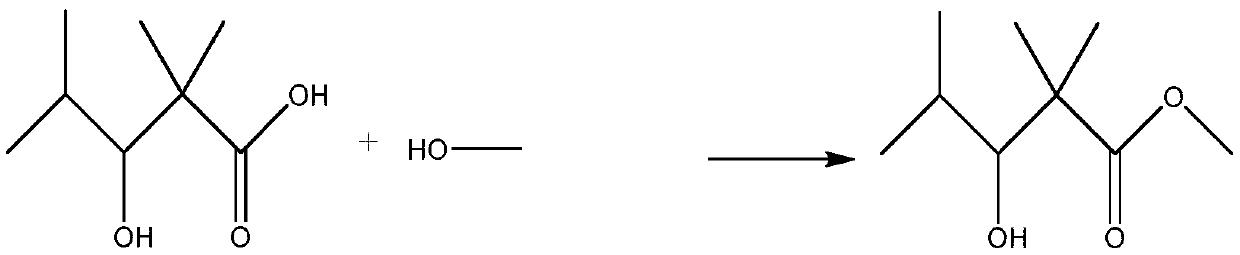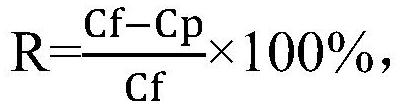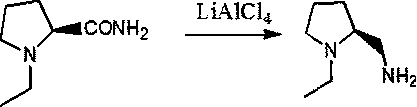Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Methyl pentanoate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methyl pentanoate, commonly known as methyl valerate, is the methyl ester of pentanoic acid (valeric acid) with a fruity odor. Methyl pentanoate is commonly used in fragrances, beauty care, soap, laundry detergents at levels of 0.1–1%.
Cracking of neo-C9 and neo-C13 carboxylic acids to either pivalic acid or methyl pivalate
InactiveUS6919474B2Organic compound preparationPreparation by carbon monoxide or formate reactionPivalic acidCarboxylic acid
A method for production of pivalic acid comprising the steps of: (a) reacting isobutylene, carbon monoxide, and a first catalyst to produce a reaction mixture; (b) contacting the reaction mixture with water, thereby producing a crude acid product having pivalic acid and oligomeric neo-carboxylic acid; (c) separating the pivalic acid and the oligomeric neo-carboxylic acid from the crude acid product; (d) reacting the oligomeric neo-carboxylic acid with a source of carbon monoxide at a temperature of less than 200° C. in the presence of a second catalyst to produce a C5 carbocation product, wherein the first and second catalyst are either the same or different; and (e) reacting the C5 carbocation product with water; thereby producing pivalic acid having an overall yield of at least 80 wt. %.
Owner:EXXONMOBIL CHEM PAT INC
Preparation method of atorvastatin calcium
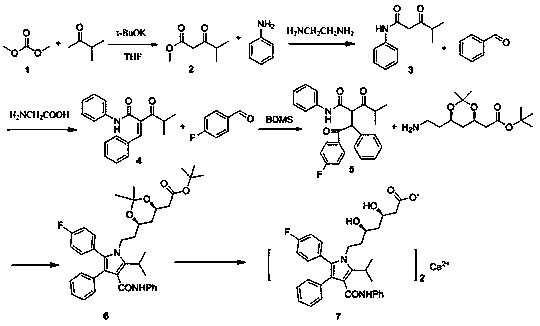
The invention belongs to the technical field of medicine preparation and particularly relates to a patent application about a novel preparation method of atorvastatin calcium. The method includes steps of preparing intermediates including: 2-methyl-3-carbonyl-methyl pentanoate, 2-methyl-3,5-dicarbonyl-5-anilino-butane, 4-methyl-3-oxo-N-phenyl-2-benzylidene pentanamide, 4-(4-fluorophenyl)-2-(2-methylpropionyl)-4-oxo-N-beta-diphenyl butyrylamide. The preparation method employs cheap and easy-to-obtained raw materials, has simple reactions and operations, and has great industrial application prospect. In conclusion, the preparation method has high reaction efficiency and product yield, is good in repeatability, is suitable for industrial production and has great application value and promotion and application significance.
Owner:TOPFOND PHARMA CO LTD
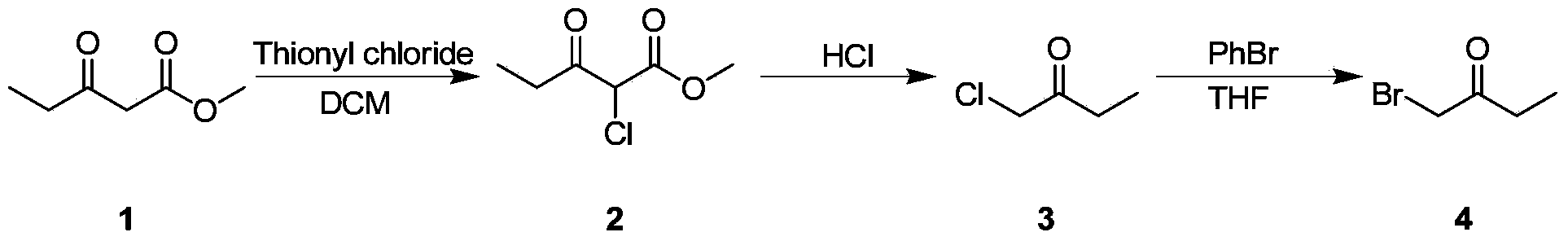
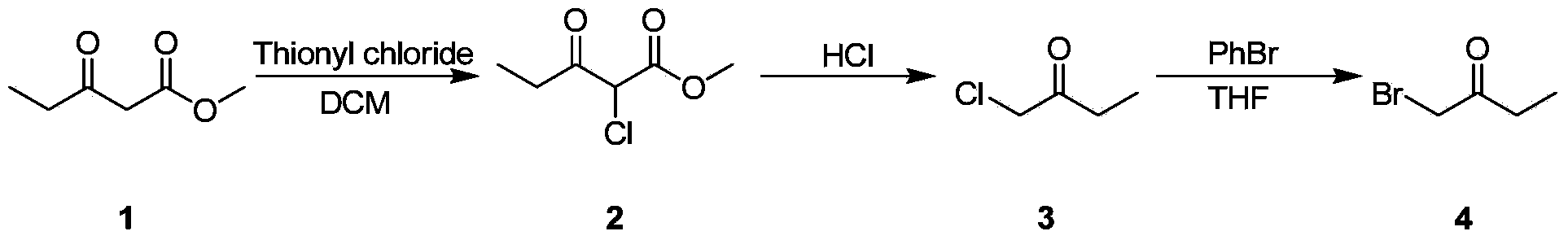
PROCESS FOR PREPARATION OF 4-FLUORO-alpha-[2METHYL-L-OXOPROPYL]-gamma-OXO-N-beta-DIPHENYLBENZENE BUTANE AMIDE
InactiveUS20130184493A1Minimize formationOrganic compound preparationCarboxylic acid amide separation/purificationDiketoneKetone
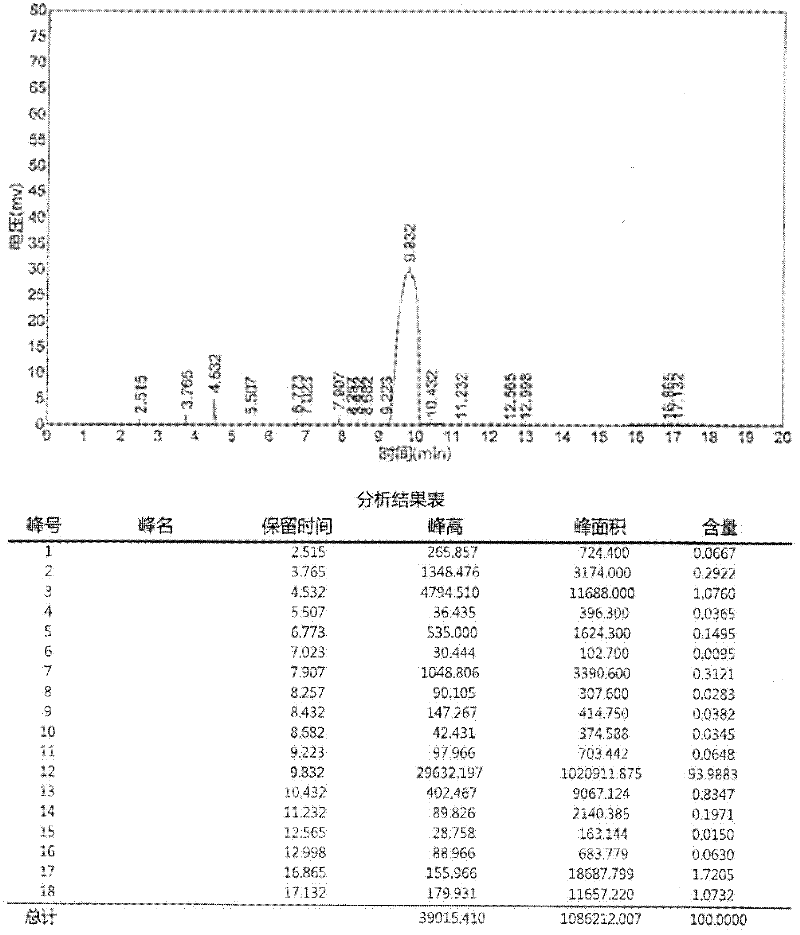
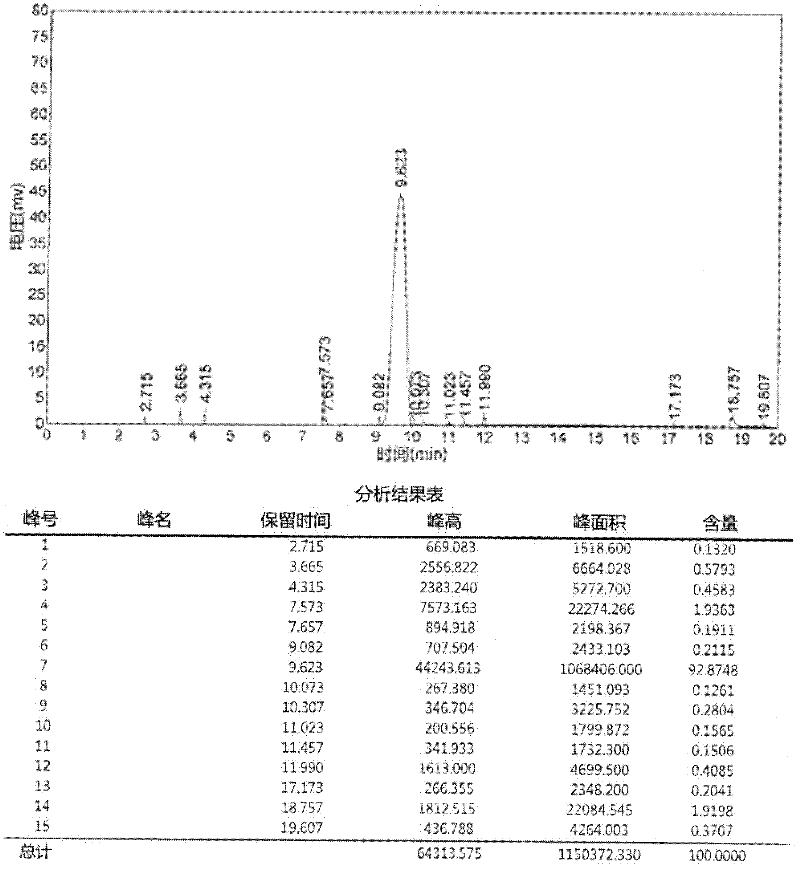
A process for preparation of 4-fluoro-α-[2-methyl-1-oxopropyl]-γ-oxo-N-β-diphenylbenzene butane amide also known as a diketone intermediate of atorvastatin, completely devoid of impurities 3,4-difluoro-α-[2-methyl-1-oxopropyl]-γ-oxo-n-β-diphenylbenzene butane amide; methyl, 2{-2[-(4-fluorophenyl)-2-oxo-1-phenylethyl)]}-4-methyl-3-oxo pentanoate; 1,4-bis(4-fluorophenyl)-2,3-diphenylbutane-1,4-dione, 1-(4-fluorophenyl)-2-phenyl ethanone; 1-(4-fluorophenyl)-2-phenyl ethanone and containing about 0.05% or less of 2-methyl-1-oxopropyl]-γ-oxo-N-β-diphenylbenzene butane amide. In that process the said diketone intermediate of formula 1 is obtained by maintaining temperature −25° C. to 50° C. during Friedel-Crafts acylation, in situ halogenation of formula II in presence of a solvent and nucleophilic substitution from a compound of formula III with formula IV in presence of a base.
Owner:VIJAYASRI ORGANICS
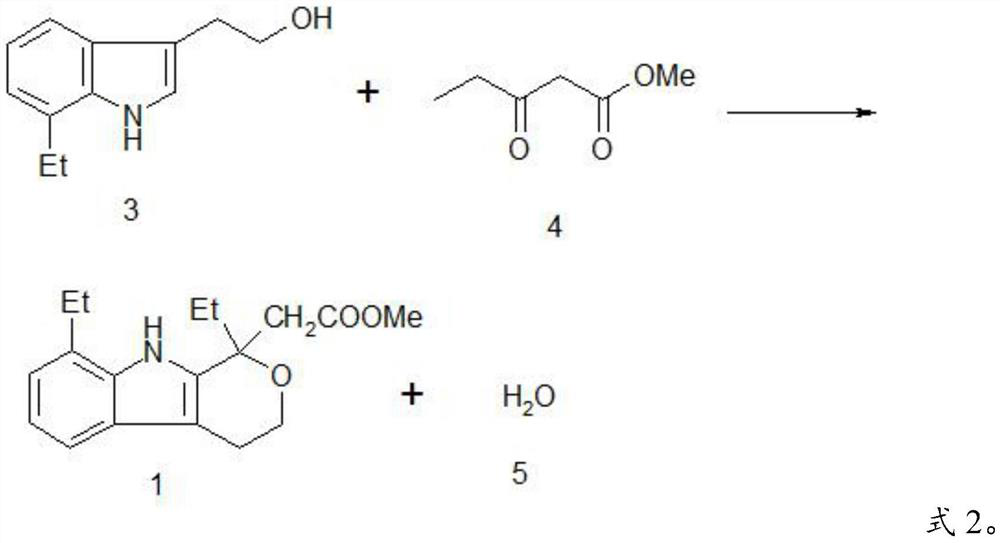
Method for preparing etodolac methyl ester
The present invention relates to new process of preparing methyl etodolate as the intermediate of non-steroid indole anti-inflammatory analgesic etodolic acid. The process includes the reaction between 7-ethyl tryptosol and 3-methyl oxy valerate inside mixed solvent of C1-C2 alcohol and benzene in the presence of acid catalyst, separating out acid layer after reaction, neutralizing the organic phase, concentrating, re-crystallizing. The process has high stability, high yield, high product quality, conversion rate near to 100 %, simple operation low cost, environment friendship and other advantages, and is suitable for industrial production.
Owner:SHANDONG XINHUA PHARMA CO LTD
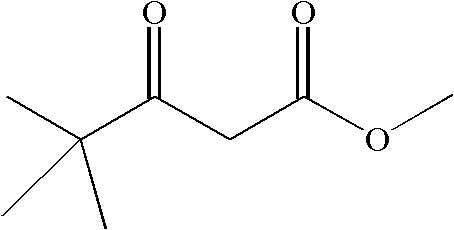
Preparation method of 4,4-dimethyl-3-keto-methyl valerate
InactiveCN102603529AReduce consumptionMild responseOrganic compound preparationCarboxylic acid esters preparationDistillationWastewater
The invention discloses a preparation method of 4,4-dimethyl-3-keto-methyl valerate, which comprises the following steps: by using pinacoline, sodium hydride and dimethyl carbonate with the water content of less than 0.2 wt.% as raw materials and using methylbenzene with the water content of less than 0.2 wt.% as a solvent, mixing to react at 55-60 DEG C, cooling, standing to stratify when the pH value is equal to 4-5, removing the solvent, and carrying out reduced pressure distillation to extract the 4,4-dimethyl-3-keto-methyl valerate. The preparation method disclosed by the invention has the advantages of relatively lower raw material consumption and less wastewater treatment amount; and the 60-62 wt.% sodium hydride is used in the reaction process, so the reaction is relatively milder in the feed process, thereby lowering the danger coefficient.
Owner:ZHANGJIAGANG CITY ZHENFANG CHEM
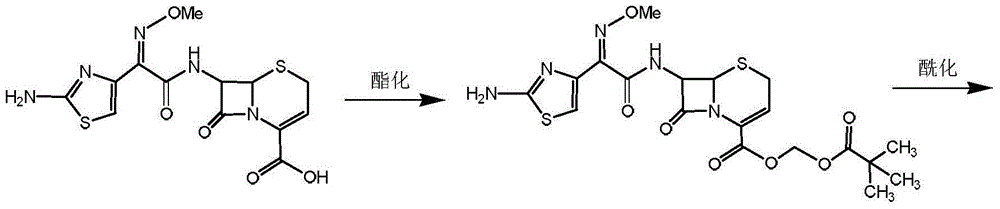
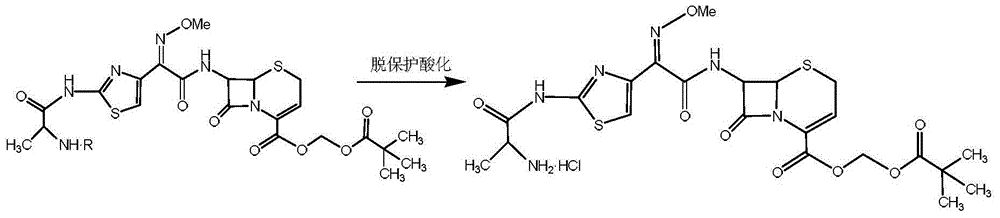
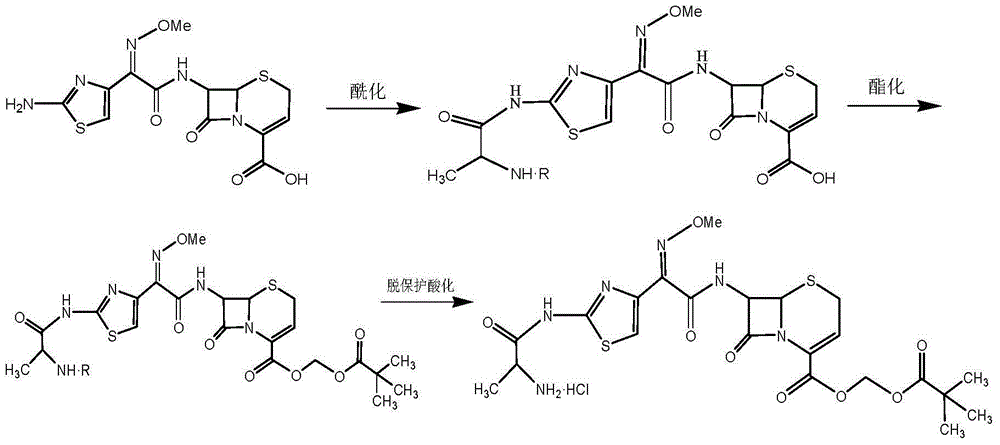
Preparation method for ceftizoxime alapivoxil, intermediates thereof and preparation method for intermediates
ActiveCN105017284ARaw materials are cheap and easy to getMild reaction conditionsOrganic chemistryCefotaximeValproic Acid
The present invention discloses a preparation method for ceftizoxime alapivoxil by using a mixture anhydrides method, intermediates 3a / 3b / 3c thereof and a preparation method for the intermediates . The method comprises: putting an ammonia cefotaxime acid ester compound in an organic solvent to react with BOC-alanine by using a mixture anhydrides method; hydrolyzing under condition of organic alcohol; generating intermediates 3a / 3b / 3c; the intermediates 3a / 3b / 3c condensation reacting with 7-ANCA in an organic solvent; generating BOC-alanyl ceftizoxime acid by esterification; the BOC-alanyl ceftizoxime acid reacting with halogenated pentanoic acid methyl ester in the presence of an acid-binding agent, to prepare BOC-alanyl ester ceftizoxime valproate; removing a BOC protection group; and after concentrating, obtaining the target compound ceftizoxime alapivoxil by crystal precipitation in the organic solvent. The preparation method for ceftizoxime alapivoxil has the advantages that raw materials are readily available, the reaction condition is mild, byproducts are fewer, purity is reliable, the yield is high, and the preparation method for ceftizoxime alapivoxil is suitable for large-scale industrial production.
Owner:ZHEJIANG YONGNING PHARMA
Water-based drilling, completion, and workover fluid composition with reduced barite sagging
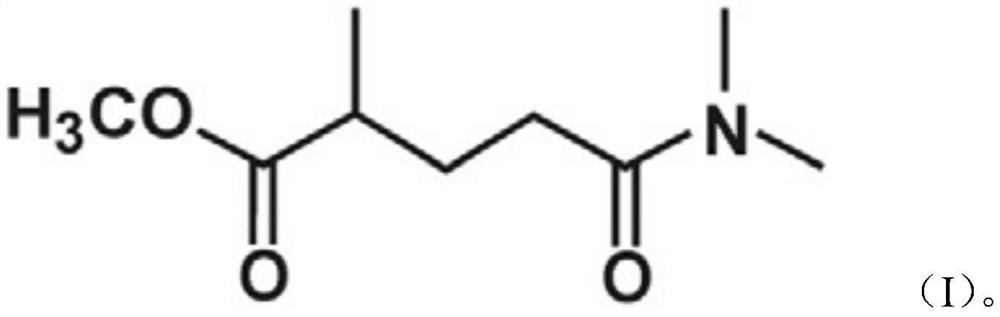
An drilling mud composition is described, which includes an aqueous base fluid, a viscosifier, and a weighting material. The drilling mud composition also includes an anti-sagging agent that comprises a dimethylamino methyl ester, such as methyl 5-(dimethylamino)-2-methyl-5-oxopentanoate. The anti-sagging agent may be used to prevent barite sagging, where barite is used as a weighting material. Small amounts of the anti-sagging agent may be used to maintain a low sag factor while drilling, and without causing unwanted increases in viscosity. The anti-sagging agent is effective for both vertical and inclined wellbores.
Owner:KING FAHD UNIVERSITY OF PETROLEUM AND MINERALS
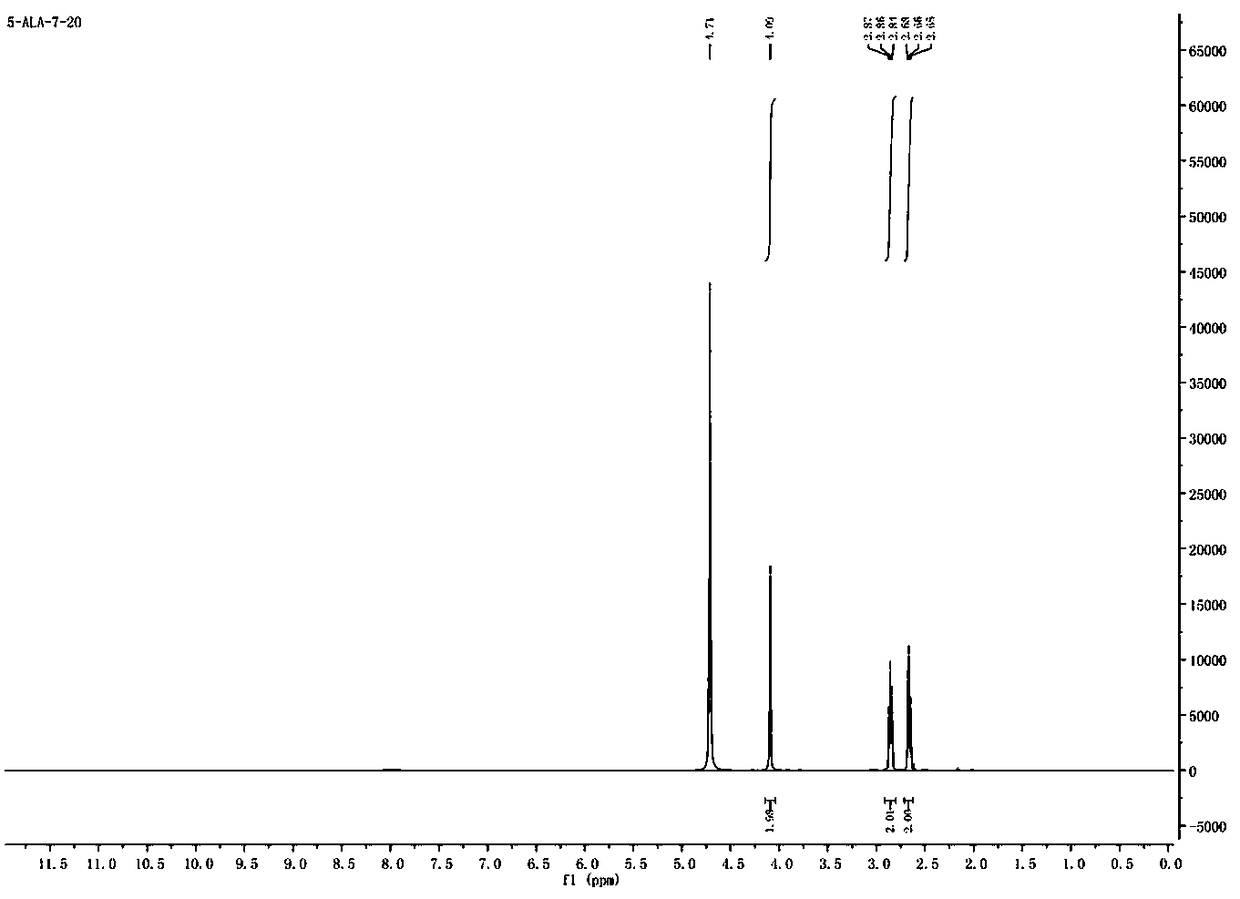
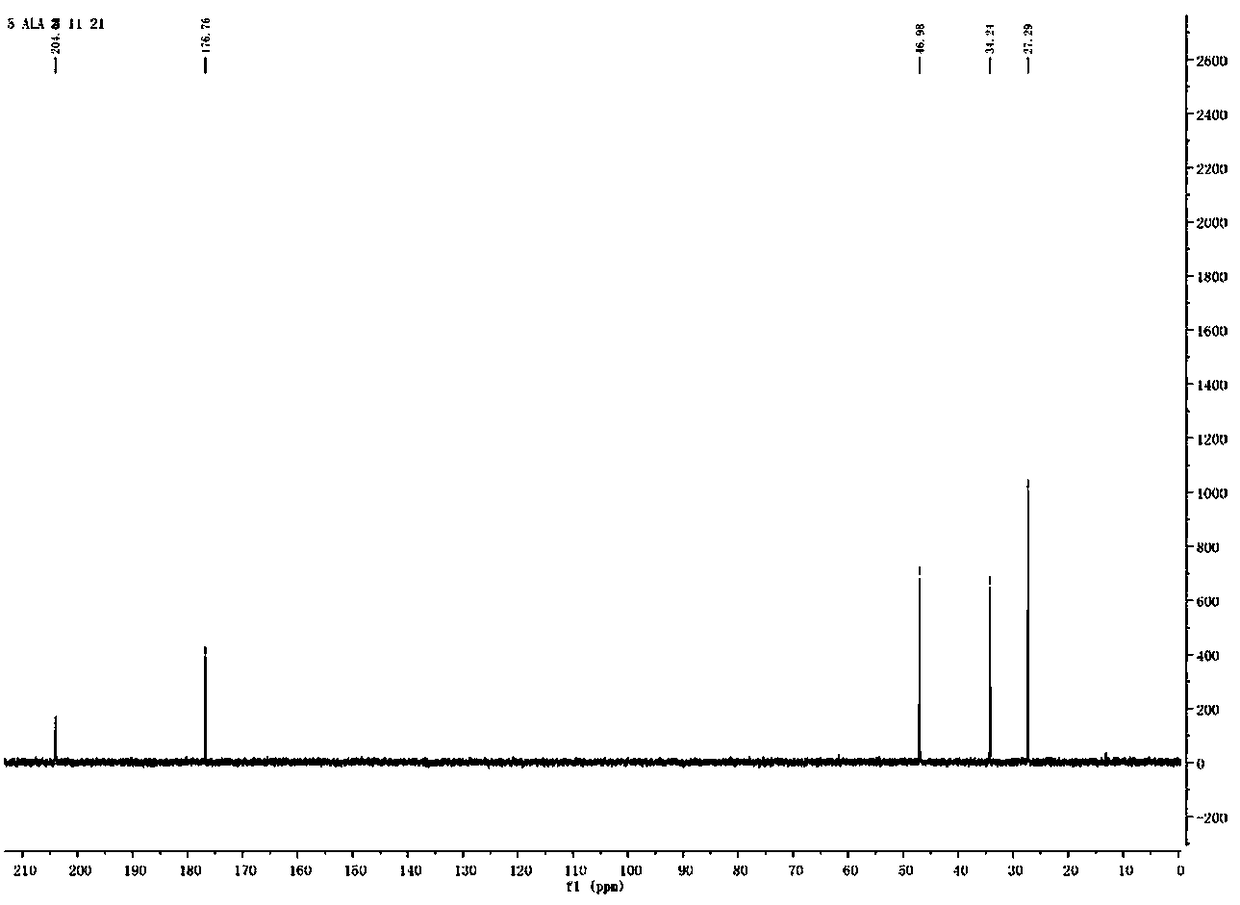
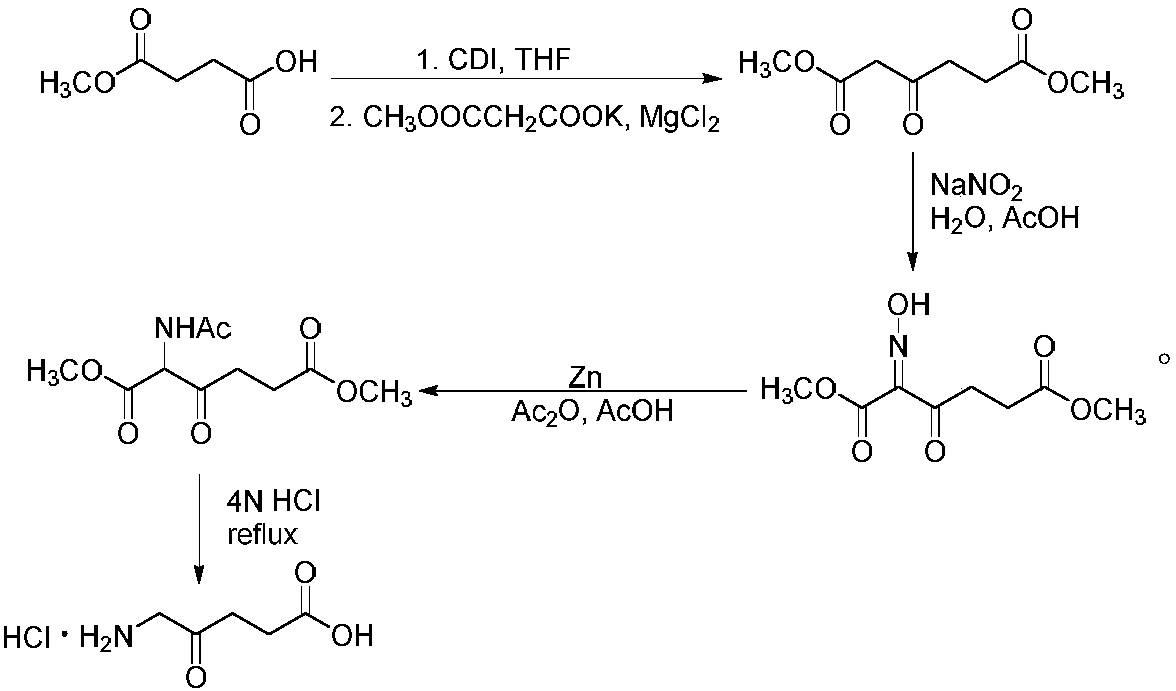
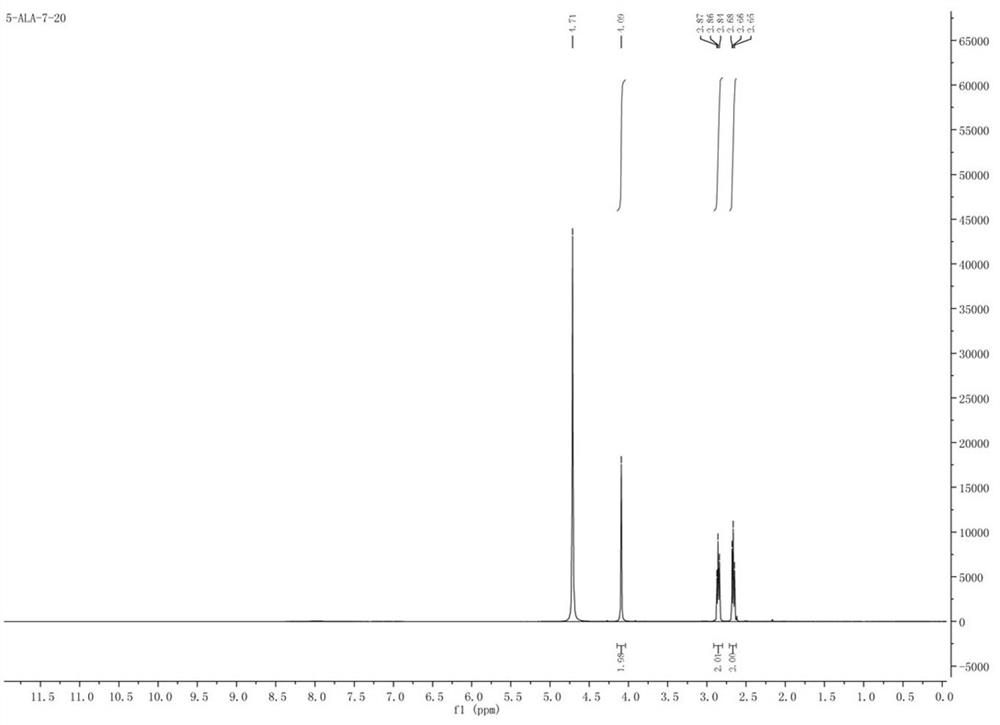
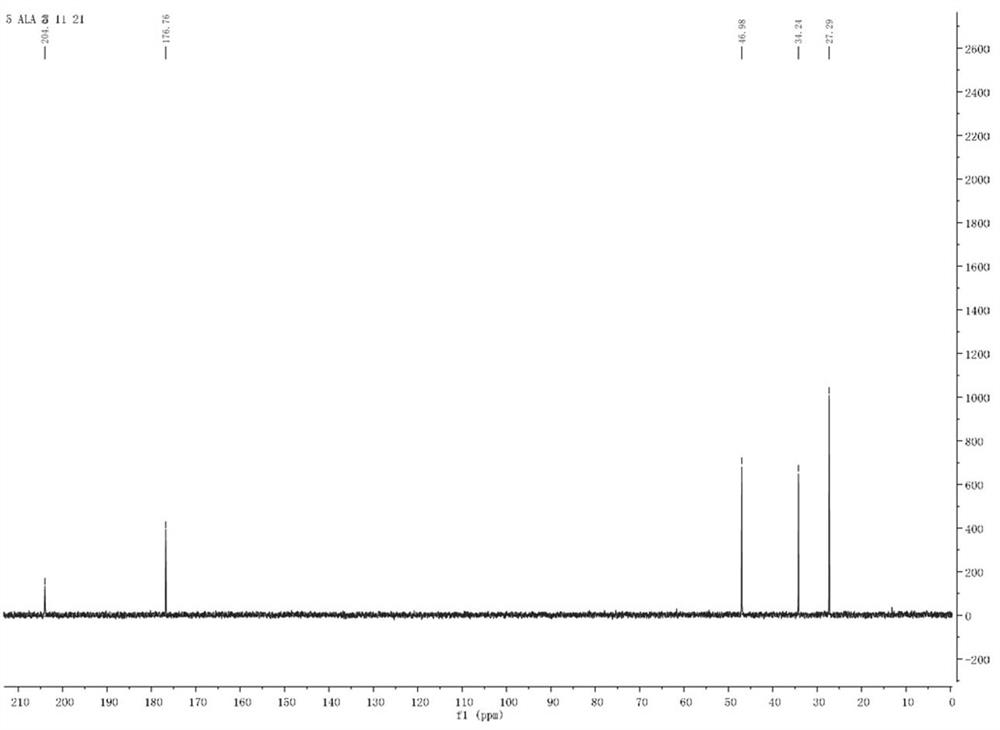
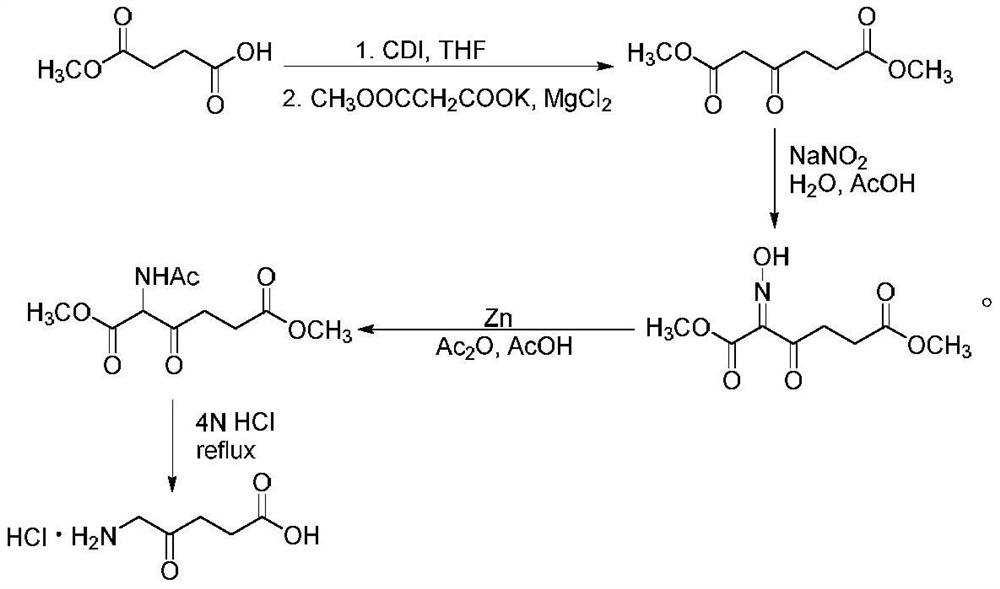
Synthesis method of 5-aminolevulinic acid hydrochloride
ActiveCN109265341ASimple processRaw materials are easy to obtainOrganic compound preparationCarboxylic acid esters preparationSynthesis methodsNitromethane
The invention relates to a synthesis method of 5-aminolevulinic acid hydrochloride. The method includes the steps that succinic anhydride is used as the raw material and subjected to monoesterification with methanol to obtain monomethyl succinate; monomethyl succinate and N,N-carbonyldiimidazole are subjected to a nucleophilic substitution reaction to obtain methyl 4-(1-imidazole)-4-oxobutanoate;methyl 4-(1-imidazole)-4-oxobutanoate and nitromethane are subjected to a nucleophilic substitution reaction under the catalysis of an alkali compound to obtain methyl 5-nitro-4-oxopentanoate; methyl5-nitro-4-oxopentanoate and a metallic reducing agent are subjected to a reduction reaction, and through hydrolysis, 5-aminolevulinic acid hydrochloride is obtained. The process is simple, the raw material is easy to obtain, no special rectification and recrystallization devices are needed for treating intermediate products, no toxic and expensive raw materials are used, the use of heavy metal reducing agents is avoided to prevent environmental pollution, the synthesis cost is low, the purity of the synthetic product can reach 97% in recrystallization detection, and the total yield is high andreaches 70% or above.
Owner:HENAN UNIVERSITY
Antistatic organic silicon self-cleaning paint
InactiveCN104449363AImprove antistatic performanceImprove insulation performanceAntifouling/underwater paintsPaints with biocidesPolymer scienceEmulsion
The invention relates to organic paints, and particularly relates to an antistatic organic silicon self-cleaning paint. The antistatic organic silicon self-cleaning paint is prepared from the following raw materials in parts by weight: 40-55 parts of hydroxyl silicone oil, 15-25 parts of silicone acrylic emulsion, 1-5 parts of acrylate, 8-16 parts of methyl pivalate, 5-10 parts of p-butylbenzoyl and 15-20 parts of diisopropyl. The antistatic organic silicon self-cleaning paint disclosed by the invention is excellent in performances such as antistatic property, insulativity, high and low-temperature resistance, water resistance, moisture resistance and chemical medium resistance.
Owner:JIANGSU NUOFEI NEW MATERIAL TECH
Bromo-butanone synthesis method
InactiveCN103449992AReduce usageEasy to purifyOrganic compound preparationCarbonyl compound preparationSynthesis methodsButanone
The invention relates to a novel bromo-butanone synthesis method. In the method, 3-methyl propionlyacetae is used as a start raw material; a key intermediate is obtained by chlorination and degreasing reactions, wherein the yield is greater than 70%; the bromo-butanone is obtained by bromination, wherein the total yield is greater than 50%. The method provided by the invention can overcome the adverse factors in the enlarged production of bromo-butanone, and has the characteristics of cheap raw materials, short path, no use of dangerous reagent, high yield and the like so that the product can be produced in a large scale and is cheap and convenient to popularize.
Owner:TIANJIN QUANHECHENG TECH
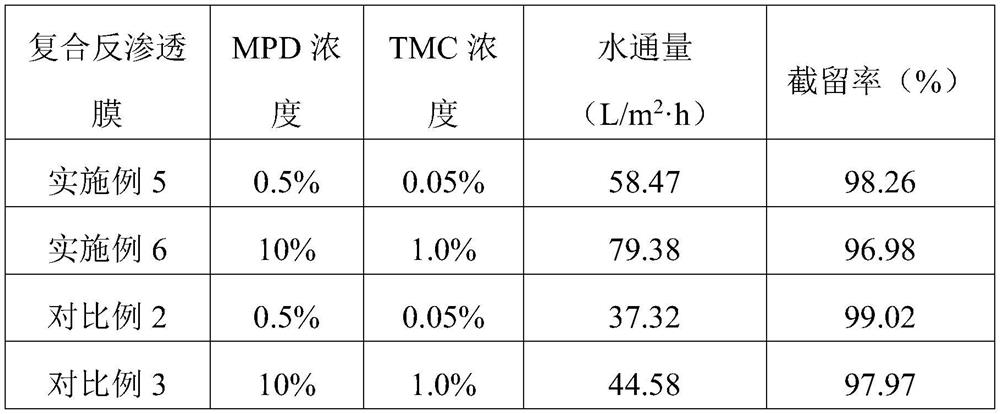
Composite reverse osmosis membrane as well as preparation method and application thereof
PendingCN112642303AEasy to separateHigh retention rateSemi-permeable membranesWater/sewage treatment bu osmosis/dialysisReverse osmosisPolyamide
The invention discloses a high-flux composite reverse osmosis membrane as well as a preparation method and application thereof. The composite reverse osmosis membrane comprises a bottom layer, a porous support layer in the middle and an active separation layer on the surface, wherein the active separation layer is a polyamide active separation layer formed by interfacial polymerization of polyamine and polyacyl chloride in the presence of methyl-5-(dimethylamino)-2-methyl-5-oxopentanoate (Polarclean). By adding a water-phase additive which is simple in variety and small in dosage, the prepared composite reverse osmosis membrane has excellent water flux and keeps good interception characteristics. In addition, the preparation method is simple and convenient to operate and has a great industrial application prospect.
Owner:CHINA PETROLEUM & CHEM CORP +1
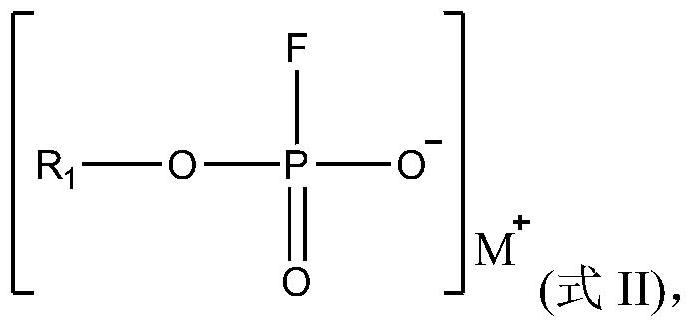
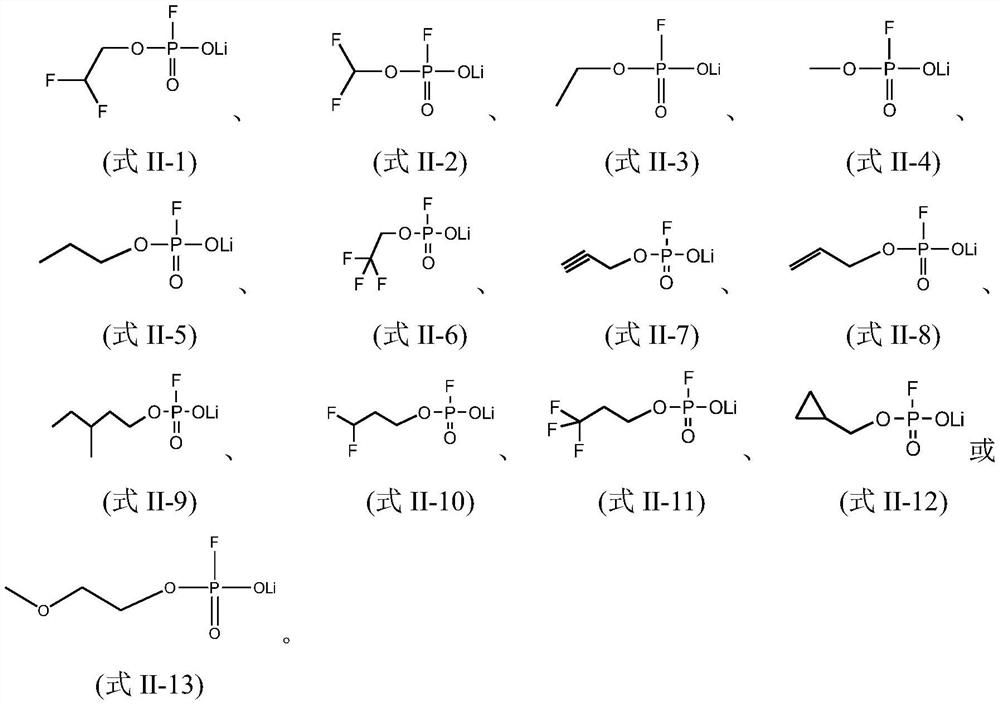
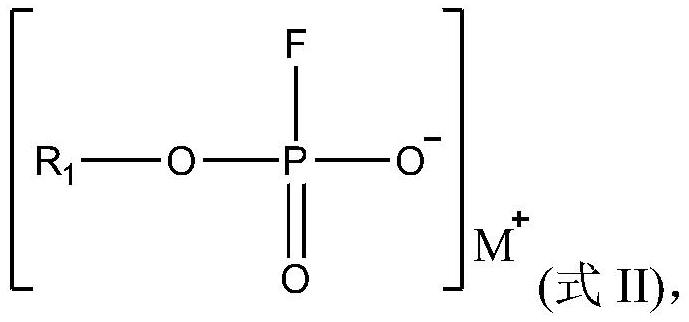
Electrolyte, and electrochemical device and electronic device using same
PendingCN114006035AGood dynamic performanceImprove high temperature circulationSecondary cells servicing/maintenanceElectrolyte immobilisation/gelificationSulfolanePropanoic acid
The present application relates to an electrolyte, and an electrochemical device and an electronic device using the same. Specifically, the invention provides the electrolyte which is characterized by comprising a compound A and a compound B. The compound A comprises at least one of dimethyl carbonate, methyl ethyl carbonate, diethyl carbonate, propylene carbonate, propyl propionate, ethyl acetate, ethyl propionate, propyl acetate, methyl acetate, methyl propyl carbonate, methyl propionate, methyl butyrate, methyl pivalate, gamma-butyrolactone, sulfolane, ethyl-propyl ether, ethylene glycol dimethyl ether, 1, 3-dioxane or 1, 4-dioxane; and the compound B comprises a compound A substituted by one or more fluorine atoms. The electrolyte provided by the invention is beneficial to improving the high-temperature circulation or storage performance of the electrochemical device.
Owner:NINGDE AMPEREX TECH
Production of 4,4-dimethyl-3-ketone-methoxycarbonyl valerate
InactiveCN101074196AHigh yieldLow costOrganic compound preparationCarboxylic acid esters preparationKetoneSolvent
Production of 4,4-methyl-3-ketone-pentoic acid carbomethoxy is carried out by anhydrous tementhyl valerate and dried acetate carbomethoxy as raw materials, taking sodium hydride as catalyst, taking toluene as solvent, agitating while reacting at 60-70 degree, cooling, regulating pH value to 4-5, laying aside, stratifying, removing solvent, decompressing, distilling and extracting to obtain final product. It costs low and has more yield.
Owner:KAILONGHUA CHEM HANGZHOU
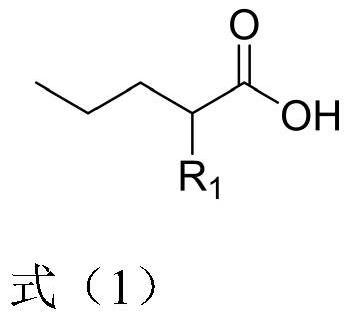
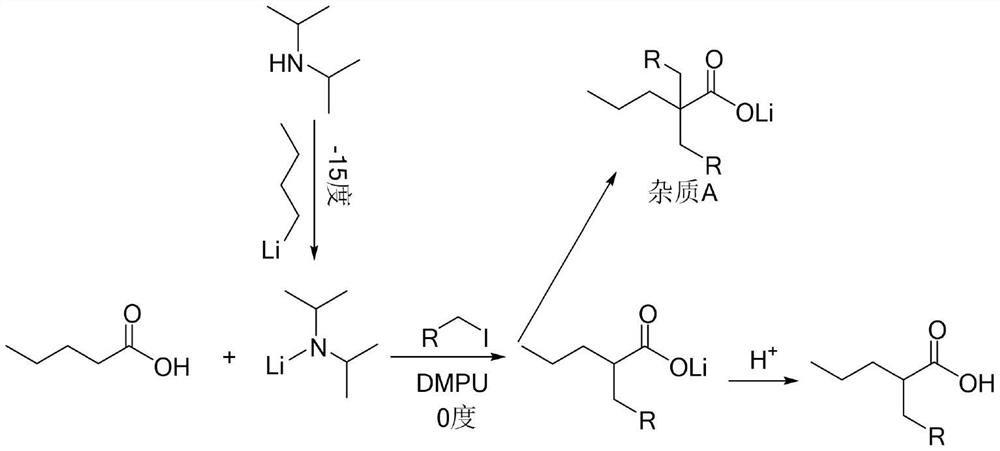
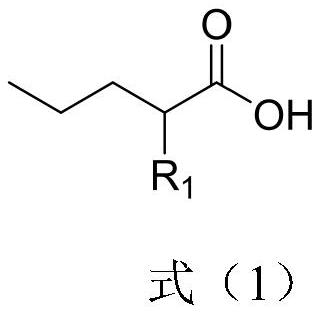
Preparation method of 2-R1 valeric acid
PendingCN113149823AReduce riskEasy to buyOrganic compound preparationPreparation from nitrilesSodium methoxideAlkane
The invention discloses a preparation method for 2-R1 valeric acid. The preparation method comprises the following steps: step 1, with methyl cyanoacetate as a starting material, adding bromopropane and sodium methoxide, carrying out a catalytic reaction, and conducting purifying to obtain 2-cyanomethyl valerate; step 2, subjecting 2-cyanomethyl valerate to a reaction under the catalysis of iodoalkane and sodium methoxide, and conducting aftertreatment to obtain 2-cyano-2-R1 methyl valerate; step 3, enabling the 2-cyano-2-R1 methyl valerate to undergo a reaction in an aqueous solution of sulfuric acid at 120-160 DEG C for 15-40 hours so as to obtain a mixture of 2-R1 valeric acid and 2-R1 methyl valerate; and step 4, hydrolyzing the mixture by using an aqueous sodium hydroxide solution to obtain 2-R1 sodium valerate and methanol, and conducting acidifying by using inorganic acid to obtain 2-R1 valeric acid. Reagents adopted in the preparation method are relatively common, and the risk of the reagents is relatively low; and reaction conditions are mild, and the temperature is easier to control relatively. The invention develops a purification process of the key intermediate 2-cyanomethyl valerate, and the process flow is simple.
Owner:SHANGHAI QINGPING PHARMA CO LTD
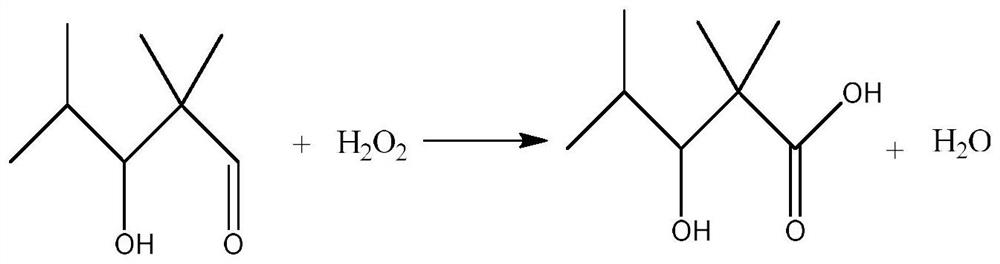
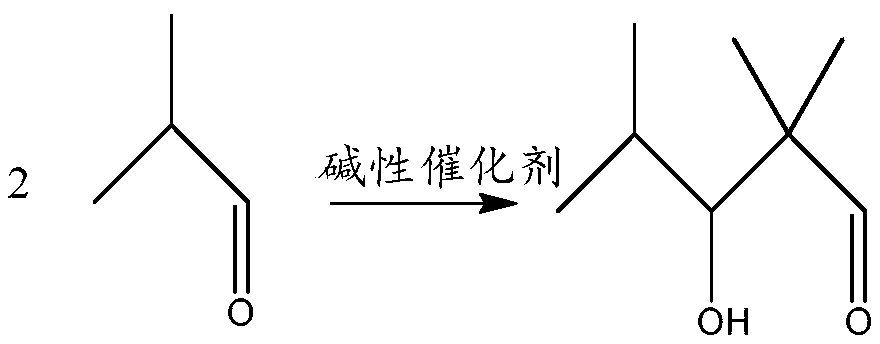
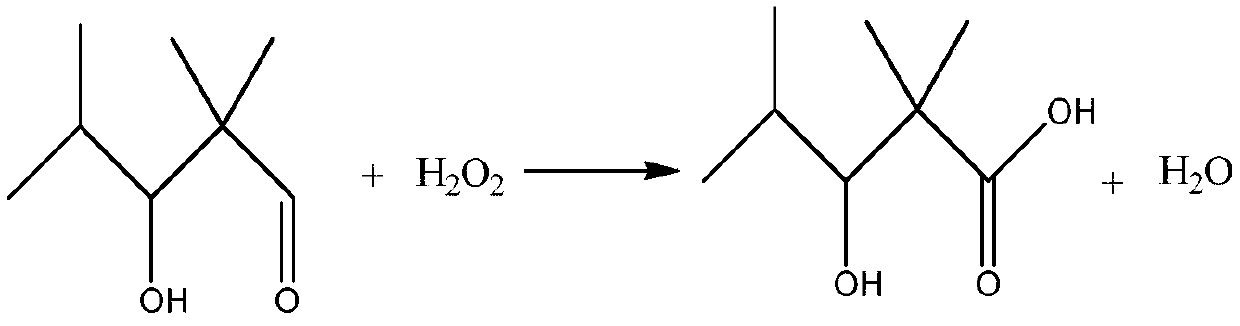
Preparation method of 2,2,4-trimethyl-3-hydroxyvaleric acid methyl ester, steel structure water-based industrial paint and preparation method thereof
ActiveCN110746297BHigh purityIncrease reaction rateOrganic compound preparationCarboxylic acid esters preparationPtru catalystStrong acids
The invention relates to the technical field of synthesis and application of organic compounds, in particular to a preparation method of methyl 2,2,4-trimethyl-3-hydroxyvalerate, a water-based industrial paint for steel structures and a preparation method thereof. The preparation method of 2,2,4-trimethyl-3 hydroxyvaleric acid methyl ester of the present invention comprises the following steps: 1) isobutyraldehyde undergoes an aldol condensation reaction under the action of a basic catalyst to generate 2,2,4 -Trimethyl-3-hydroxypentanal; the 2,2,4-trimethyl-3-hydroxypentanal obtained above was mixed with H 2 o 2 Oxidation reaction occurs to obtain 2,2,4-trimethyl-3 hydroxyvaleric acid; 2 ) reacting the 2,2,4-trimethyl-3 hydroxyvaleric acid and methanol prepared in step 1) under the action of a strong acid catalyst to obtain methyl 2,2,4-trimethyl-3 hydroxyvaleric acid. The preparation method of 2,2,4-trimethyl-3 hydroxyvaleric acid methyl ester of the present invention, the reaction product is single, does not need to carry out multi-step separation, and is simple to operate, and the present invention also provides a kind of containing above-mentioned 2,2, A water-based industrial paint for steel structures of 4-trimethyl-3-hydroxyvaleric acid methyl ester and a preparation method thereof.
Owner:RUNTAI CHEM TAIXING CO LTD +1
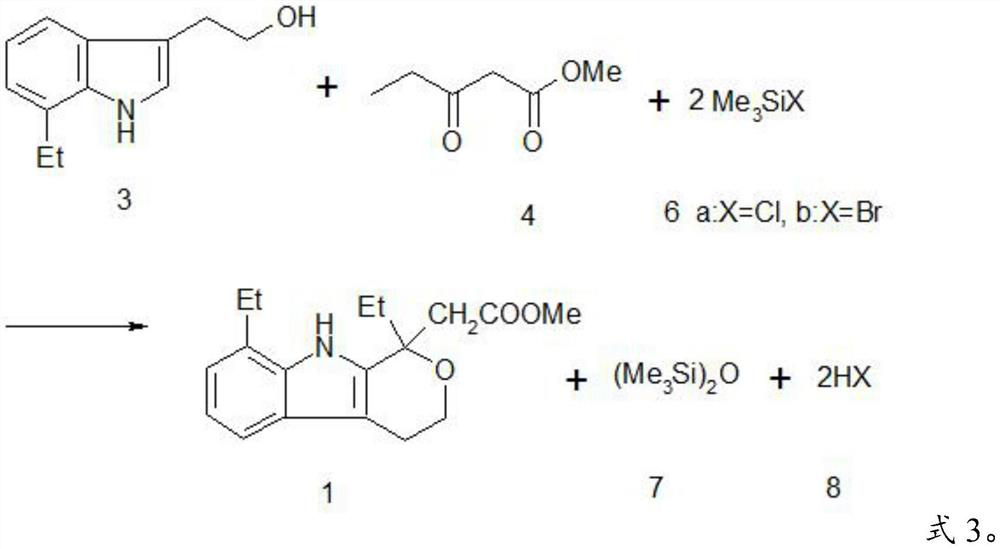
A kind of method preparing etodolac methyl ester
ActiveCN111303172BReduce workloadHigh yieldGroup 4/14 element organic compoundsOrganic synthesisEthyl group
The invention provides a method for preparing etodolac methyl ester, which relates to the technical field of organic synthesis. The method provided by the invention comprises the following steps: (1) after the reaction raw materials are mixed, a ring closure reaction is carried out at 20-25° C. to obtain a reaction liquid; the reaction raw materials include 7-ethyltryptol, 3-oxopentanoic acid Methyl ester, trimethylhalosilane and methanol, excluding concentrated sulfuric acid; the trimethylhalosilane is trimethylchlorosilane or trimethylbromosilane; (2) cooling the reaction solution to 10-15°C , and filtered to obtain etodolac methyl ester and mother liquor. The method provided by the invention has high yield and does not use concentrated sulfuric acid which is extremely harmful and easily causes product oxidation. Further, the present invention further improves the product yield through the continuous application of the mother liquor, can obtain almost quantitative products, reduces the workload of solvent treatment, and is easy to operate; and the trimethylhalosilane is easy to recover and recycle.
Owner:ZHEJIANG INT STUDIES UNIV
A kind of synthetic method of 5-aminolevulinic acid hydrochloride
ActiveCN109265341BSimple processRaw materials are easy to obtainOrganic compound preparationCarboxylic acid esters preparationPropanoic acidButanedioic acid
The invention relates to a synthesis method of 5-aminolevulinic acid hydrochloride, comprising: using succinic anhydride as a raw material, performing monoesterification with methanol to obtain monomethyl succinate; monomethyl succinate and N, N'-carbonyldiimidazole undergoes a nucleophilic substitution reaction to obtain 4-(1-imidazole)-4-oxobutanoic acid methyl ester; 4-(1-imidazole)-4-oxobutanoic acid methyl ester and nitromethane A nucleophilic substitution reaction occurs under the catalysis of a basic compound to obtain methyl 5-nitro-4-oxopentanoate; methyl 5-nitro-4-oxopentanoate undergoes a reduction reaction with a metal reducing agent and undergoes hydrolysis 5-aminolevulinic acid hydrochloride is obtained. The process of the present invention is simple, the raw materials are easy to obtain, no special rectification and recrystallization devices are needed to process the intermediate products, no toxic and expensive raw materials are used, and the use of heavy metal reducing agents is avoided, so as not to pollute the environment, and the synthesis cost It is cheap, and the purity of the synthesized product can reach 97% after recrystallization detection, and the total yield is high, which can reach more than 70%.
Owner:HENAN UNIVERSITY
The preparation method of ceftizoxime propivoxil and its intermediate and the preparation method of the intermediate
ActiveCN105017284BRaw materials are cheap and easy to getMild reaction conditionsOrganic chemistryCefotaximeValproic Acid
The present invention discloses a preparation method for ceftizoxime alapivoxil by using a mixture anhydrides method, intermediates 3a / 3b / 3c thereof and a preparation method for the intermediates . The method comprises: putting an ammonia cefotaxime acid ester compound in an organic solvent to react with BOC-alanine by using a mixture anhydrides method; hydrolyzing under condition of organic alcohol; generating intermediates 3a / 3b / 3c; the intermediates 3a / 3b / 3c condensation reacting with 7-ANCA in an organic solvent; generating BOC-alanyl ceftizoxime acid by esterification; the BOC-alanyl ceftizoxime acid reacting with halogenated pentanoic acid methyl ester in the presence of an acid-binding agent, to prepare BOC-alanyl ester ceftizoxime valproate; removing a BOC protection group; and after concentrating, obtaining the target compound ceftizoxime alapivoxil by crystal precipitation in the organic solvent. The preparation method for ceftizoxime alapivoxil has the advantages that raw materials are readily available, the reaction condition is mild, byproducts are fewer, purity is reliable, the yield is high, and the preparation method for ceftizoxime alapivoxil is suitable for large-scale industrial production.
Owner:ZHEJIANG YONGNING PHARMA
Preparation method of 4, 4-dimethyl-3-oxo-methyl pentanoate
InactiveCN106397200AImprove distillation yieldHigh purityOrganic compound preparationCarboxylic acid esters preparationHydrogenDistillation
The invention discloses a preparation method of 4, 4-dimethyl-3-oxo-methyl pentanoate. The method includes: taking pinacolone, sodium hydrogen and dimethyl carbonate as the raw materials, and using toluene as the solvent, carrying out stirring reaction at 55-60DEG C, then performing cooling, and at a pH of 4-5, preparing a 4, 4-dimethyl-3-oxo-methyl pentanoate crude product; and subjecting the 4, 4-dimethyl-3-oxo-methyl pentanoate crude product to distillation under the conditions of a vacuum degree of less than 10mm Hg and a temperature of 90-95DEG C. By means of the method provided by the invention, the 4, 4-dimethyl-3-oxo-methyl pentanoate has high distillation yield, the product purity is high, the main content is up to more than 98%, and the total yield is up to 96%.
Owner:ZHANGJIAGANG CITY ZHENFANG CHEM
Pharmaceutical Compositions
Owner:CIPLA LTD
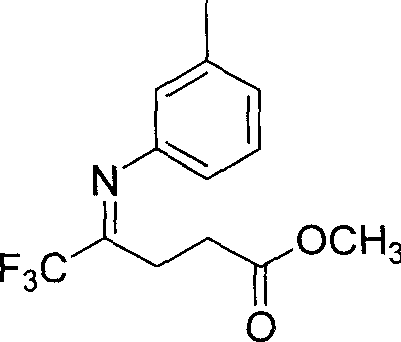
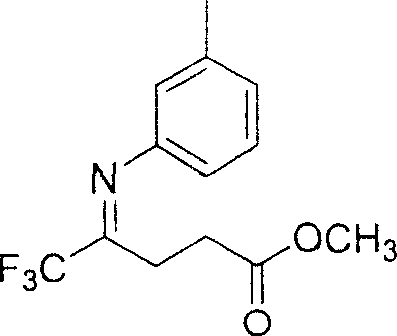
5,5,5-trifluoro-4-(3-methyl phenylimino) methyl valerate and its preparation method
InactiveCN1970533ARaw materials are easy to getSimple and fast operationImino compound preparationBenzeneNitrogen
The invention discloses a 5, 5, 5-trifluoro-4-(3-methyl benzaimino) valerianic methyl ester and making method, which comprises the following steps: adopting benzene or toluene as solvent; adding 5, 5, 5-trifluoro-4-carbonyl valerianic methyl ester and 3-methyl phenylamine in the reacting container protected by nitrogen as well as benzene or toluene; heating to 48-72h acted by catalyst; heating to 100-120 deg.c or 130-150 deg.c; stirring continuously; decompressing; evaporating solvent; separating crude product through silica gel column layer technique; obtaining light yellow compound.
Owner:JIANGSU TIANCHENG BIOCHEM PROD
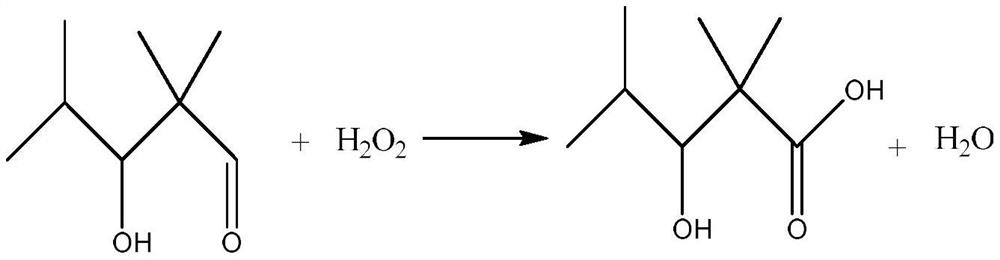
Preparation method of 2,2,4-trimethyl-3-hydroxypentanoate, waterborne industrial paint for steel structure and preparation method of waterborne industrial paint
ActiveCN110746297AHigh purityIncrease reaction rateOrganic compound preparationCarboxylic acid esters preparationPtru catalystMeth-
The invention relates to the technical field of organic compound synthesis and application and in particular relates to a preparation method of 2,2,4-trimethyl-3-hydroxypentanoate, a waterborne industrial paint for a steel structure and a preparation method of the waterborne industrial paint. The preparation method of the 2,2,4-trimethyl-3-hydroxypentanoate comprises the following steps: 1) performing a hydroxyaldehyde condensation reaction on iso-butyraldehyde under the action of an alkaline catalyst so as to produce 2,2,4-trimethyl-3-hydroxypentanaldehyde, and performing an oxidation reaction on the obtained 2,2,4-trimethyl-3-hydroxypentanaldehyde with H2O2 so as to obtain 2,2,4-trimethyl-3-hydroxypentanoic acid; and 2) enabling the 2,2,4-trimethyl-3-hydroxypentanoate obtained in the step 1) and methanol to react under the action of a strong acid catalyst, so as to obtain 2,2,4-trimethyl-3-hydroxypentanoate. The preparation method of the 2,2,4-trimethyl-3-hydroxypentanoate, which isprovided by the invention, is single in reaction product, needs no multiple steps of separation and is simple to operate. The invention further provides the waterborne industrial paint with the 2,2,4-trimethyl-3-hydroxypentanoate for the steel structure and the preparation method of the waterborne industrial paint.
Owner:RUNTAI CHEM TAIXING CO LTD +1
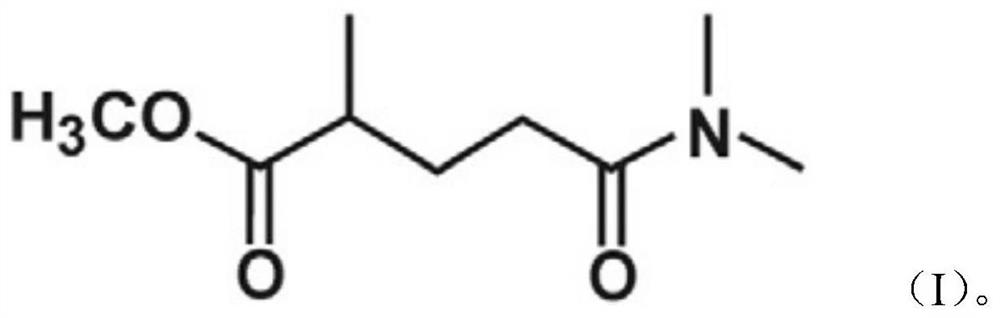
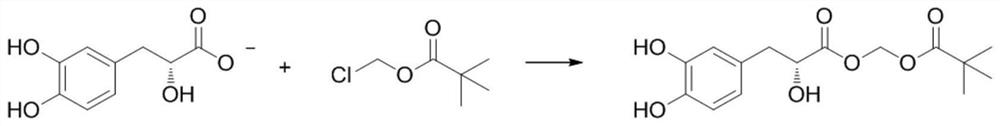
Tanshensu methyl pivalate derivative and preparation method thereof
PendingCN114394901AImprove bioavailabilityImprove reaction efficiencyOrganic compound preparationOrganic chemistry methodsOrganic solventCombinatorial chemistry
The invention relates to the technical field of medicinal chemistry, and particularly discloses a tanshinol methyl pivalate derivative. The tanshinol methyl pivalate derivative has a structure as shown in a formula I which is described in the specification. The preparation method of the tanshinol methyl pivalate derivative specifically comprises the following steps: dissolving sodium tanshinol in an organic solvent, then adding chloromethyl pivalate and an acid-binding agent, reacting at 40-60 DEG C under the protection of inert gas, and obtaining the tanshinol methyl pivalate derivative after the reaction is finished. Researches show that the tanshinol methyl pivalate derivative prepared by modifying tanshinol with chloromethyl pivalate can greatly improve the bioavailability of tanshinol.
Owner:广东医科大学附属第二医院
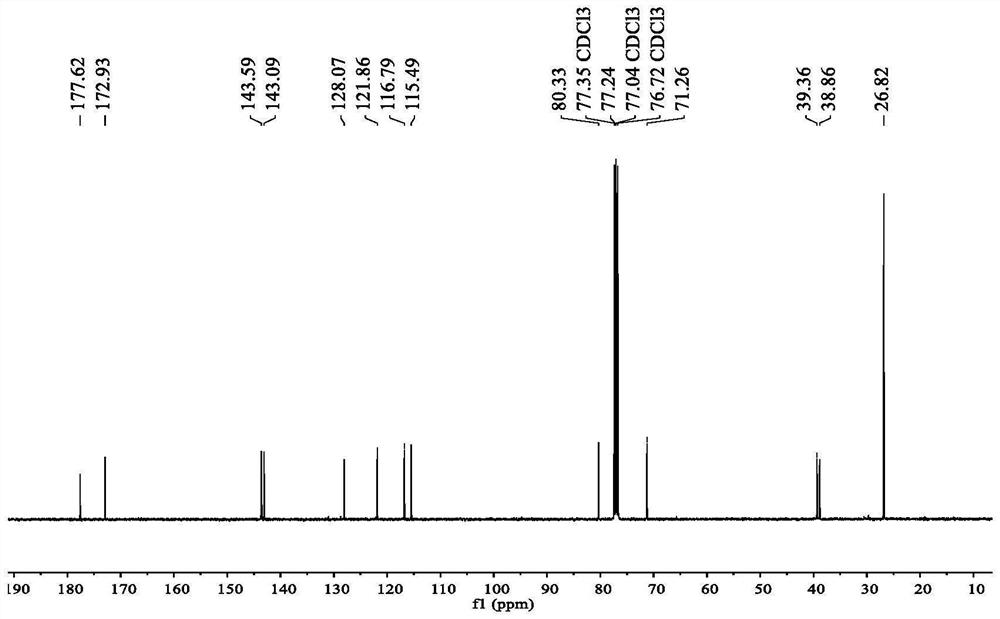
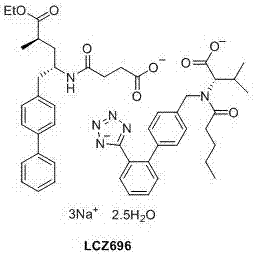
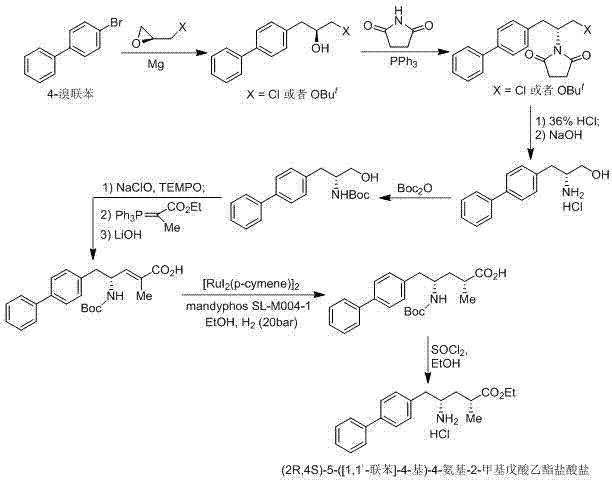
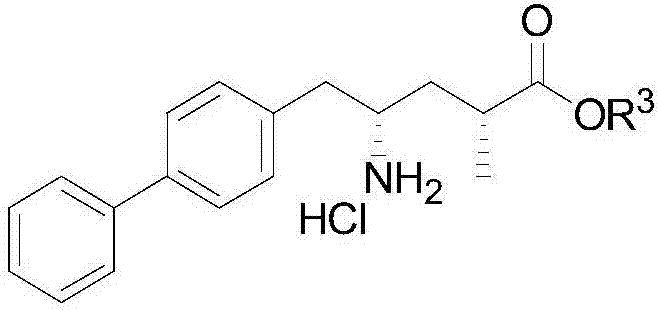
Preparation of key intermediates of lcz696
ActiveCN105601524BEasy to achieve commercial mass productionOrganic compound preparationAmino-carboxyl compound preparationMetal catalystMedicinal chemistry
Owner:WISDOM PHARM CO LTD
Composite reverse osmosis membrane as well as preparation method and application thereof
The invention discloses a composite reverse osmosis membrane as well as a preparation method and application thereof. The composite reverse osmosis membrane comprises a bottom layer, a middle porous support layer and a surface active separation layer, wherein the active separation layer is a polyamide active separation layer formed by performing interfacial polymerization on a water phase containing polyamine and an organic phase containing 5-(dimethylamino)-2-methyl-5-oxovalerate and polyacyl chloride. By adding the organic phase additive which is simple in variety and small in dosage, the prepared composite reverse osmosis membrane has excellent water flux and keeps good interception characteristic. In addition, the preparation method is simple and convenient to operate and has great industrial application prospects.
Owner:CHINA PETROLEUM & CHEM CORP +1
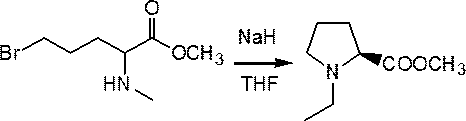
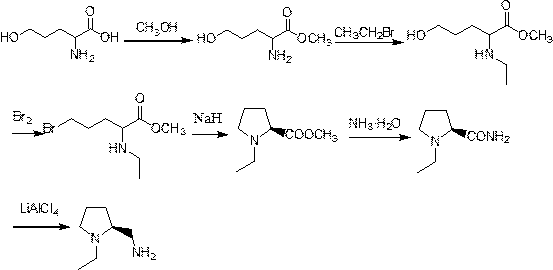
Preparation method of (s)-N-ethyl-2-aminomethylpyrrolidine
InactiveCN111153845AHigh yieldThe reaction route is simpleOrganic chemistry methodsBiochemical engineeringEthyl group
The invention provides a preparation method of (s)-N-ethyl-2-aminomethylpyrrolidine. 2-amino-5-hydroxyvaleric acid used as a raw material undergoes esterification protection to obtain methyl 2-amino-5-hydroxypentanoate, N-ethylation is further achieved through bromoethane, and then the N-ethyl-2-aminomethylpyrrolidine is prepared through hydroxyl halogenation, substitution cyclization, ammonolysisand reduction six-step reaction. High-cost raw materials and an N,O-diethylation reaction are successfully avoided, and the method has the advantages of easiness in implementation of the above reaction route, few byproducts, high yield reaching 64.2%, and suitableness for industrialization.
Owner:BEIJING VENTUREPHARM BIOTECH
Method for preparing etodolac methyl ester
The present invention relates to a new process of preparing methyl etodolate as the intermediate of non-steroid indole anti-inflammatory analgesic etodolic acid. The process includes the reaction between 7-ethyl tryptosol and 3-methyl oxy valerate inside mixed solvent of C1-C2 alcohol and benzene in the presence of acid catalyst, separating out acid layer after reaction, neutralizing the organic phase, concentrating, re-crystallizing. The process has high stability, high yield, high product quality, conversion rate near to 100 %, simple operation low cost, environment friendship and other advantages, and is suitable for industrial production.
Owner:SHANDONG XINHUA PHARMA CO LTD
Method for synthesizing gamma-alkyl butyrolactone from L-glutamic acid
The invention discloses a method for synthesizing gamma-alkyl butyrolactone from L-glutamic acid. The method disclosed by the invention comprises the following steps: (1) the L-glutamic acid is hydroxylated to obtain (S)-5-oxo tetrahydrofuran-2-formic acid, and the (S)-5-oxo tetrahydrofuran-2-formic acid is esterified to obtain (R)-2-hydroxy-1, 6-diethyl adipate; (2) the (R)-2-hydroxy-1, 6-diethyl adipate is reduced to obtain (S)-5-hydroxymethyl dihydrofuran-2 (3H)-ketone; (3) the (S)-5-hydroxymethyl dihydrofuran-2 (3H)-ketone is sulfonated to obtain (S)-(5-oxo tetrahydrofuran group)-4-methyl p-methyl toluenesulfonate, and the (S)-(5-oxo tetrahydrofuran group)-4-methyl p-methyl toluenesulfonate is epoxidized to obtain (S)-4, 5-epoxy-methyl pentanoate; and (4) the (S)-4, 5-epoxy-methyl pentanoate is used as a raw material for synthesizing the gamma-alkyl butyrolactone. The method disclosed by the invention has the characteristics that the production cost is low, production can be carried out according to the needs of customers, the flexibility is very high, the safety is good, the requirement on equipment is low, and the method is very suitable for industrial production.
Owner:龙海市贝特利生物科技有限公司
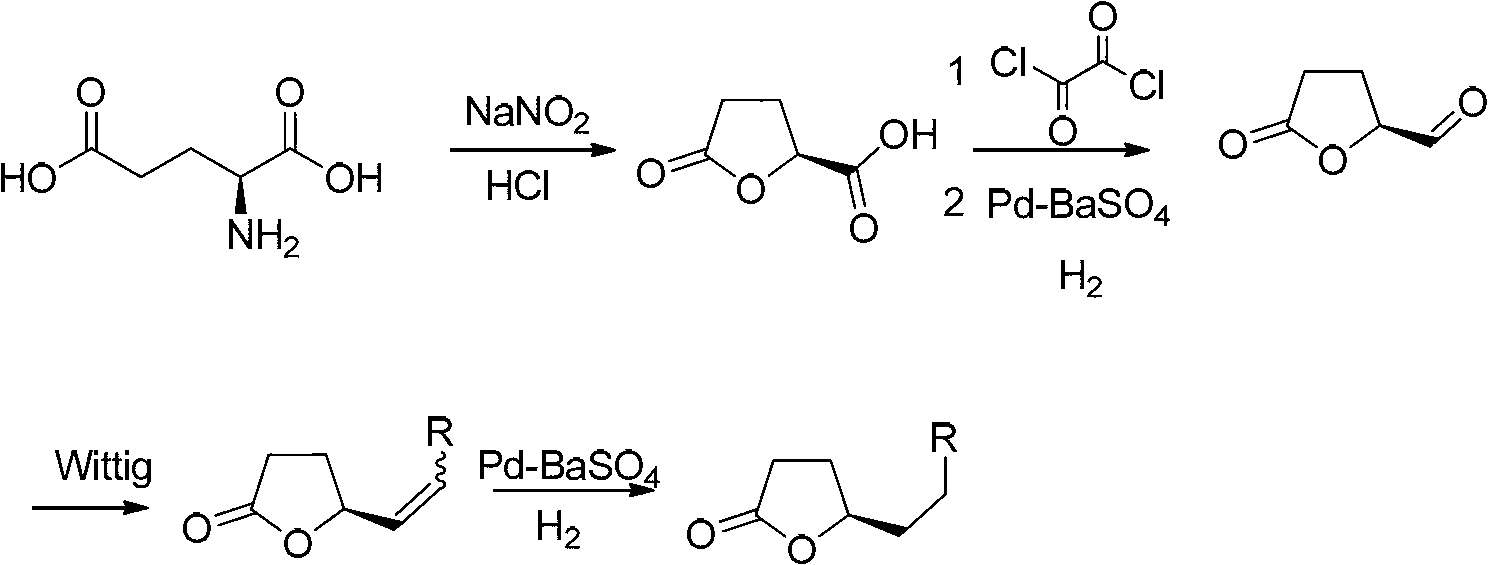
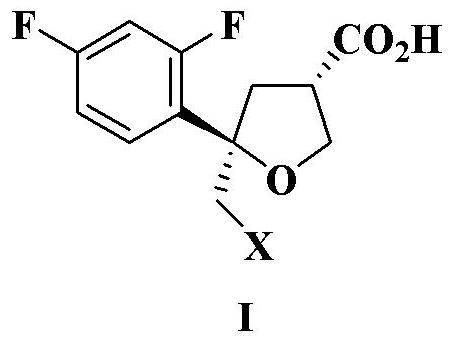
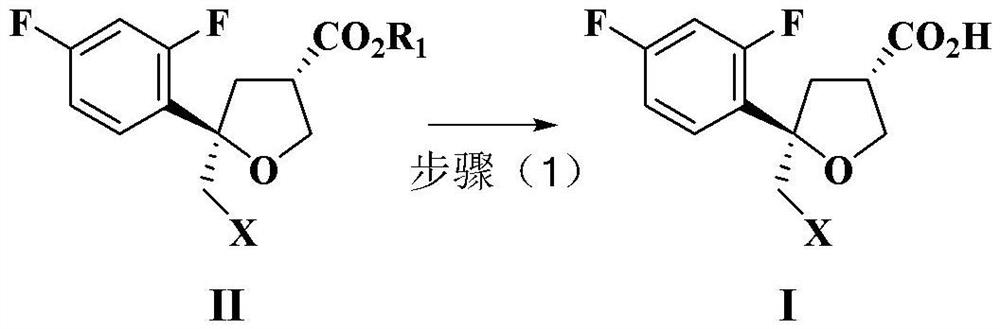
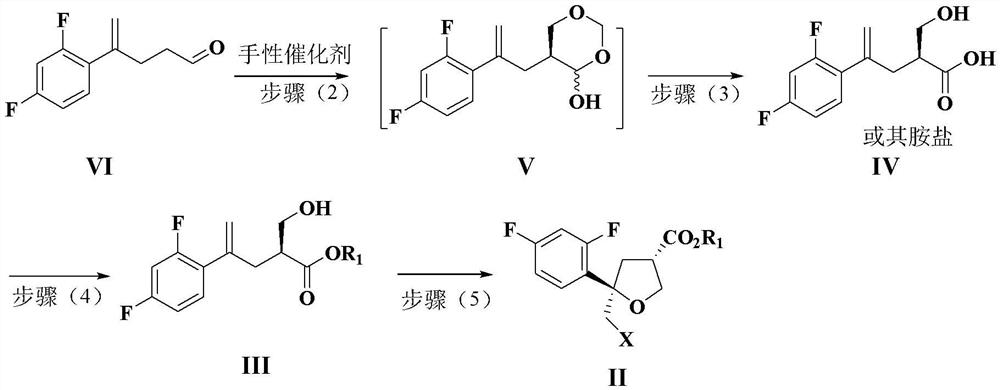
Preparation method of intermediate compound for synthesizing posaconazole and intermediate compound prepared by preparation method
PendingCN114591272AHigh yieldHigh purityOrganic compound preparationCarboxylic acid esters preparationPtru catalystHydrolysis
The invention relates to a preparation method of an intermediate compound for synthesizing medicine posaconazole and the intermediate compound prepared by the preparation method. The preparation method of the compound as shown in the formula I comprises the following steps: by taking 4-(2, 4-difluorophenyl)-4-enylpentanoic acid methyl ester or a reduction product thereof 4-(2, 4-difluorophenyl)-4-enylpentanecarboxaldehyde as an initial raw material, reacting with formaldehyde or paraformaldehyde in the presence of a chiral catalyst, and then carrying out characteristic oxidation, esterification reaction and halogenation cyclization reaction to obtain the compound as shown in the formula I. The compound as shown in the formula I is prepared by the following steps of: reacting the initial raw material with methyl 4-(2, 4-difluorophenyl)-4-enylpentanoic acid methyl ester or the reduction product thereof. And finally carrying out hydrolysis and acid treatment. The preparation method has the advantages of mild reaction conditions, simple reaction process, high total yield of target products, high purity and the like, and is suitable for industrial production.
Owner:ZHEJIANG AUSUN PHARMA
Method for synthesizing trifluoromethoxybenzene
The invention relates to a new method for synthesizing trifluoromethoxybenzene, which comprises the following steps of: under the protection of nitrogen, adding a certain amount of 5,5,5-trifluoro-4-oxopentanoate and 2-methyl-4-methoxybenzenamine into a reaction container by taking benzene or methylbenzene as a solvent; reacting in the presence of a catalyst, heating and refluxing for 24 hours, and constantly stirring for complete reaction; after reaction, steaming to remove the solvent under reduced pressure; separating a crude product by using silica gel column chromatography; and finally obtaining a light yellow liquid target compound, namely 5,5,5-trifluoro-4-(2-methyl-4-methoxybenze-imido)methyl pentanoate. The method has the advantages that: raw materials are readily available; the process is simple; and the product is prepared by a two-step method, so the method is stable and reliable, and the production efficiency is high.
Owner:TIANJIN TIANCHENG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



![PROCESS FOR PREPARATION OF 4-FLUORO-alpha-[2METHYL-L-OXOPROPYL]-gamma-OXO-N-beta-DIPHENYLBENZENE BUTANE AMIDE PROCESS FOR PREPARATION OF 4-FLUORO-alpha-[2METHYL-L-OXOPROPYL]-gamma-OXO-N-beta-DIPHENYLBENZENE BUTANE AMIDE](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/37d2ab3d-394d-44ae-9485-bdb31c6d67c8/US20130184493A1-20130718-D00001.png)
![PROCESS FOR PREPARATION OF 4-FLUORO-alpha-[2METHYL-L-OXOPROPYL]-gamma-OXO-N-beta-DIPHENYLBENZENE BUTANE AMIDE PROCESS FOR PREPARATION OF 4-FLUORO-alpha-[2METHYL-L-OXOPROPYL]-gamma-OXO-N-beta-DIPHENYLBENZENE BUTANE AMIDE](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/37d2ab3d-394d-44ae-9485-bdb31c6d67c8/US20130184493A1-20130718-D00002.png)
![PROCESS FOR PREPARATION OF 4-FLUORO-alpha-[2METHYL-L-OXOPROPYL]-gamma-OXO-N-beta-DIPHENYLBENZENE BUTANE AMIDE PROCESS FOR PREPARATION OF 4-FLUORO-alpha-[2METHYL-L-OXOPROPYL]-gamma-OXO-N-beta-DIPHENYLBENZENE BUTANE AMIDE](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/37d2ab3d-394d-44ae-9485-bdb31c6d67c8/US20130184493A1-20130718-D00003.png)