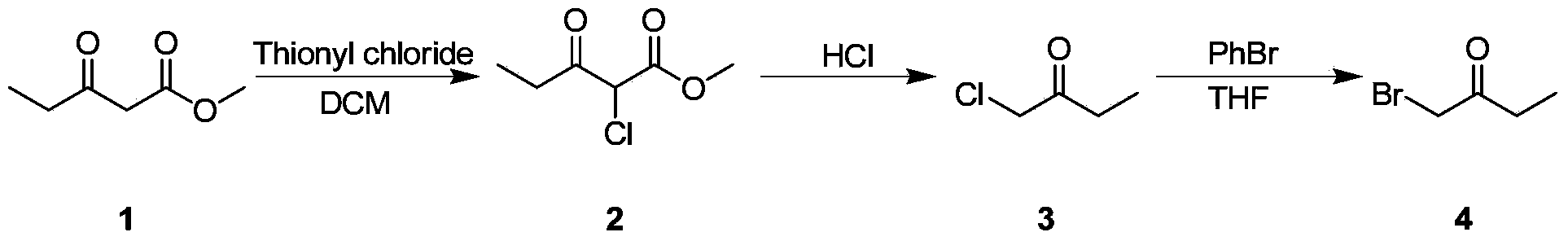
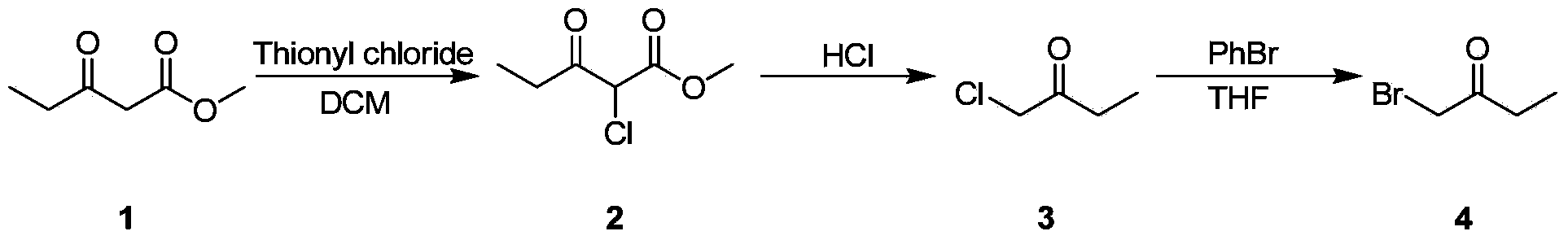
Bromo-butanone synthesis method
A technology of bromobutanone and methyl oxopentanoate, applied in the field of biomedicine, can solve problems such as hindering the development and development of downstream products, and achieve the effects of easy promotion, easy availability of raw materials and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: laboratory synthesis
[0017] 1. Chlorination reaction
[0018] Dissolve methyl 3-oxopentanoate (10g) in anhydrous dichloromethane (50mL), drop the temperature to 0°C and add thionyl chloride (10.4g) dropwise, after the dropwise addition, warm up to room temperature 25°C, stir After 8 hours, TLC detected that the reaction was complete. The reaction solution was directly concentrated to obtain product 2 (11.4 g), with a yield of 90%.
[0019] 2. Degreasing reaction
[0020] Compound 2 (10 g) was dissolved in hydrochloric acid (20 mL), stirred evenly, heated to 80° C. and stirred for 4 hours, and TLC detected that the reaction was complete. Stop heating and drop to room temperature, add water, extract with dichloromethane, combine organic phases, wash with sodium bicarbonate solution, adjust to alkalinity, separate liquid with separatory funnel, wash with saturated sodium chloride, dry over anhydrous sodium sulfate, Product 3 (5.4 g) was obtained after c...
Embodiment 2
[0023] Embodiment 2: small test
[0024] 1. Chlorination reaction
[0025] Dissolve methyl 3-oxopentanoate (100g) in anhydrous dichloromethane (500mL), drop the temperature to 0°C and add thionyl chloride (104g) dropwise. Hours, TLC detected that the reaction was complete. The reaction solution was directly concentrated to obtain product 2 (114g), with a yield of 90%.
[0026] 2. Degreasing reaction
[0027] Compound 2 (100 g) was dissolved in hydrochloric acid (200 mL), stirred evenly, heated to 80° C. and stirred for 4 hours, and TLC detected that the reaction was complete. Stop heating and drop to room temperature, add water, extract with dichloromethane, combine organic phases, wash with sodium bicarbonate solution, adjust to alkalinity, separate liquid with separatory funnel, wash with saturated sodium chloride, dry over anhydrous sodium sulfate, Product 3 (54 g) was obtained after concentration with a yield of 84%.
[0028] 3. Bromination reaction
[0029] Compound...
Embodiment 3
[0030] Embodiment 3: pilot test
[0031] 1. Chlorination reaction
[0032] Dissolve methyl 3-oxopentanoate (1kg) in anhydrous dichloromethane (5L), drop the temperature to 0°C and add thionyl chloride (1.04kg) dropwise, after the dropwise addition, warm up to room temperature 25°C, stir After 8 hours, TLC detected that the reaction was complete. The reaction solution was directly concentrated to obtain product 2 (1.14kg), with a yield of 90%.
[0033] 2. Degreasing reaction
[0034] Compound 2 (1kg) was dissolved in hydrochloric acid (2L), stirred evenly, heated to 80°C and stirred for 4 hours, and the reaction was complete as detected by TLC. Stop heating and drop to room temperature, add water, extract with dichloromethane, combine organic phases, wash with sodium bicarbonate solution, adjust to alkalinity, separate liquid with separatory funnel, wash with saturated sodium chloride, dry over anhydrous sodium sulfate, Product 3 (540 g) was obtained after concentration wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com