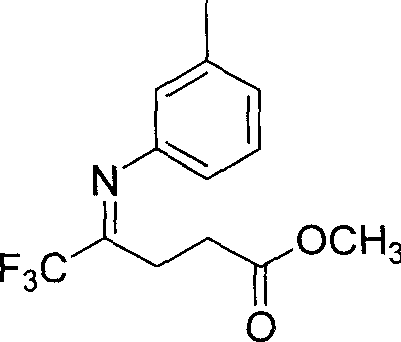
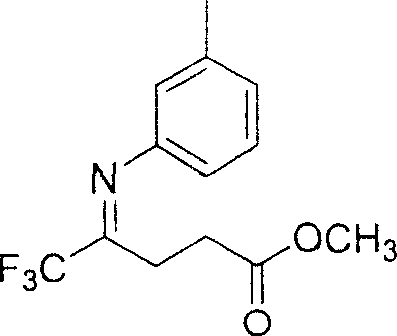
5,5,5-trifluoro-4-(3-methyl phenylimino) methyl valerate and its preparation method
A technology of methyl aniline and methyl valerate, applied in the field of organic compound synthesis technology, can solve the problems such as limitation of physiological activity of fluorine-containing amino acids, lack, lack of synthesis means, etc., and achieves high yield, easy operation, raw material easy-to-get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The processing steps in the present embodiment are as follows:
[0022] Under the protection of nitrogen, add 5,5,5-trifluoro-4-carbonyl-pentanoic acid methyl ester 10mmol, 1.9 g in a three-necked flask with a volume of 50 ml; 3-methylaniline 10 mmol, 1.1 g; solvent toluene 25 ml; and catalyst p-toluenesulfonic acid 1 mmol, 0.20 g. Then install a water separator and a reflux condenser, heat to reflux for 72 hours, and the heating temperature is 140°C; at the same time, stir continuously to make the reaction complete; after the reaction is completed, the solvent is evaporated under reduced pressure; the crude product is separated by silica gel column chromatography, and the silica gel column layer Ethyl acetate in the analysis: sherwood oil=1: 4; Finally, 2.0 grams of light yellow target compound 5,5,5-trifluoro-4-(3-methylphenylimino) methyl pentanoate was obtained. The rate is 73%.
Embodiment 2
[0024] The processing steps in the present embodiment are as follows:
[0025] Under nitrogen protection, in a three-necked flask with a volume of 100 ml, 10 mmol, 1.9 grams of 5,5,5-trifluoro-4-carbonyl-valeric acid methyl ester were added; 15 mmol, 1.6 grams of 3-methylaniline; the solvent toluene 50 ml; and catalyst p-toluenesulfonic acid 1 mmol, 0.20 g. Then install a water separator and a reflux condenser, heat and reflux for 80 hours, and the heating temperature is 140°C; at the same time, stir continuously to make the reaction complete; after the reaction is completed, the solvent is evaporated under reduced pressure; the crude product is separated by silica gel column chromatography, and the silica gel column layer Ethyl acetate in the analysis: sherwood oil=1: 4; Finally, 2.2 grams of light yellow target compound 5,5,5-trifluoro-4-(3-methylphenylimino) methyl pentanoate was obtained. The rate is 81%.
Embodiment 3
[0027] The processing steps in the present embodiment are as follows:
[0028] Under the protection of nitrogen, add 5,5,5-trifluoro-4-carbonyl-pentanoic acid methyl ester 10mmol, 1.9 g in a three-necked flask with a volume of 250 ml; 3-methylaniline 3 mmol, 3.2 g; solvent toluene 100 ml; and catalyst p-toluenesulfonic acid 1 mmol, 0.20 g. Then install a water separator and a reflux condenser, heat and reflux for 100 hours, and the heating temperature is 140°C; at the same time, stir continuously to make the reaction complete; after the reaction is completed, the solvent is evaporated under reduced pressure; the crude product is separated by silica gel column chromatography, and the silica gel column layer Ethyl acetate in the analysis: sherwood oil=1: 4; Finally, 2.2 grams of light yellow target compound 5,5,5-trifluoro-4-(3-methylphenylimino) methyl pentanoate was obtained. The rate is 81%.
[0029] The product obtained in the above examples was detected by infrared spectr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com