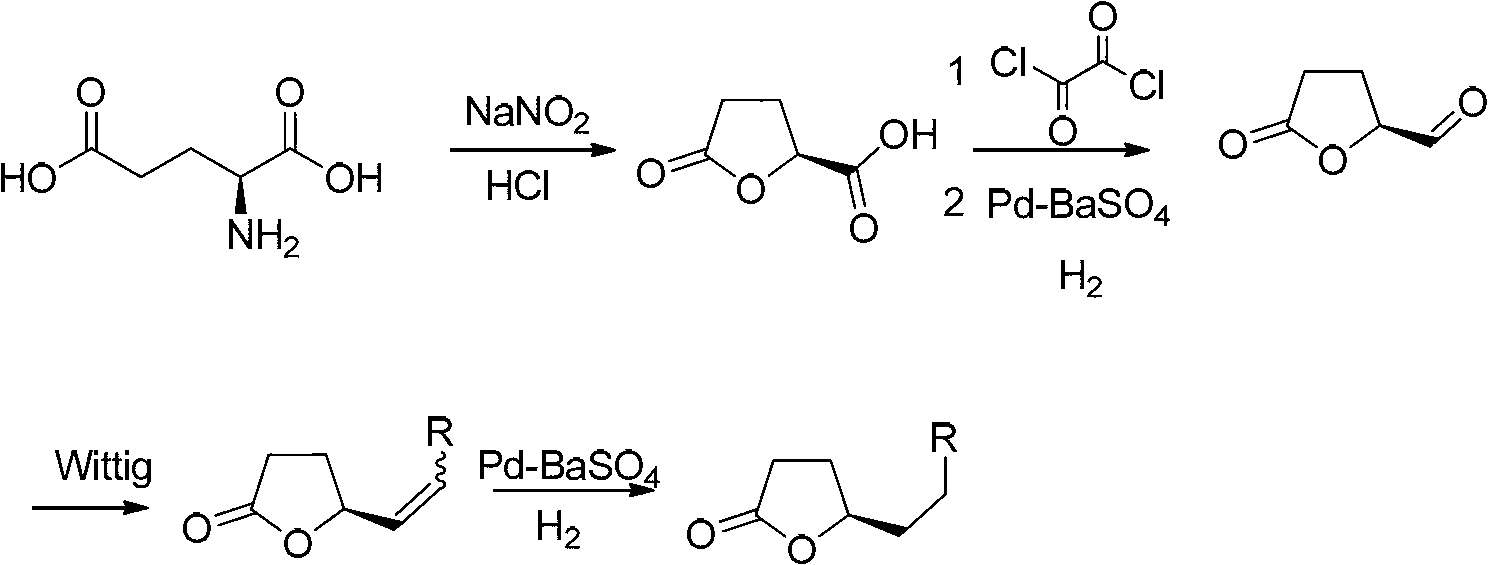
Method for synthesizing gamma-alkyl butyrolactone from L-glutamic acid
A technology of alkyl butyrolactone and glutamic acid, applied in the field of perfume industry, can solve the problems of triphenylphosphorus being difficult to handle, unsuitable for industrial production, dangerous in operation, etc., and achieves low price, low equipment requirements, and reduced production cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1. Preparation of epoxy compounds
[0038] 1. Add 400g of glutamic acid, 600mL of water and 600mL of concentrated hydrochloric acid into the reaction flask, then dropwise add 600mL of sodium nitrite solution (containing 280g of sodium nitrite), keep the reaction temperature at 0-5°C, and finish dropping in 6-8 hours , after the dropwise addition was completed, it was raised to room temperature and stirred overnight, and the water was evaporated under reduced pressure below 50°C to obtain a white salt-like viscous solid, which was extracted with 400 mL of ethyl acetate three times, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. Obtain (S)-5-oxotetrahydrofuran-2-carboxylic acid; add 1L absolute ethanol and 50-80mL concentrated sulfuric acid to the crude (S)-5-oxotetrahydrofuran-2-carboxylic acid obtained above, and stir for 8~14 hours After the reaction was completed, anhydrous sodium carbonate was added to neutralize to pH = 7...
Embodiment 2
[0050] 1. Preparation of epoxy compounds
[0051] 1. Add 400g of glutamic acid, 600mL of water and 600mL of concentrated hydrochloric acid into the reaction flask, then dropwise add 600mL of sodium nitrite solution (containing 280g of sodium nitrite), keep the reaction temperature at 0-5°C, and finish dropping in 6-8 hours , after the dropwise addition was completed, it was raised to room temperature and stirred overnight, and the water was evaporated under reduced pressure below 50°C to obtain a white salt-like viscous solid, which was extracted with 400 mL of ethyl acetate three times, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. Obtain (S)-5-oxotetrahydrofuran-2-carboxylic acid; add 1L absolute ethanol and 50-80mL concentrated sulfuric acid to the crude (S)-5-oxotetrahydrofuran-2-carboxylic acid obtained above, and stir for 8~14 hours After the reaction was completed, anhydrous sodium carbonate was added to neutralize to pH = 7...
Embodiment 3
[0063] 1. Preparation of epoxy compounds
[0064] 1. Add 400g of glutamic acid, 600mL of water and 600mL of concentrated hydrochloric acid into the reaction flask, then dropwise add 600mL of sodium nitrite solution (containing 280g of sodium nitrite), keep the reaction temperature at 0-5°C, and finish dropping in 6-8 hours , after the dropwise addition was completed, it was raised to room temperature and stirred overnight, and the water was evaporated under reduced pressure below 50°C to obtain a white salt-like viscous solid, which was extracted with 400 mL of ethyl acetate three times, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. Obtain (S)-5-oxotetrahydrofuran-2-carboxylic acid; add 1L absolute ethanol and 50-80mL concentrated sulfuric acid to the crude (S)-5-oxotetrahydrofuran-2-carboxylic acid obtained above, and stir for 8~14 hours After the reaction was completed, anhydrous sodium carbonate was added to neutralize to pH = 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com