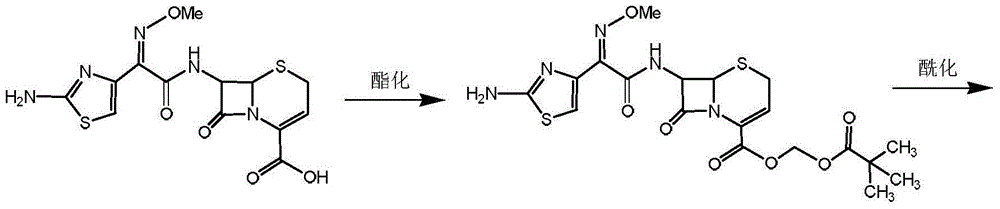
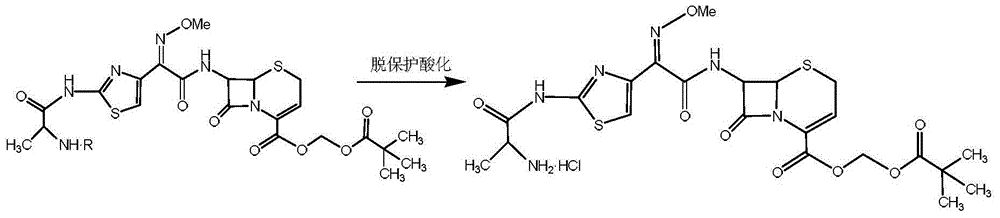
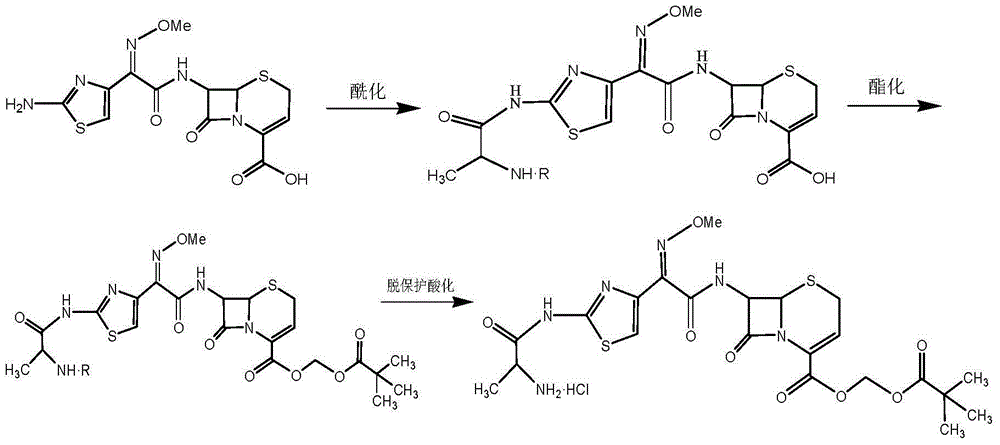
Preparation method for ceftizoxime alapivoxil, intermediates thereof and preparation method for intermediates
A technology of cefizoxime propiproxil and esterification, which is applied in the field of preparation of intermediates and intermediates, and can solve the problems of high cost, harsh reaction conditions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Synthesis of BOC-alanylaminothioxime acetate (1c): Weigh 60 g (0.32 mol) of BOC-alanine and add it to 840 ml of ether, stir and dissolve, then cool down to -15°C, and add four drops within 30 minutes. 35.376 g (0.35 mol) of methylguanidine, and 36.16 g (0.33 mol) of ethyl chloroformate were added dropwise. After the dropwise addition, stir for 2 hours, add 65.52 g (0.29 mol) of ethyl aminothioxime acetate, continue the reaction for 1 hour, then raise the temperature to -5°C and stir for 20 hours. After the reaction, wash with 300ml of 1N HCl and 200ml of saturated saline. The organic layer was dried and concentrated to dryness under reduced pressure. After vacuum drying, 84 g of light yellow BOC-alanylaminothioxime acetate (1C) solid was obtained, with a yield of 128.21% and a purity of 99.27%.
[0076] Embodiment 2-10 according to the method for embodiment 1, raw and auxiliary material molar ratio is also the same as embodiment 1:
[0077]
Embodiment 11
[0079] The hydrolysis of BOC-alanylaminothioxime acetate (1c): Weigh the BOC-alanylaminothioxime acetate (1c) 84g (0.21mol) that step 1 makes and join in 62.5ml methanol, Then add 2N NaOH400ml, stir to dissolve, and continue to stir for 8 hours. After the reaction, concentrated hydrochloric acid was added dropwise to the reaction solution to adjust the pH to 8.5, methanol was evaporated to dryness, then 250ml of water and 300ml of dichloromethane were added to the reaction solution for extraction, the layers were left to stand, and the collected water layer was adjusted to pH 8.5 with 3N HCl. The pH was reached to 0.8, stirred rapidly for 1 hour, filtered, and the filter cake was dried to obtain 78.47 g of white BOC-alanylaminothioxime acetic acid (2a) as a solid (93.41% yield) with a purity of 98.83%.
Embodiment 12
[0081] Preparation of active ester Ⅰ (3a): Weigh 40 g (0.11 mol) of BOC-alanylaminothioxime acetic acid (2a) and 22.83 g (0.15 mol) of 1-hydroxybenzotriazole (HOBT), and add In 175ml of dimethylformamide (DMF), stir until dissolved at 20°C, then add 41.09g of dicyclohexylcarbodiimide (DCC) dropwise within 40 minutes, stir for 1 hour after adding, filter, 20ml of dimethylformamide Amide (DMF) washes the filter cake, combines the filtrate, adds water 120ml, separates out a large amount of solids, filters, and a small amount of dimethylformamide (DMF) solution (DMF:H 2 O=1:3) washed the filter cake and dried in vacuum to obtain 49.87 g (yield 124.68%) of light yellow active ester I (3a) solid with a purity of 98.22%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com