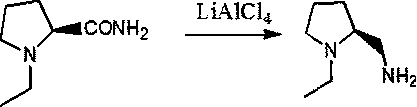Preparation method of (s)-N-ethyl-2-aminomethylpyrrolidine
A technology of aminomethylpyrrolidine and ethylpyrrolidine, which is applied in the field of synthesis of (s)-N-ethyl-2-aminomethylpyrrolidine, can solve unfavorable industrialized production, difficult reaction, L-co-ammonia The problem of high acid cost, to achieve the effect of less by-products, easy reaction route and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
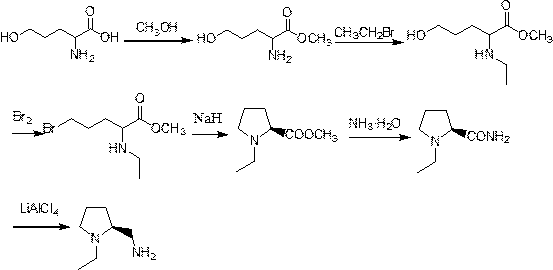
[0022] Example 1 Preparation of 2-amino-5-hydroxypentanoic acid methyl ester
[0023] Using 2-amino-5-hydroxyvaleric acid as a raw material, the esterification reaction with methanol is used to obtain 2-amino-5-hydroxyvaleric acid methyl ester. Specifically, methanol is added to the raw material, the molar ratio of 2-amino-5-hydroxypentanoic acid and methanol is 1:1.5, 2-amino-5-hydroxypentanoic acid and concentrated H 2 SO 4 The mass ratio is 1:0.2, reflux stirring 3h, 2-amino-5-hydroxypentanoic acid methyl ester yield is 90%, and its reaction equation is as follows:
[0024]
Embodiment 2
[0025] Example 2 Preparation of 2-ethylamino-5-hydroxypentanoic acid methyl ester
[0026] Get 2-ethylamino-5-hydroxyvaleric acid methyl ester product 10.0 g among the embodiment 1 and bromoethane 10.4 g are mixed in DMF (100ml), in N 2 Add NaH in batches under protection, stir at room temperature for 4-5 h, rinse with water after the reaction, extract with ethyl acetate, and distill under reduced pressure to obtain 9.8 g of the product with a yield of 82%. The reaction equation is as follows:
[0027]
Embodiment 3
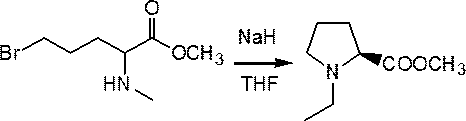
[0028] Example 3 Preparation of methyl 5-bromo-2-ethylaminovalerate
[0029] Take 8.0 g of the product of Example 2 and add it to carbon tetrachloride (50ml) solvent, stir, add 8.7 g of liquid bromine dropwise, stir at 60°C for 2 hours, cool, and remove the solvent by rotary evaporation. 2 Cl 2 Wash the residue with water, separate the liquids, and distill under reduced pressure if there are signs, and obtain the target product, methyl 5-bromo-2-ethylaminovalerate, with a mass of 7.0 g and a yield of 64%. Its reaction equation is as follows:
[0030]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com