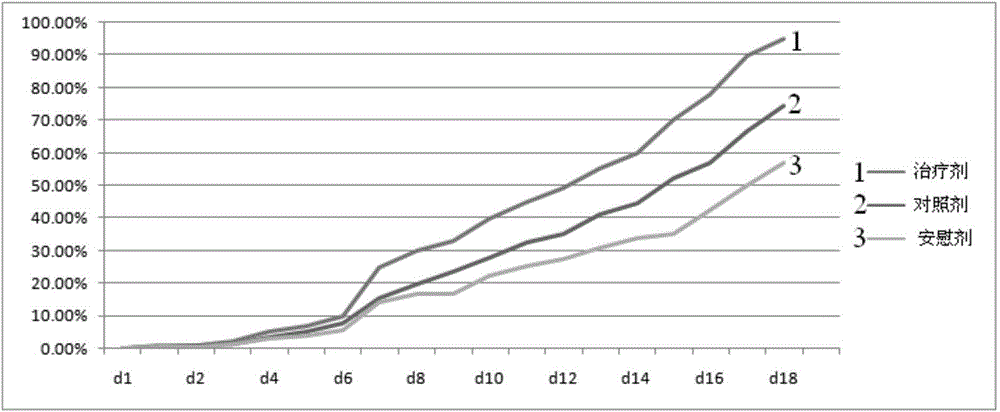
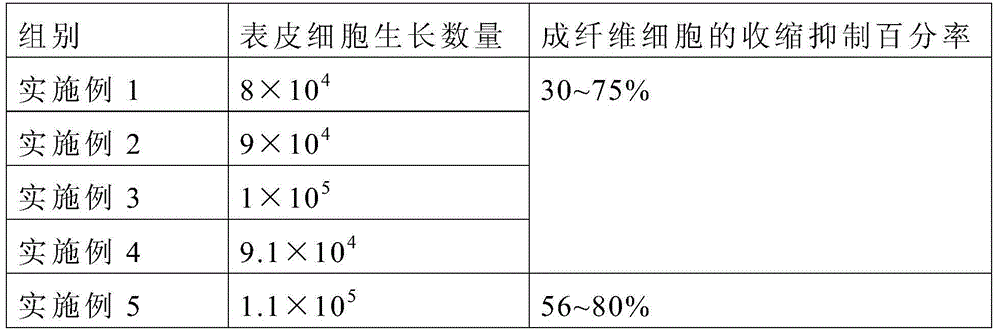
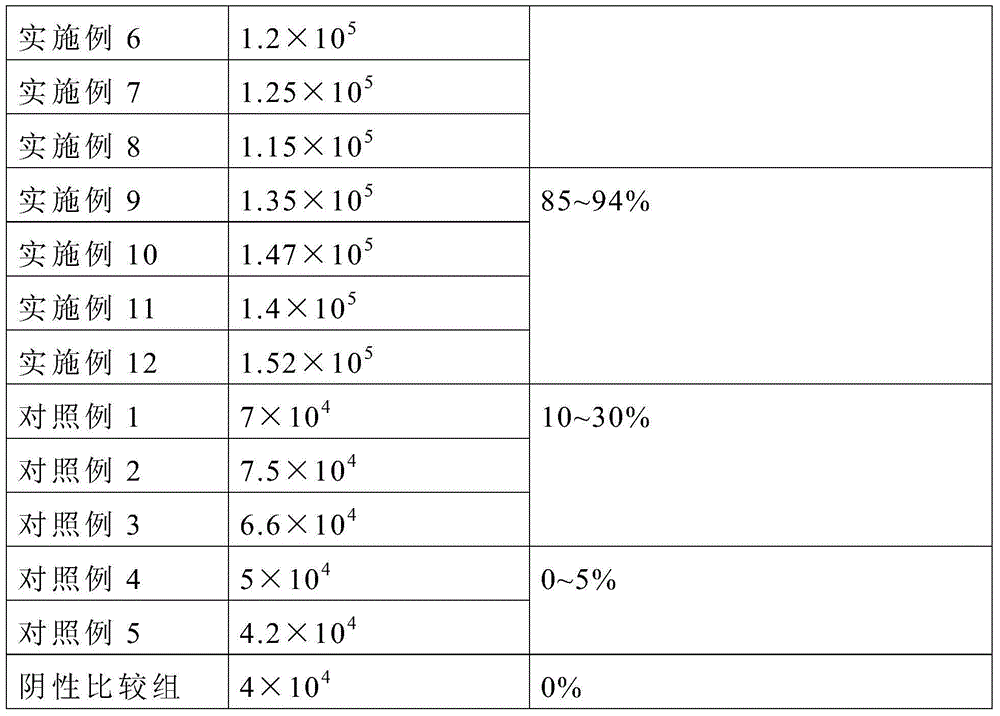
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
192 results about "Granulocyte macrophage colony-stimulating factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Granulocyte-macrophage colony-stimulating factor (GM-CSF), also known as colony-stimulating factor 2 (CSF2), is a monomeric glycoprotein secreted by macrophages, T cells, mast cells, natural killer cells, endothelial cells and fibroblasts that functions as a cytokine. The pharmaceutical analogs of naturally occurring GM-CSF are called sargramostim and molgramostim.
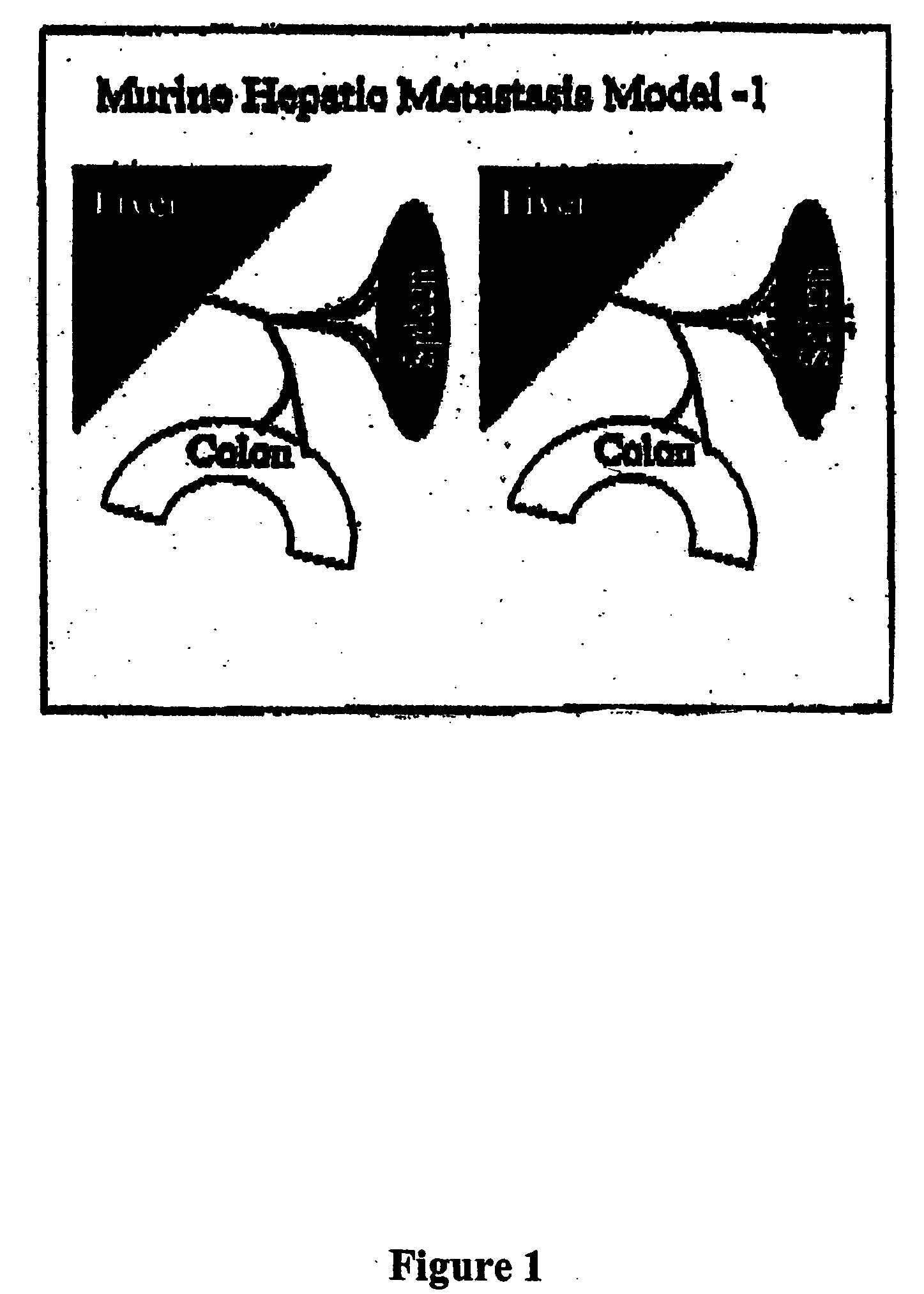
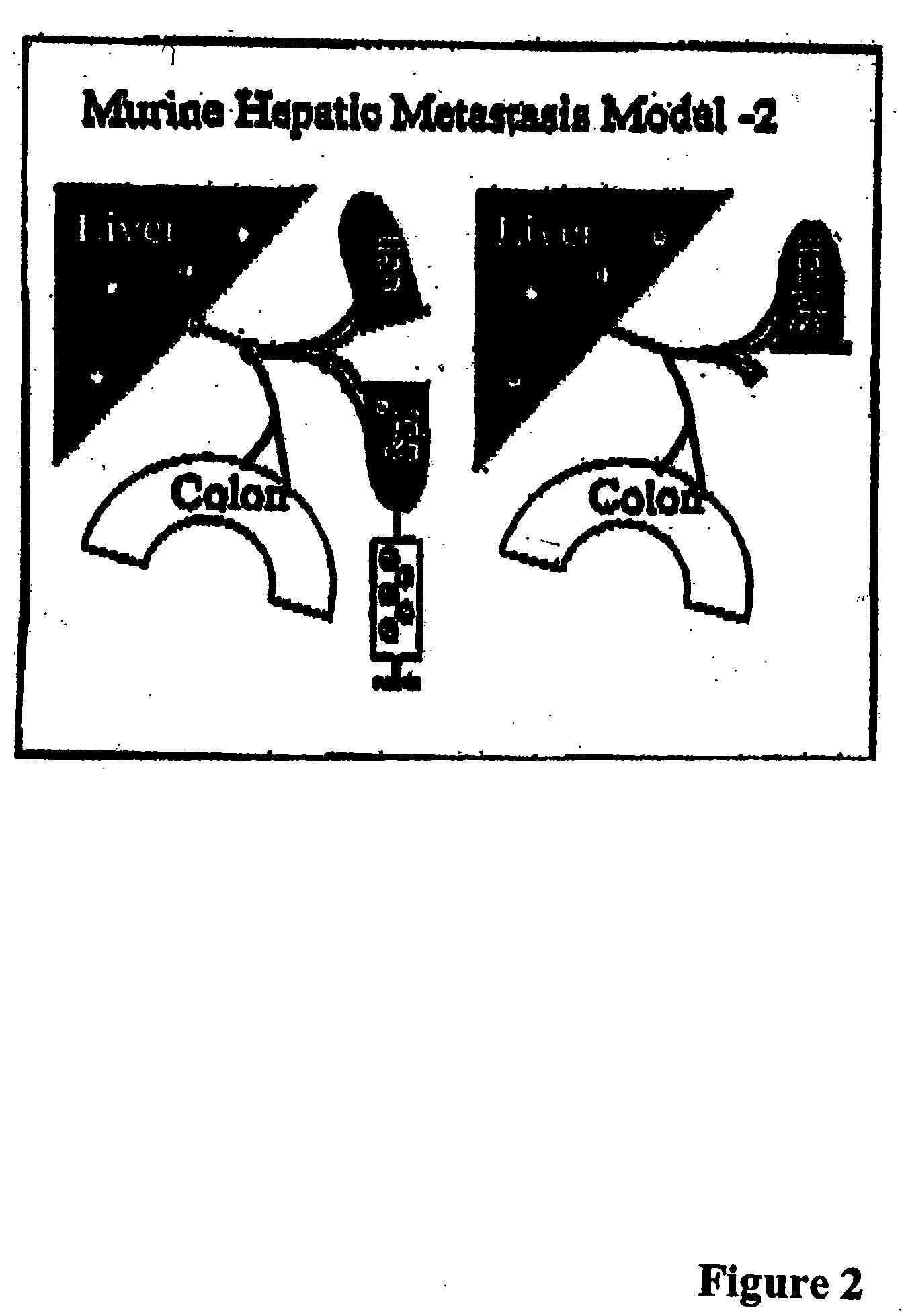
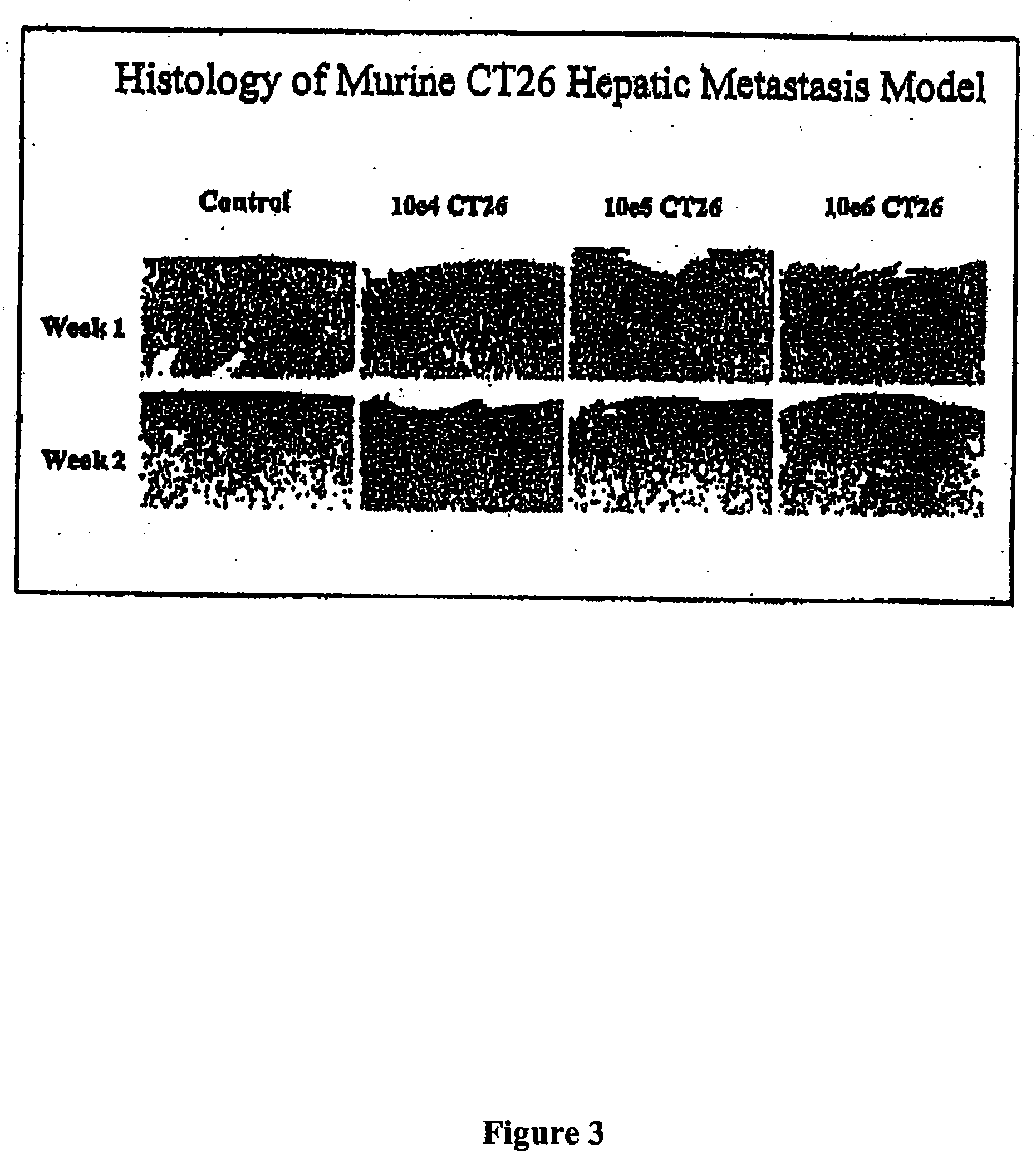
Methods and compositions for the targeting of a systemic immune response to specific organs or tissues
The invention provides methods and compositions for targeting a separately generated immune response to a specific organ or tissue, e.g. one affected by cancer, using one or more agents with a tropism for the organ or tissue or that can be specifically localized to the desired organ or tissue. For example, the invention provides methods ands compositions for treating liver metastases from colorectal cancer using a combination of a granulocyte / macrophage colony stimulating factor (GM-CSF) augmented tumor cell vaccination and Listeria monocytogenes (LM) infection.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method of isolating and culturing mesenchymal stem cell derived from umbilical cord blood
InactiveUS20070092967A1Increase success rateArtificial cell constructsSkeletal/connective tissue cellsInterleukin 6G-csf therapy
The present invention relates to a method of isolating and culturing mesenchymal stem cells using umbilical cord blood that is most ideal for cell therapy. The method comprises adding an anti-coagulant to umbilical cord blood having a volume of more than 45 ml per unit, which is obtained within 24 hours after parturition; diluting the resulting mixture of the anti-coagulant and umbilical cord blood with an αMEM (alpha-minimum essential medium), followed by centrifugation to harvest monocytes; and subjecting the obtained monocytes into suspension culture in the αMEM containing Stem Cell Factor, GM-CSF (granulocyte-macrophage colony-stimulating factor), G-CSF (granulocyte colony-stimulating factor), IL-3 (interleukin-3) and IL-6 (interleukin-6).
Owner:HAN HOON
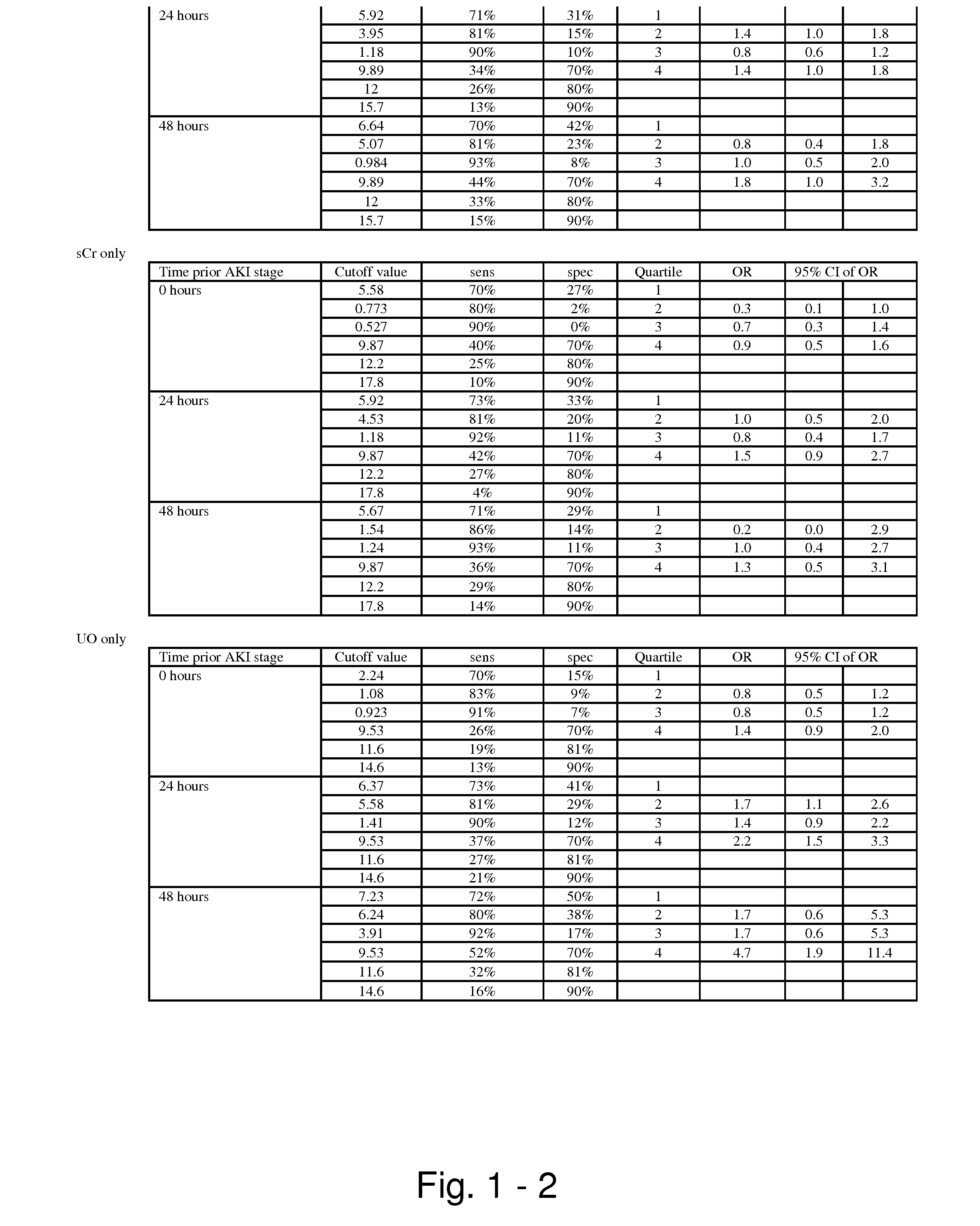
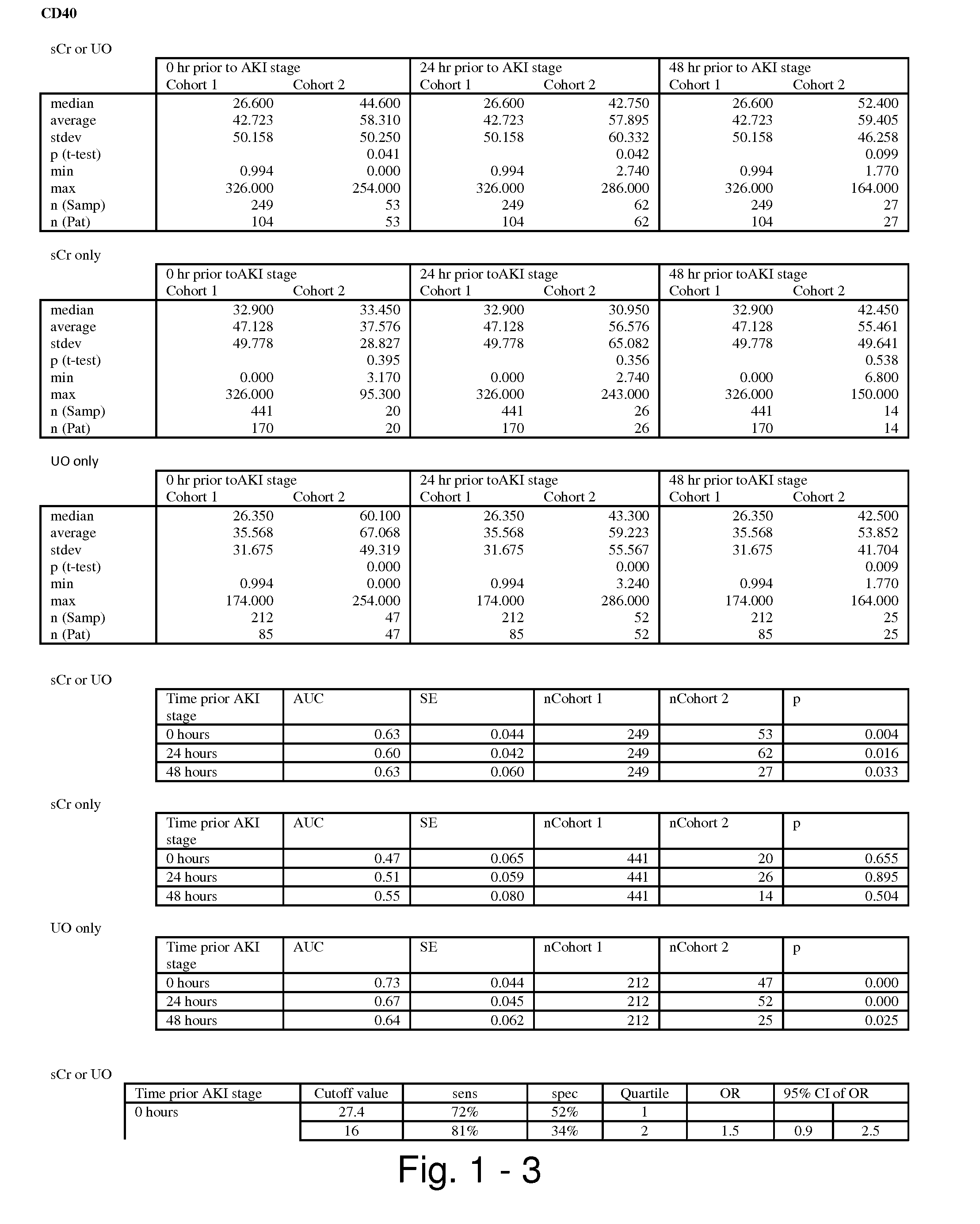
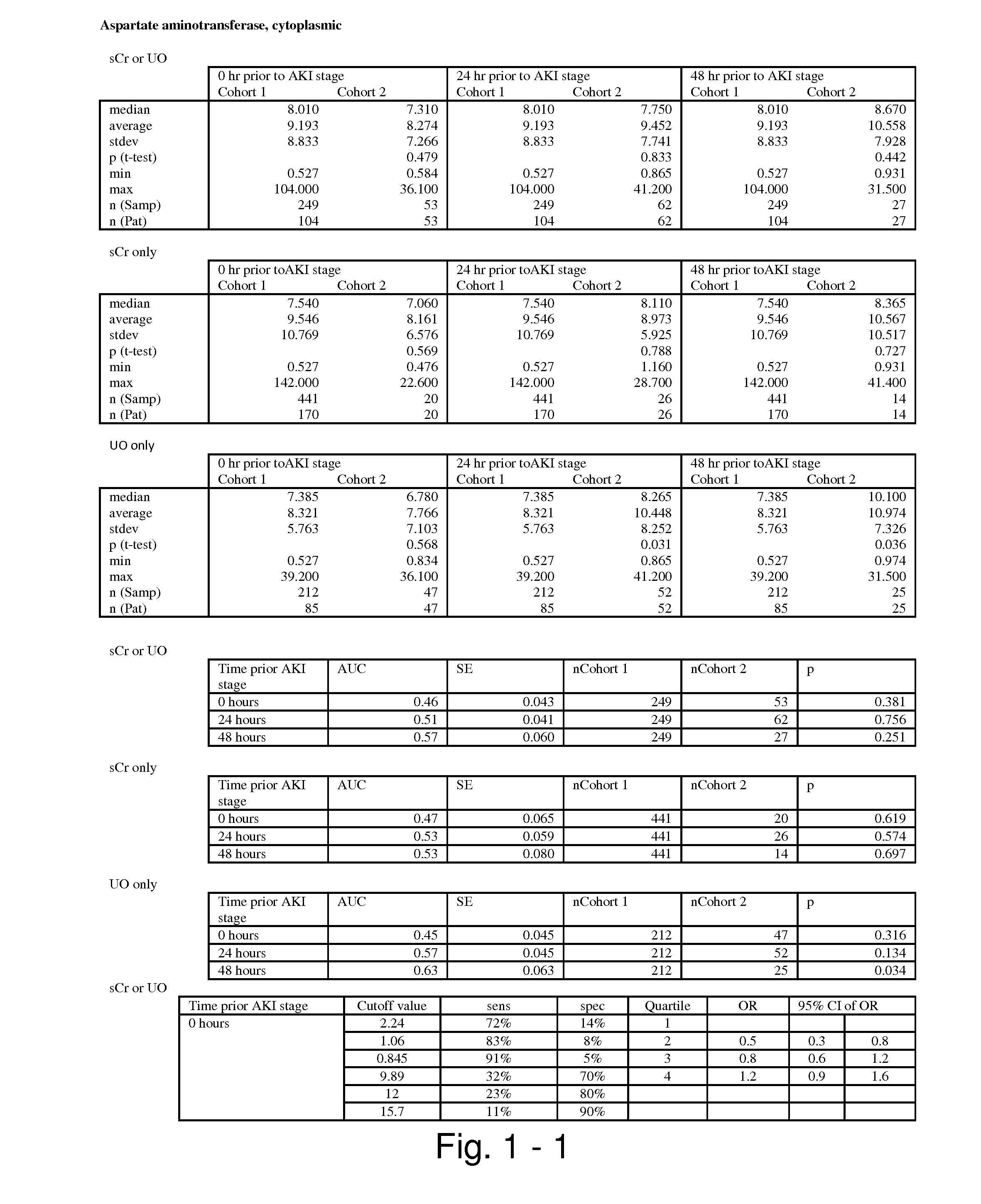
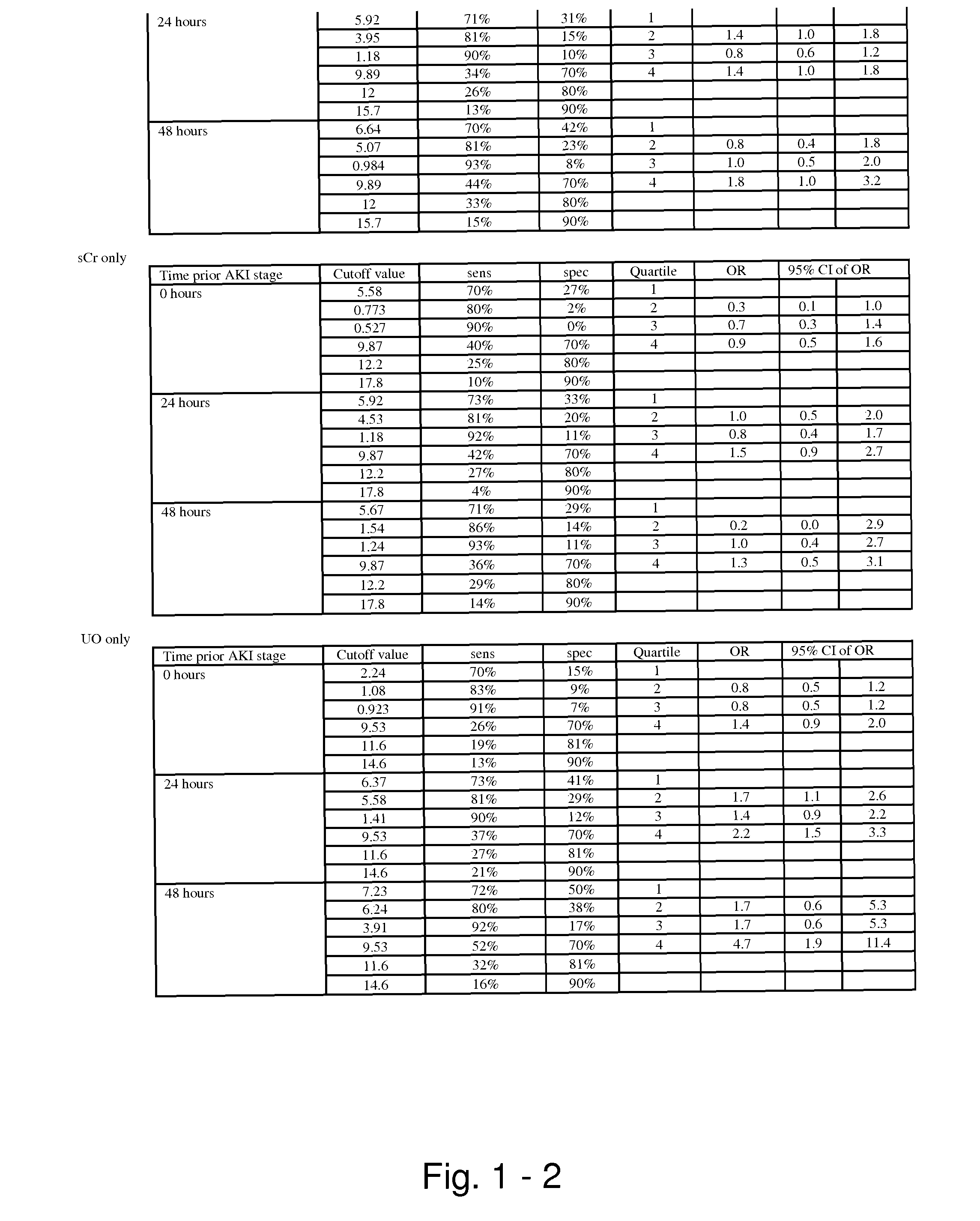
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
ActiveUS20110201038A1Easy to adaptMicrobiological testing/measurementDisease diagnosisInterleukin 10Soluble P-Selectin
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using assays that detect one or more markers selected from the group consisting of Cytoplasmic aspartate aminotransferase, soluble Tumor necrosis factor receptor superfamily member 5, soluble CD40 Ligand, soluble C-X-C Motif chemokine 16, S100-A12, Eotaxin, soluble E-selectin, Fibronectin, Granulocyte colony-stimulating factor, Granulocyte-macrophage colony-stimulating factor, Heparin-binding growth factor 2, soluble Hepatocyte growth factor receptor, Interleukin-1 receptor antagonist, Interleukin-1 beta, Interleukin-10, Interleukin-15, Interleukin-3, Myeloperoxidase, Nidogen-1, soluble Oxidized low-density lipoprotein receptor 1, Pappalysin-1, soluble P-selectin glycoprotein ligand 1, Antileukoproteinase, soluble Kit ligand, Tissue inhibitor of metalloproteinase 1, Tissue inhibitor of metalloproteinase 2, soluble Tumor necrosis factor, soluble Vascular cell adhesion molecule 1, and Vascular endothelial growth factor A as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
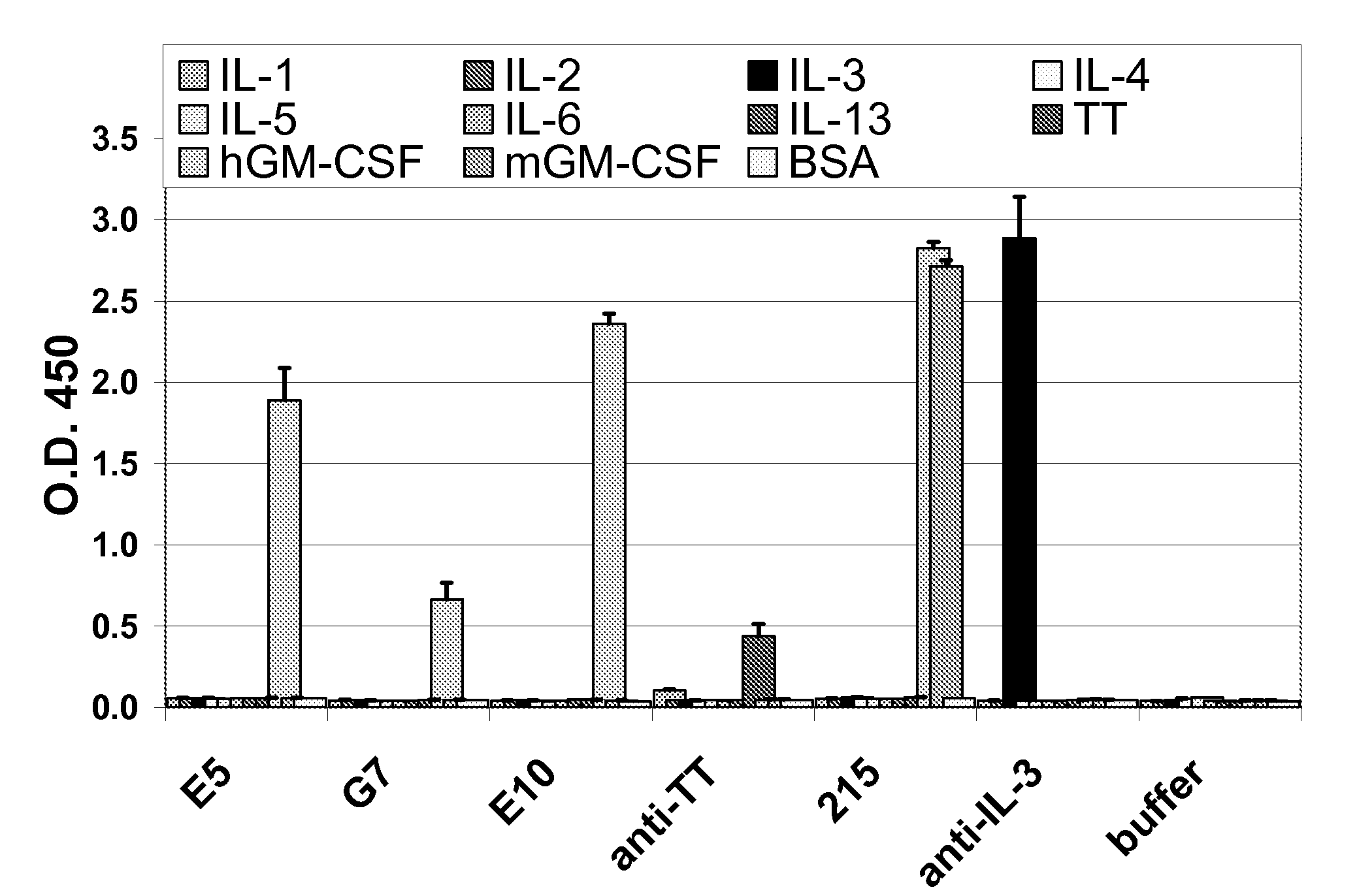
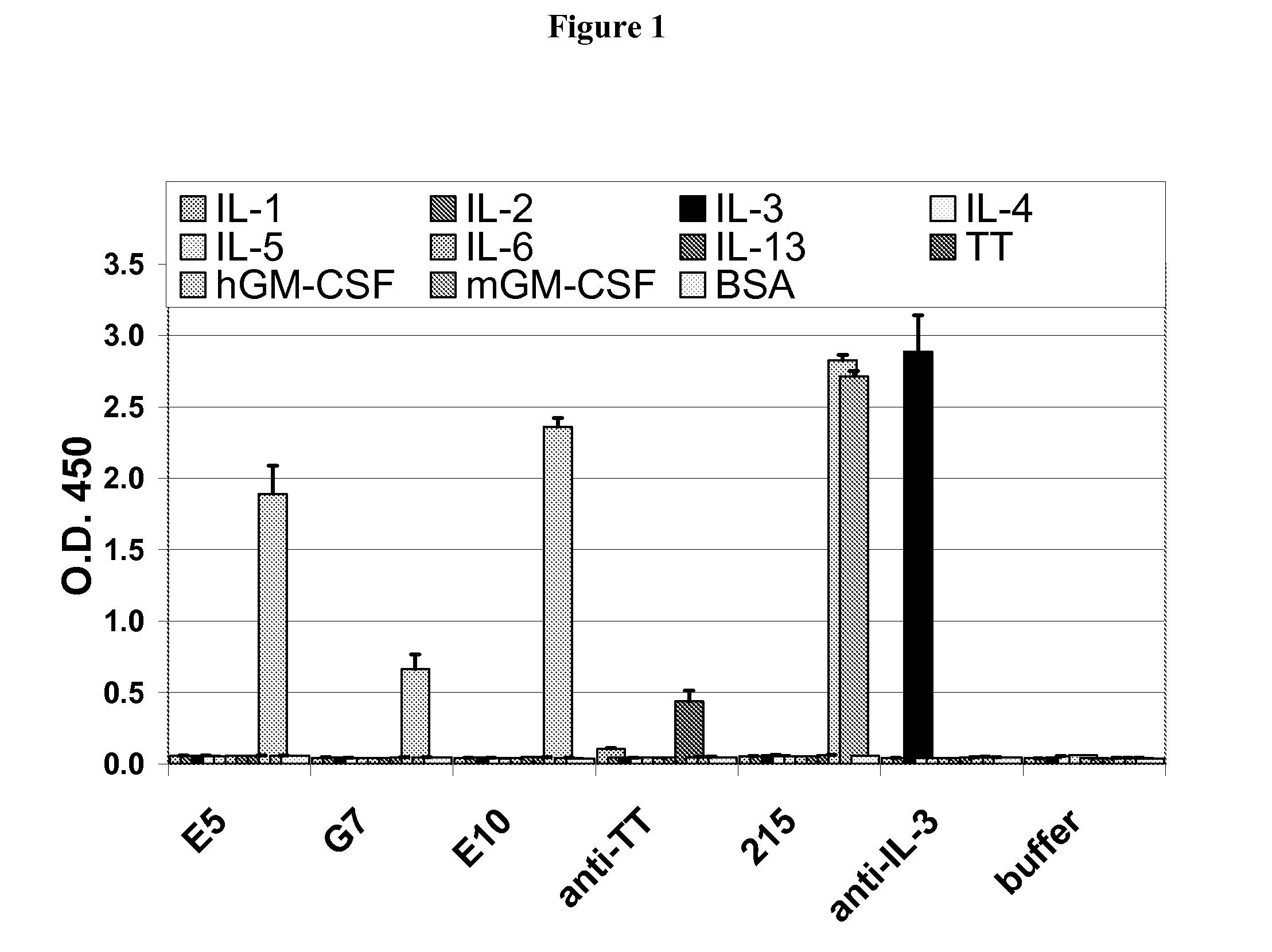
Antigenic GM-CSF peptides and antibodies to GM-CSF
Hybridoma lines that secrete human monoclonal antibodies with high binding specificity and biological activity, particularly neutralizing activity against granulocyte-macrophage colony stimulating factor, and methods of generating the hybridoma lines are provided. Target antigens and epitopes are also provided. The antibodies may be used in therapeutic methods, for example in the treatment of cancer, infectious disease, or autoimmune disease.
Owner:EISAI INC
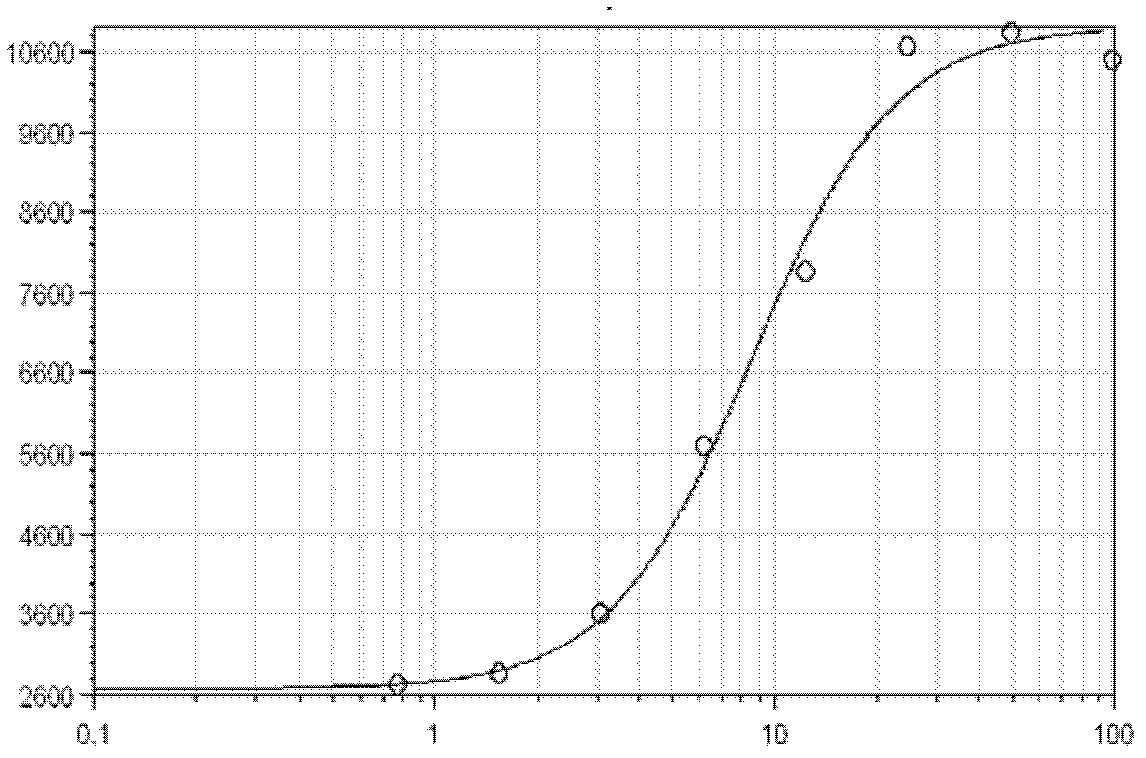
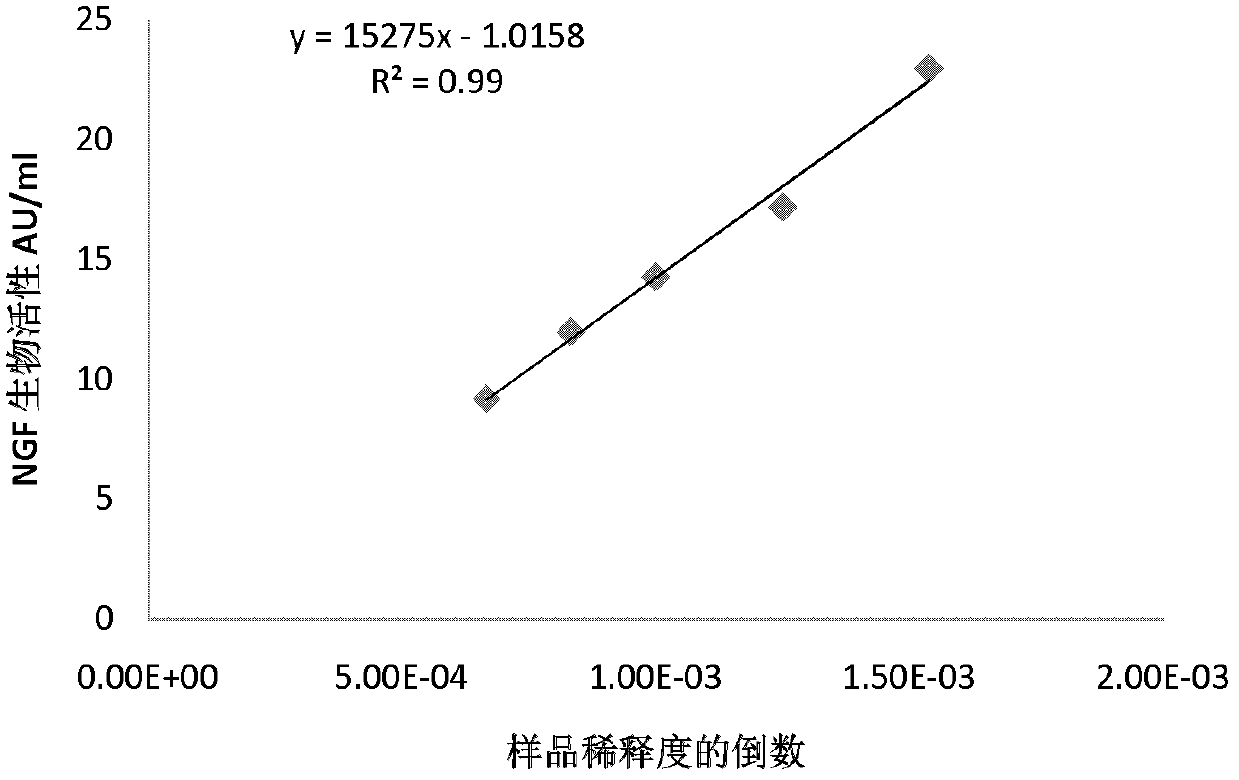
Quantitative determination method for activity of nerve growth factor
The invention discloses a quantitative determination method for activity of a nerve growth factor. The method comprises the following steps of: (1) washing TF-1 cells in a logarithmic phase by a basal culture medium that contains no serum and recombined human granulocyte-macrophage colony stimulating factors, and then resuspending the TF-1 cells to obtain TF-1cell suspension liquid; (2) respectively adding the TF-1cell suspension liquid into a gradient-diluted nerve growth factor standard sample and a sample to be tested, and incubating at 37 DEG C in presence of 5% of CO2; then respectively adding an indicating agent to detect the absorbance / fluorescence value and drawing a standard curve line; and (3) calculating the activity concentration of the nerve growth factor of the sample to be tested according to the standard curve line and the absorbance / fluorescence value of the sample. The quantitative determination method for activity of the nerve growth factor disclosed by the invention has good accuracy and precision.
Owner:STAIDSON (BEIJING) BIOPHARMACEUTICALS CO LTD
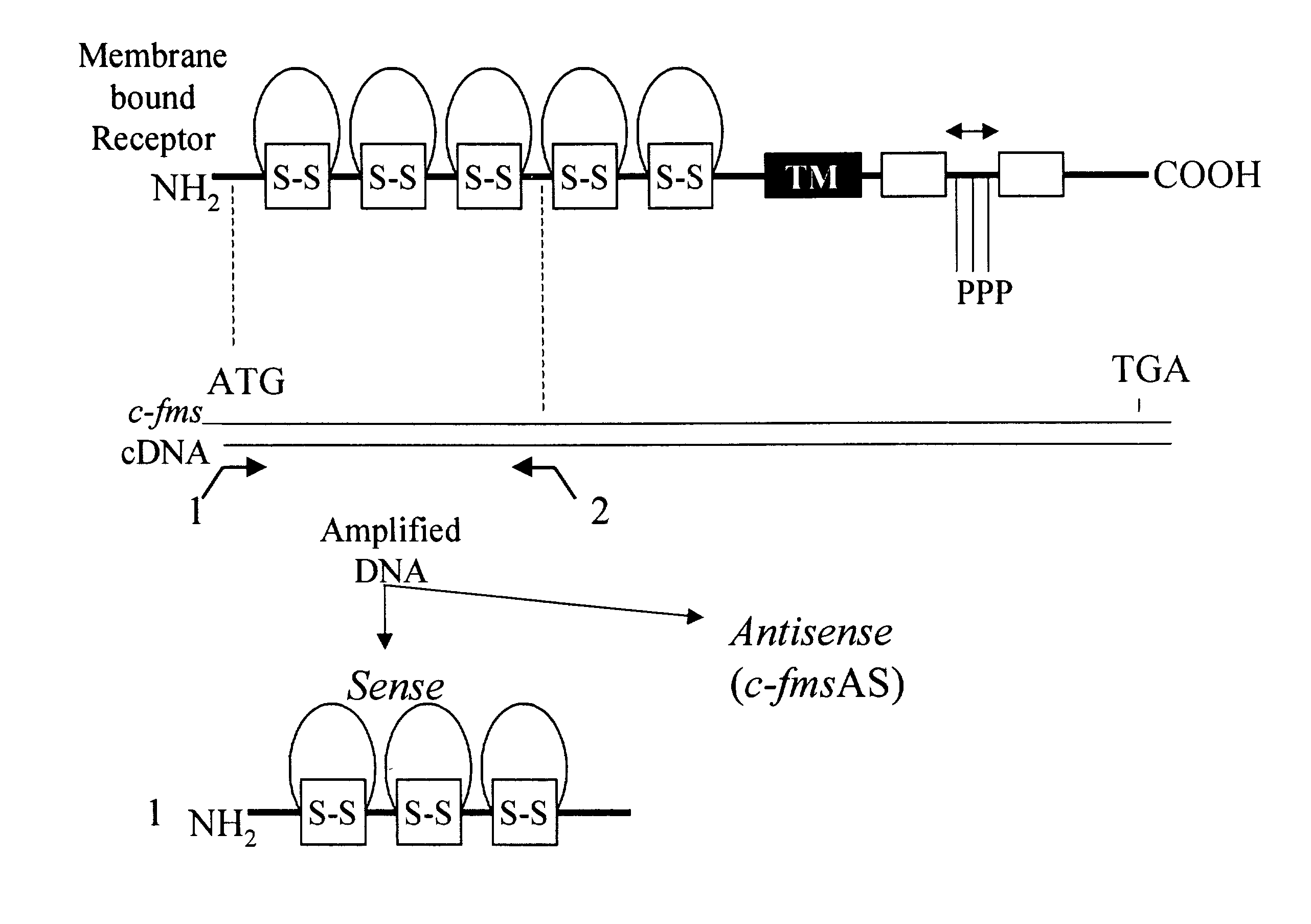
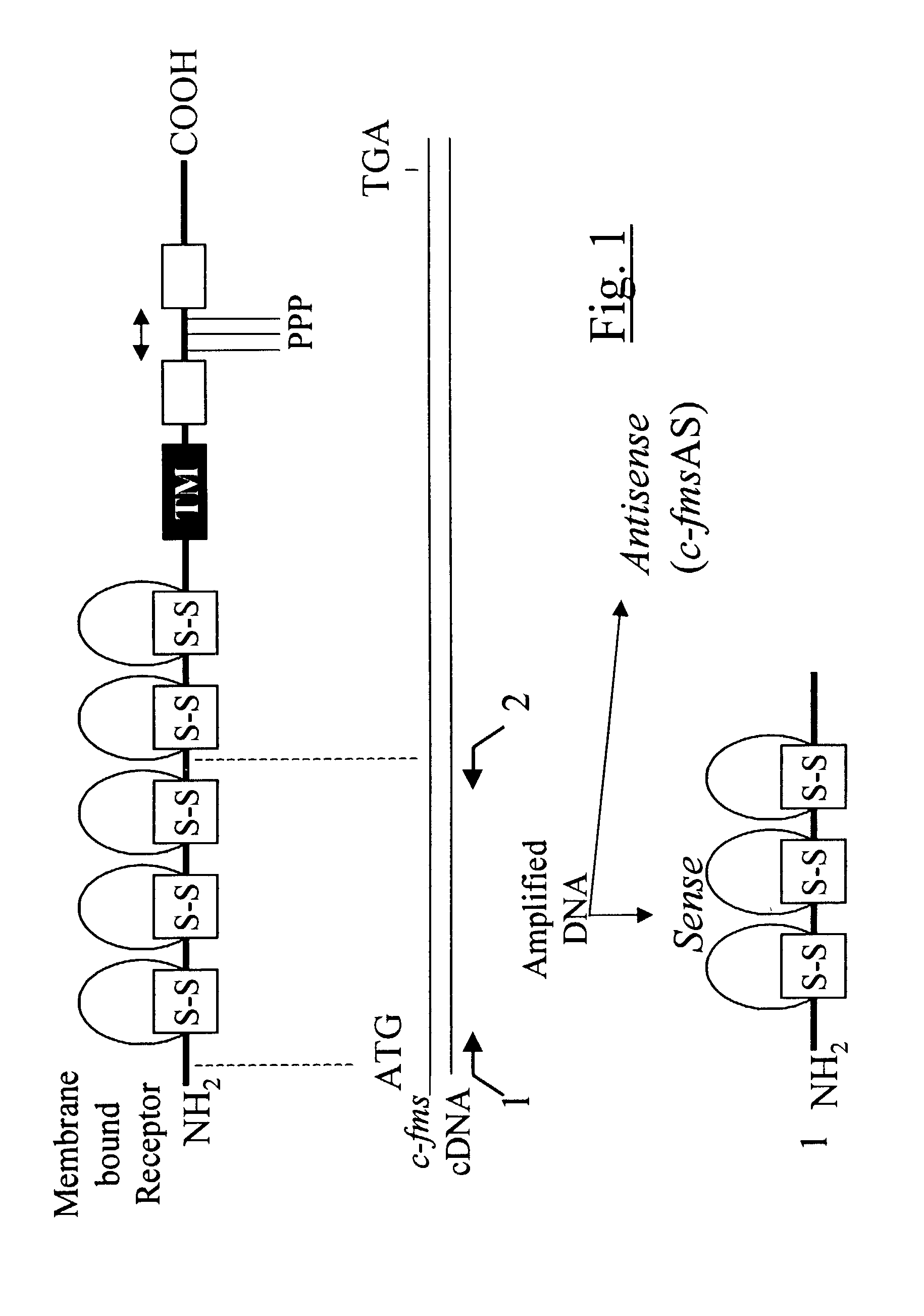
Methods for inhibiting macrophage colony stimulating factor and c-FMS-dependent cell signaling
Owner:RAJAVASHISTH TRIPATHI
Recombinant II type herpes simplex virus vector, preparation method of recombinant II type herpes simplex virus vector, recombinant virus, medicinal composition and application
ActiveCN102146418AGenetic material ingredientsViral/bacteriophage medical ingredientsCurative effectRecombinant virus vaccine
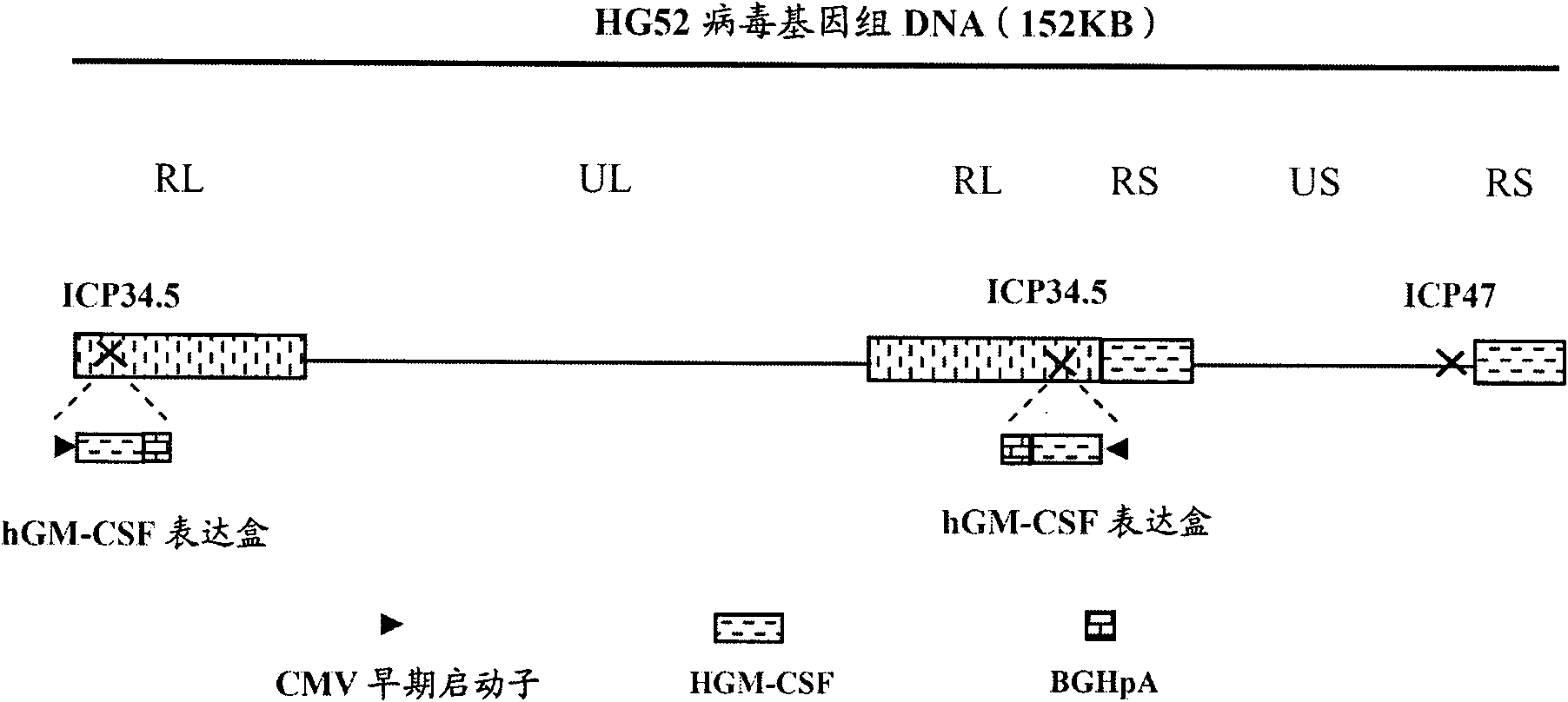
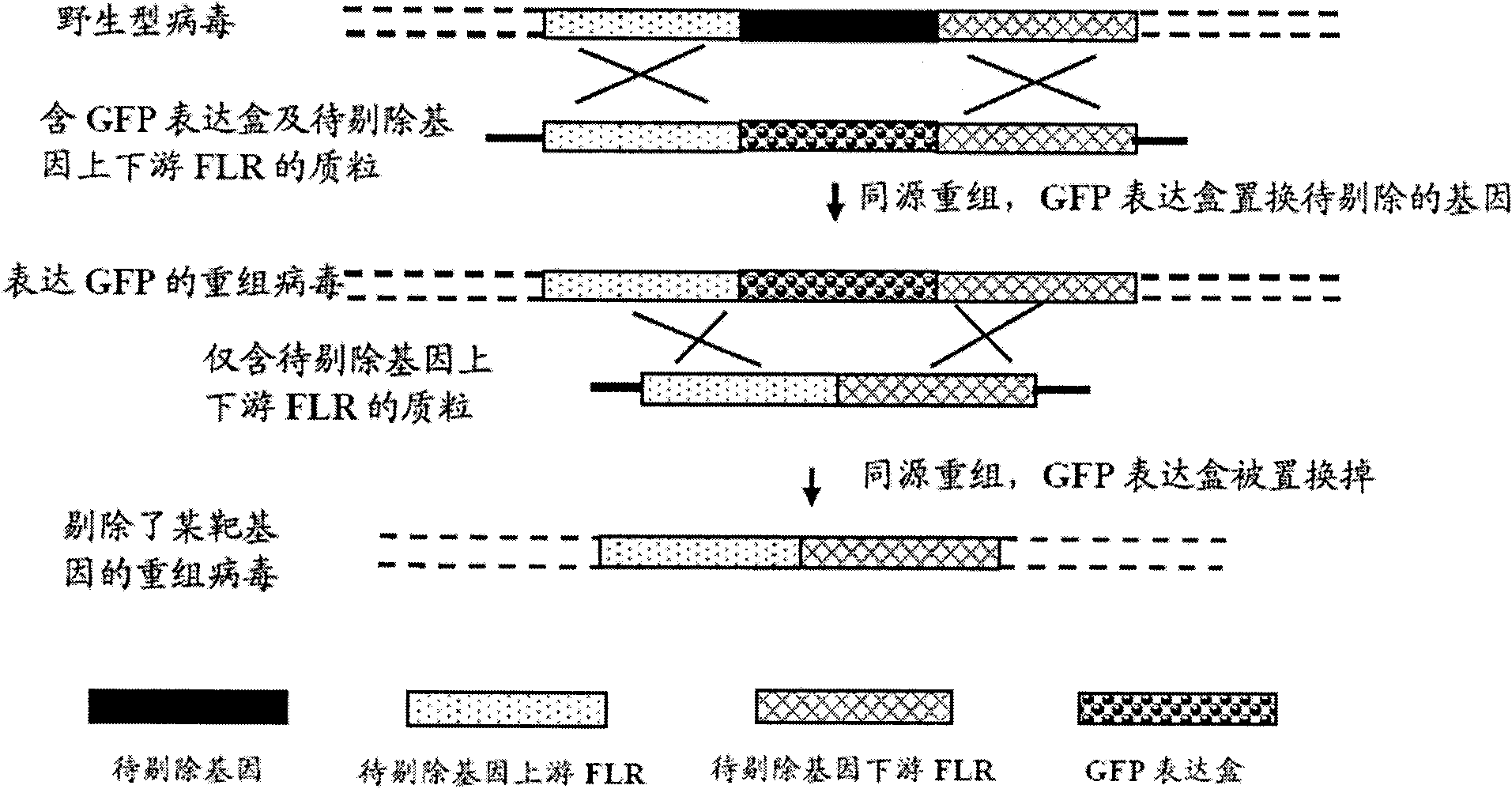
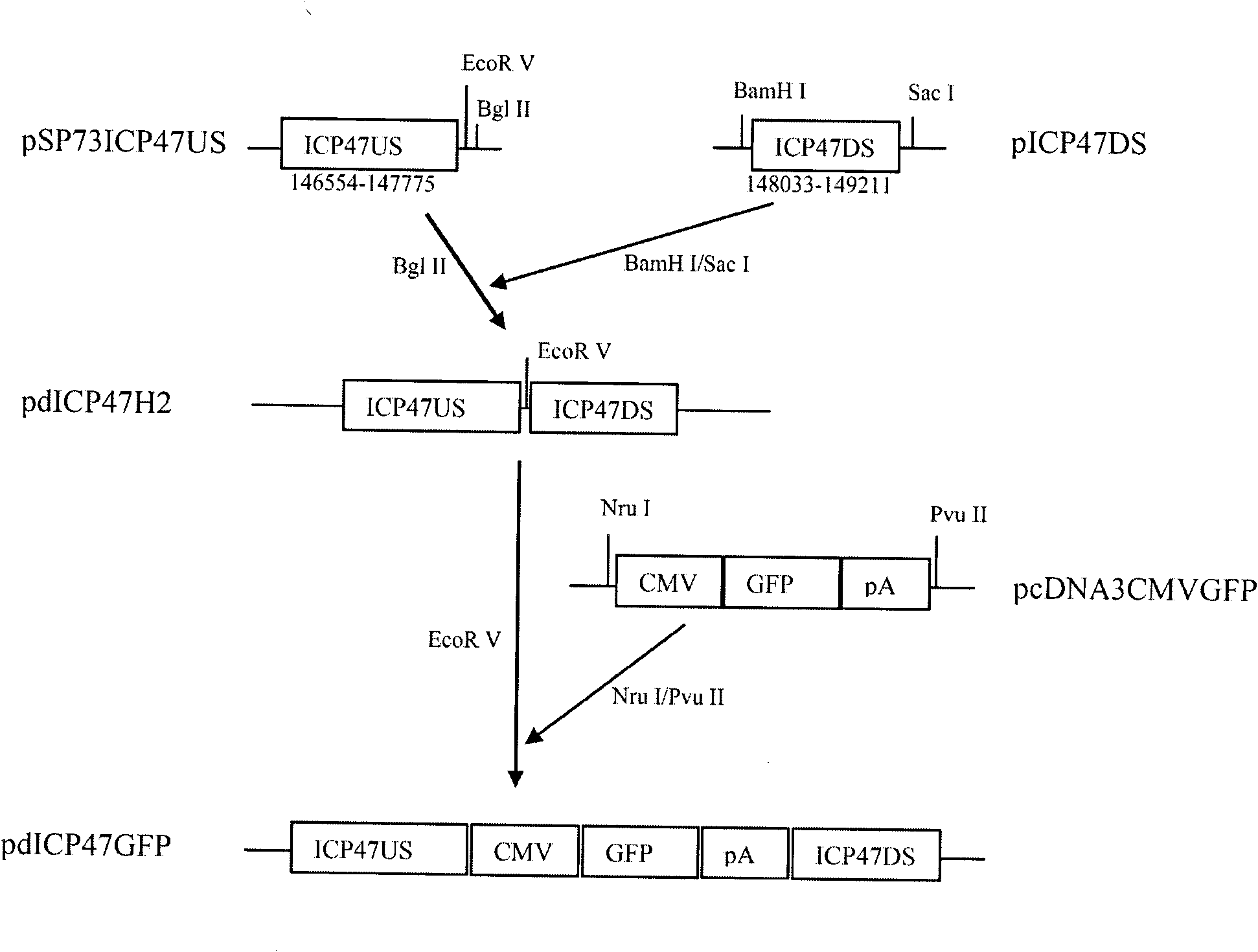
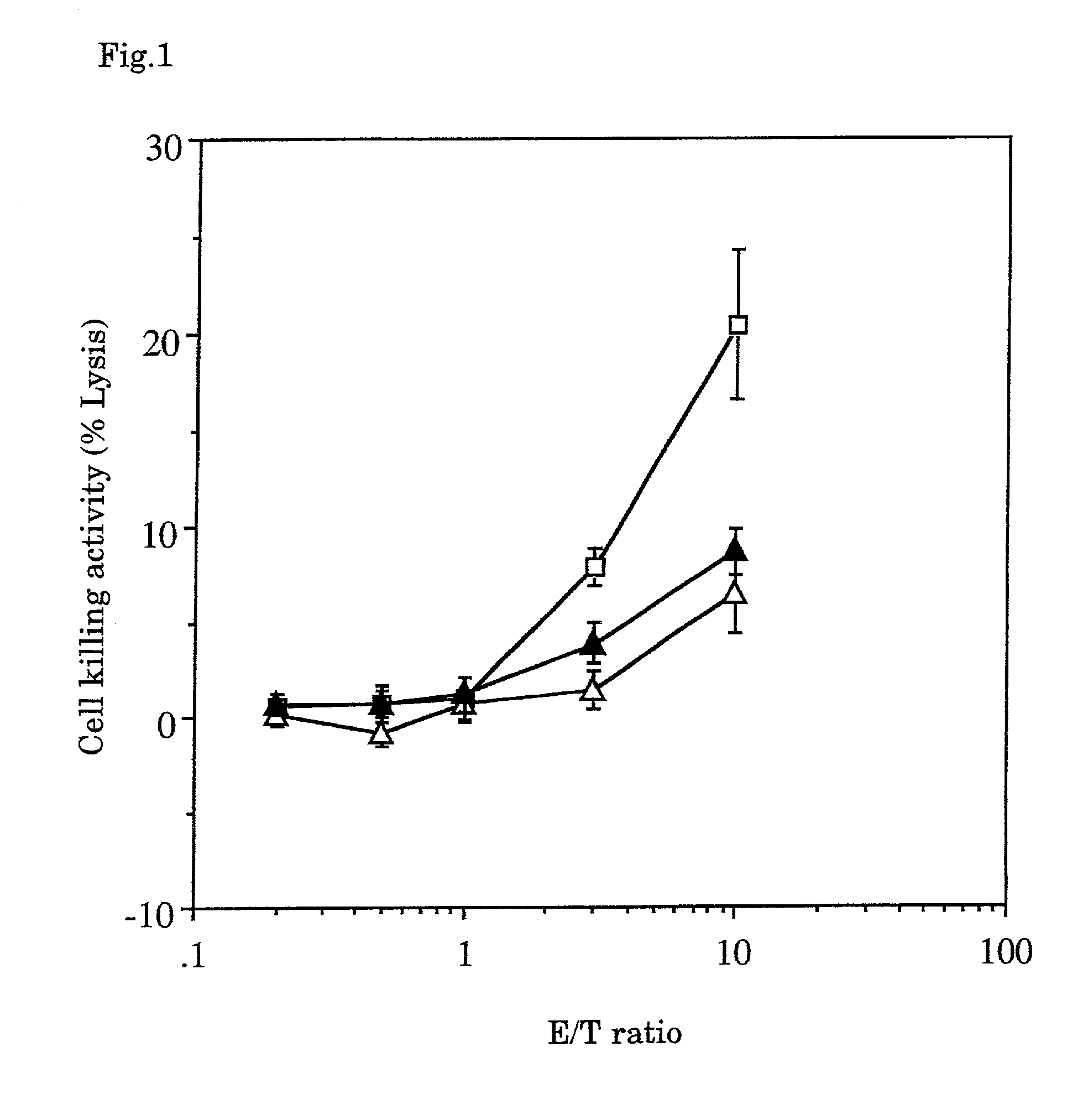
The invention provides a recombinant II type herpes simplex virus vector. An ICP34.5 gene and an ICP47 gene of a wild II type herpes simplex virus HG52 strain are removed in the virus vector, and preferably a human granulocyte macrophage-colony stimulating factor (hGM-CSF) expression box is inserted into the position where the ICP34.5 gene is removed. The invention also provides a preparation method of the recombinant II type herpes simplex virus vector, a recombinant virus using the recombinant II type herpes simplex virus as a vector, a medicinal composition consisting of the recombinant II type herpes simplex virus vector and a pharmaceutically acceptable vector or excipient, and application of the recombinant II type herpes simplex virus vector in preparation of a gene medicament for treating tumors. As the ICP34.5 gene is removed in the recombinant II type herpes simplex virus vector provided by the invention, the oncolysis virus is safe and can selectively grow and propagate in tumor cells; the ICP47 gene is removed to promote immune response and enhance oncolysis activity; and the curative effect of the recombinant II type herpes simplex virus vector is superior to that of the conventional recombinant I type herpes simplex virus vector, and the recombinant II type herpes simplex virus vector has high safety.
Owner:WUHAN BINHUI BIOTECH CO LTD
Antibodies to GM-CSF
Owner:EISAI INC
Tumor vaccines
InactiveUS7247310B1Easy to handleImprove anti-tumor effectPeptide/protein ingredientsMammal material medical ingredientsAbnormal tissue growthGranulocyte colony-stimulating factor
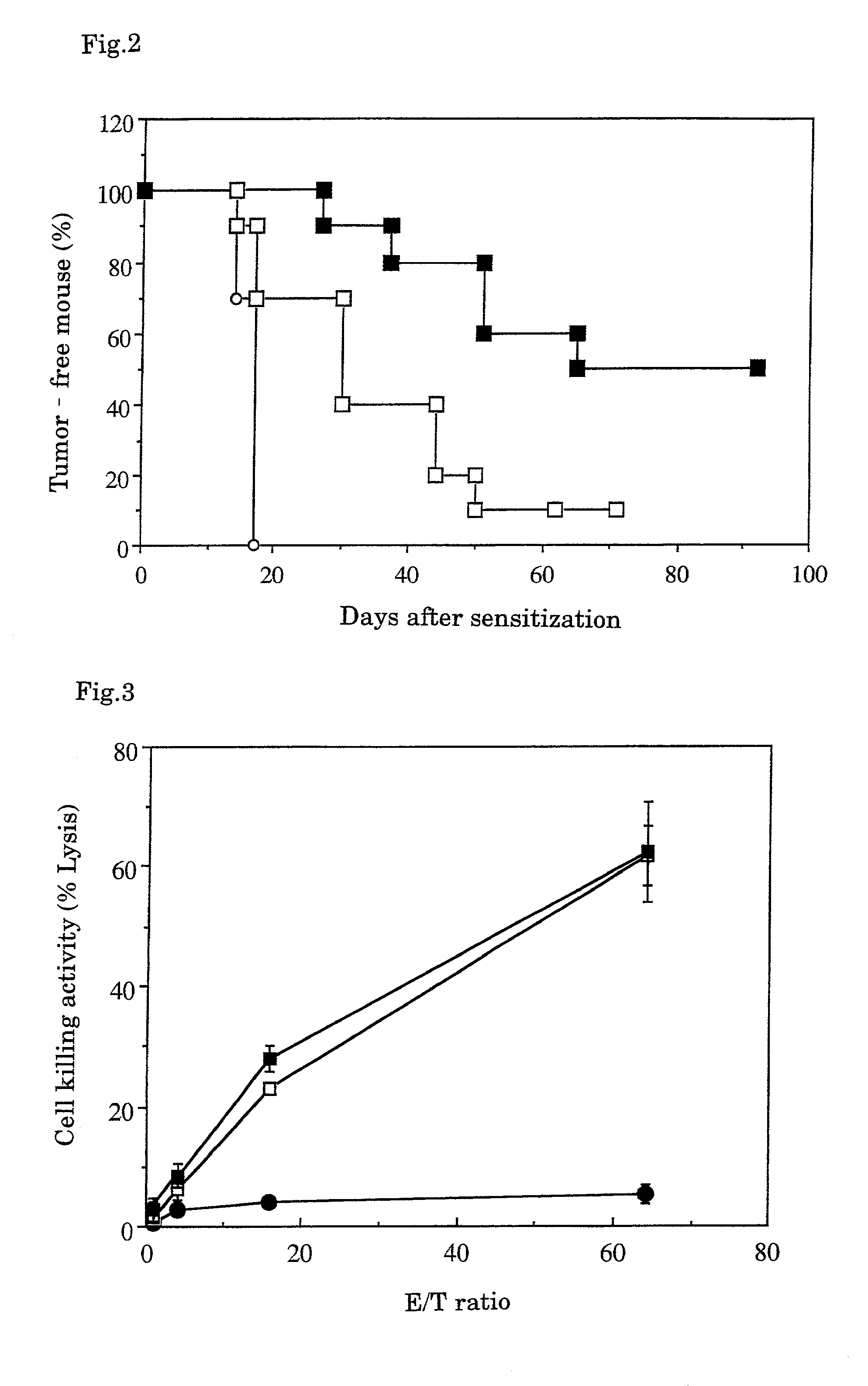
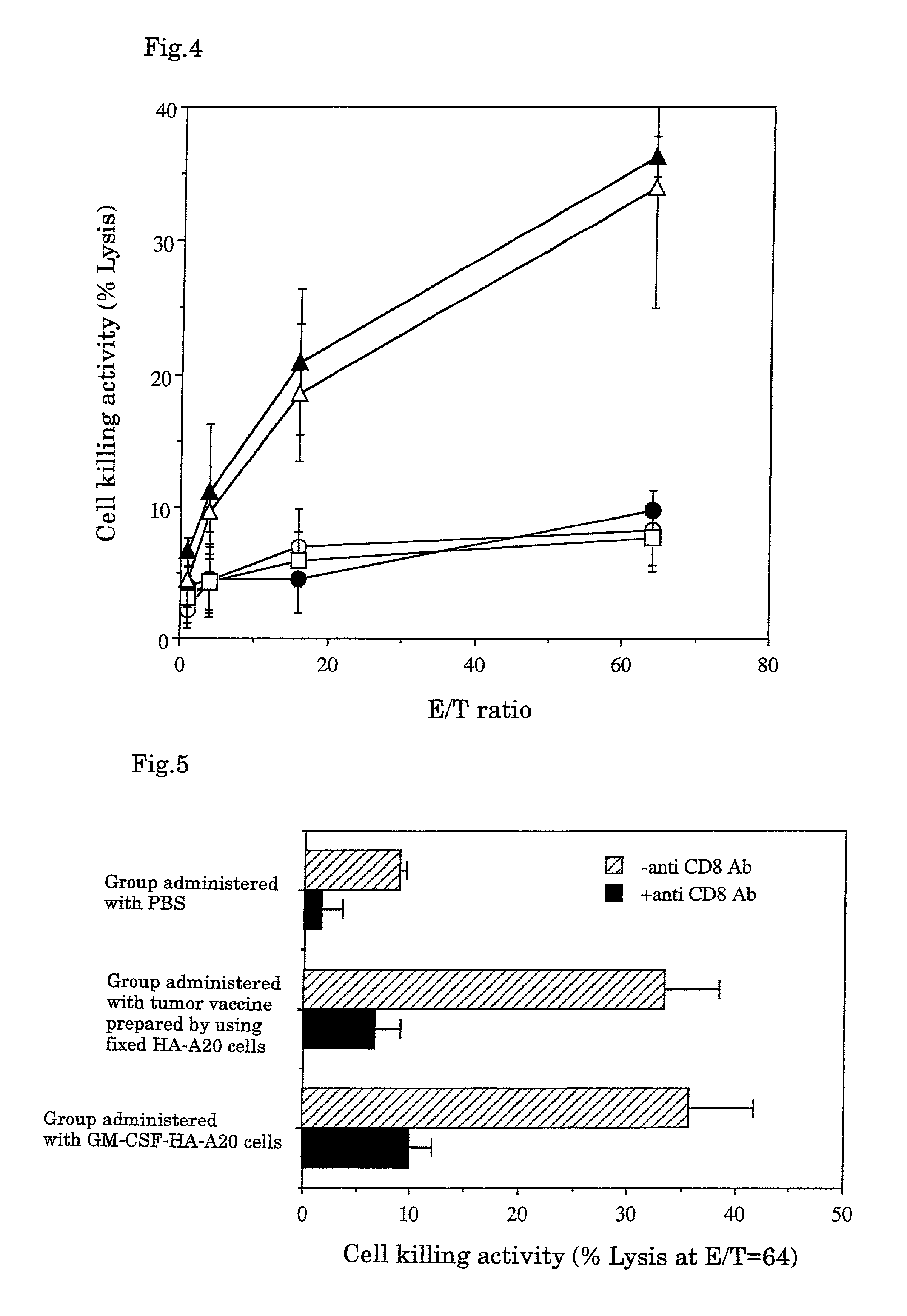
A tumor vaccine which comprises a microparticle or a lysate prepared from a solidified tumor material selected from the group consisting of a tumor tissue, a tumor cell, and a component thereof, and at least one cytokine and / or cytokine-inducing agent (e.g., a granulocyte-macrophage-colony stimulating factor and / or interleukin-2 and the like), and optionally an adjuvant. The vaccine can be easily prepared and widely applied for prevention of recurrence, inhibition of metastasis and therapeutic treatment regardless of a type of a tumor, and has excellent antitumor effect.
Owner:RIKEN +1
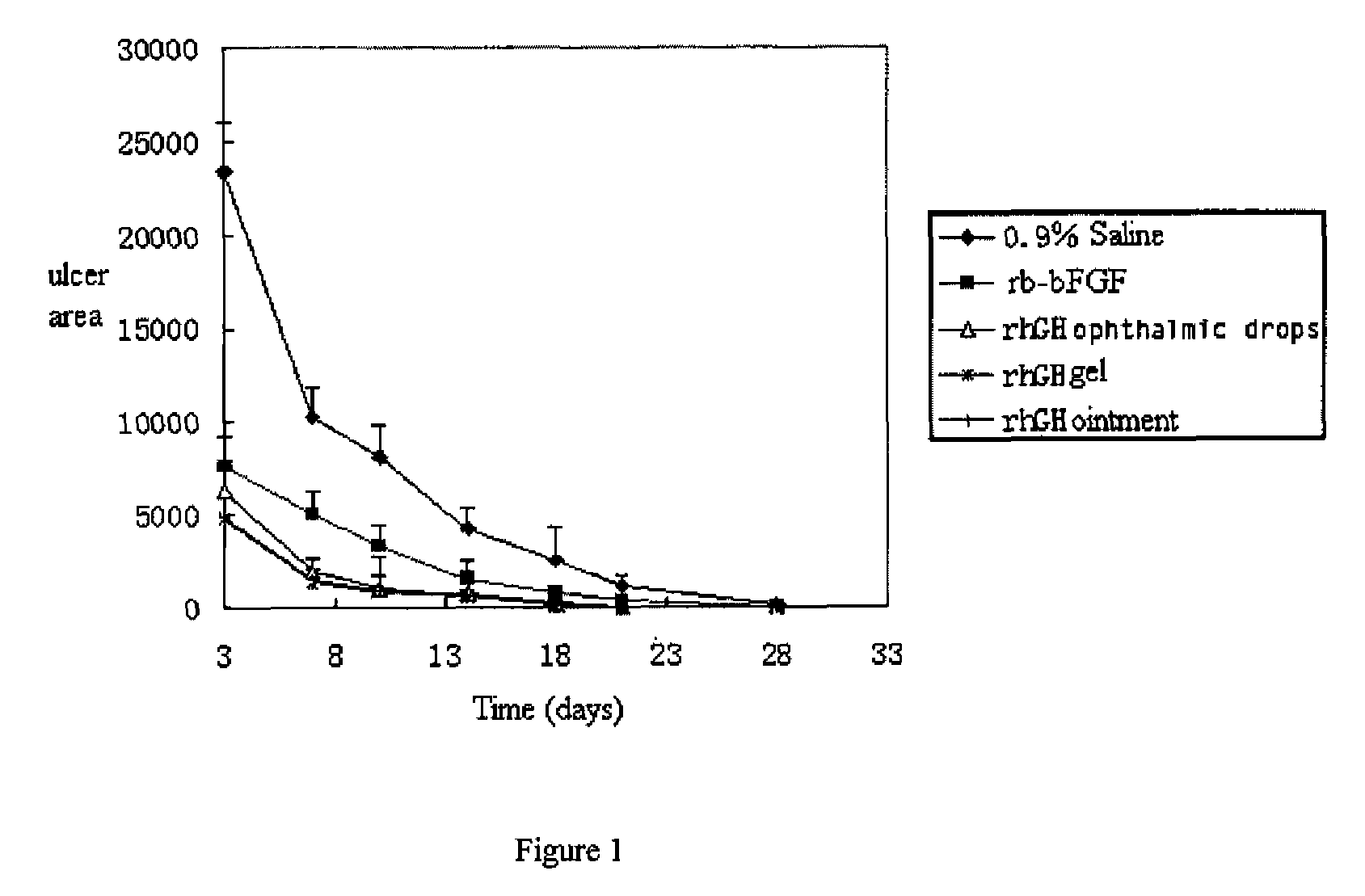
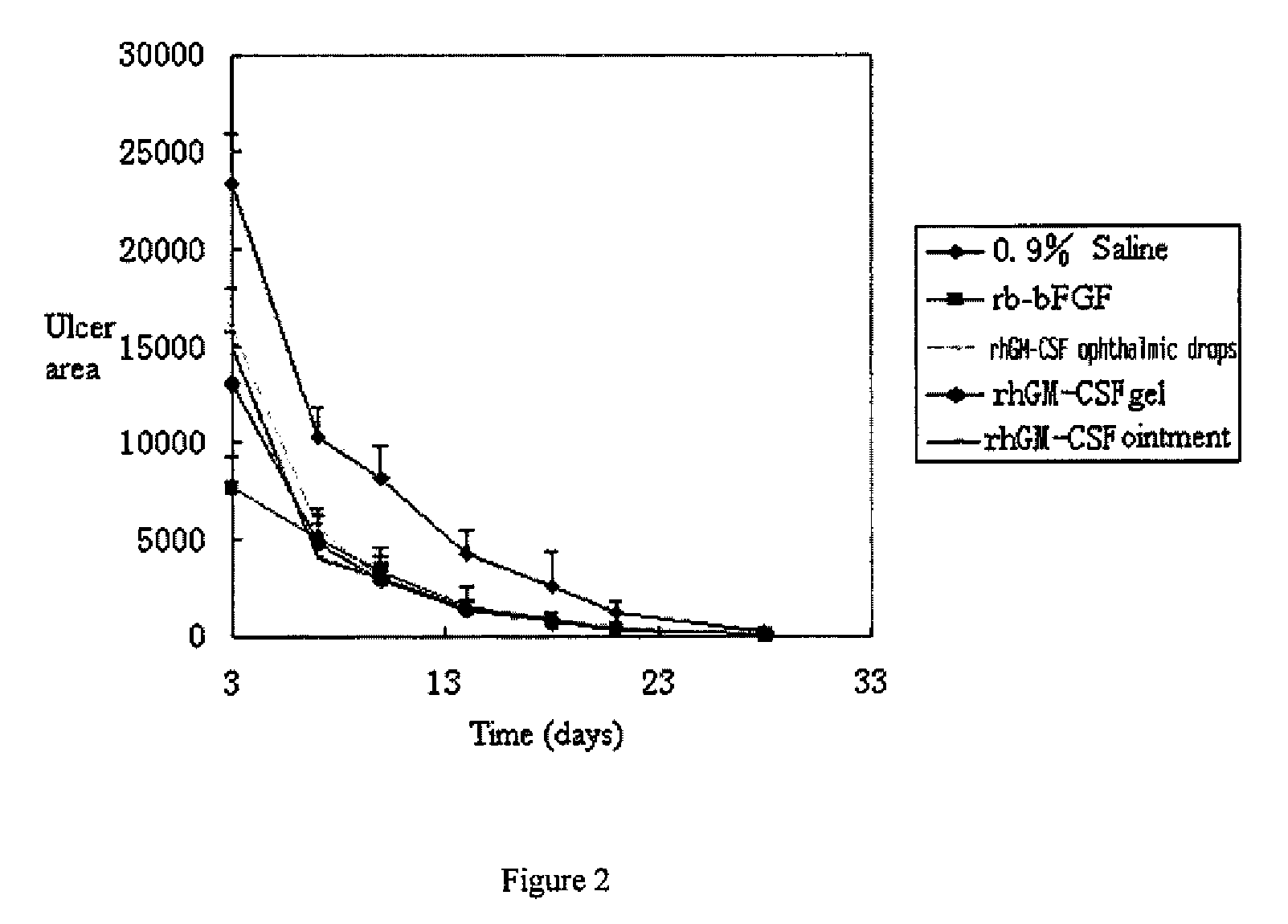
Ophthalmic hGM-CSF preparation
InactiveUS7858582B2Avoid excessive viscosityBenefit to drugSenses disorderPeptide/protein ingredientsGynecologyHuman growth hormone
Owner:CHANGCHUN GENESCIENCE PHARM CO LTD
Human monoclonal antibody binding to hGM-CSF and its antigen binding portion
ActiveUS7935795B2High affinityPrevent proliferationSenses disorderAntipyreticComplementarity determining regionAutoimmune disease
The present invention provides a human monoclonal antibody, and antigen-binding portions thereof, capable of binding to human granulocyte-macrophage colony stimulating factor (hGM-CSF) and neutralizing the bioactivity of the hGM-CSF, wherein the anti-hGM-CSF monoclonal antibody has a light chain (L chain) including an amino acid sequence SEQ ID NO:1 and a heavy chain (H chain) including an amino acid sequence SEQ ID NO: 2. Also provided are human monoclonal anti-hGM-CSF antibodies, and antigen-binding portions thereof, characterized by complementarity determining regions (CDRs) or H chain and L chain variable regions related to SEQ ID NO:1 and SEQ ID NO:2. Antibodies, and antigen-binding portions thereof, of the invention are useful in the treatment of diseases associated with overproduction of hGM-CSF, including allergic disease, graft rejection and graft-versus-host disease (GVHD), and autoimmune diseases.
Owner:EVEC
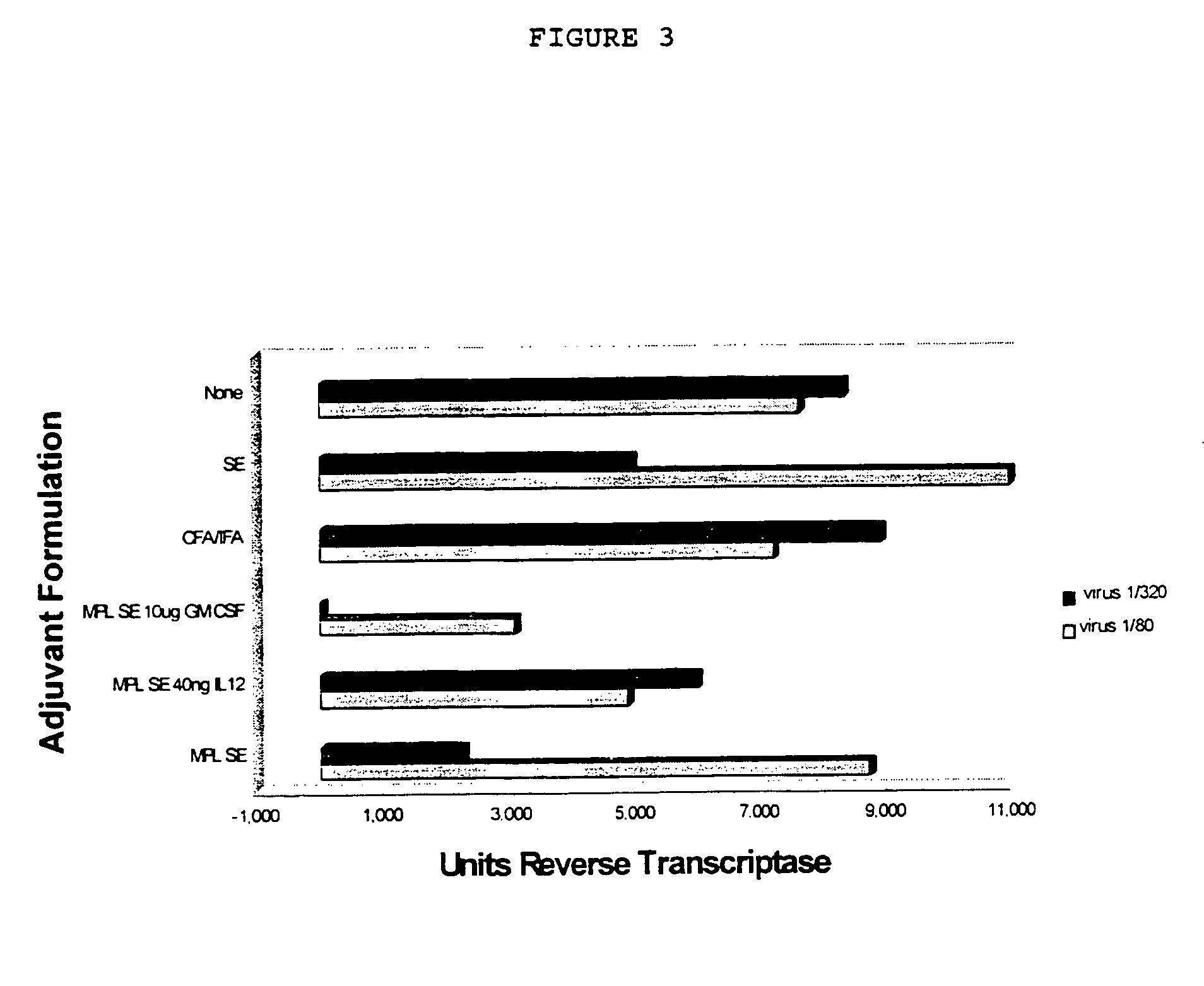
Adjuvant combination formulations
InactiveUS7611721B1Improve abilitiesSsRNA viruses negative-senseAntibacterial agentsWhite blood cellVertebrate Animals
The use of 3-O-deacylated monophosphoryl lipid A or monophosphoryl lipid A and derivatives and analogs thereof, in combination with a cytokine or lymphokine such as granulocyte macrophage colony stimulating factor or interleukin-12 is useful as an adjuvant combination in an antigenic composition to enhance the immune response in a vertebrate host to a selected antigen.
Owner:ZOETIS SERVICE LLC
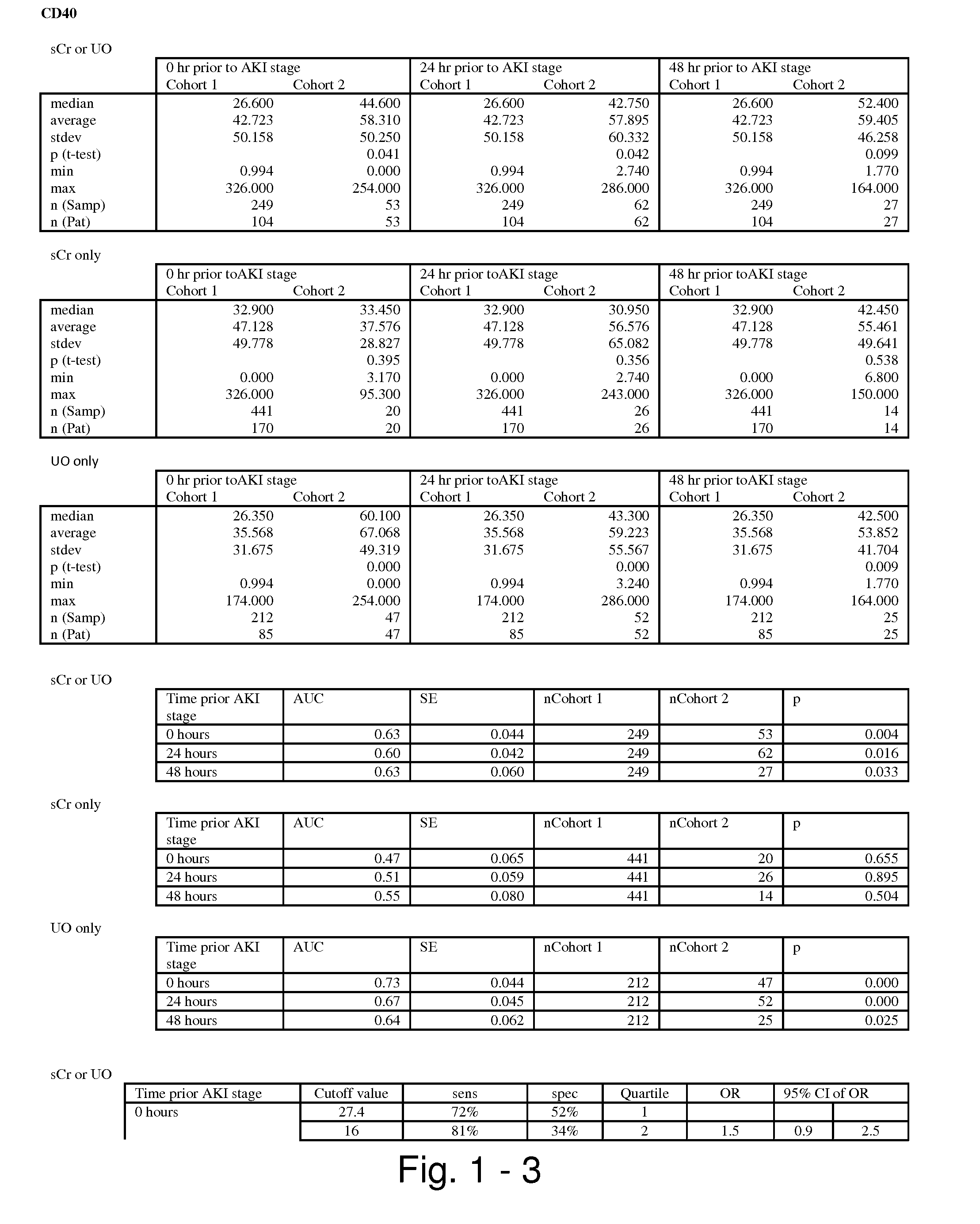
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
ActiveUS8778615B2Easy to adaptMicrobiological testing/measurementAntibody ingredientsInterleukin 10Soluble P-Selectin
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using assays that detect one or more markers selected from the group consisting of Cytoplasmic aspartate aminotransferase, soluble Tumor necrosis factor receptor superfamily member 5, soluble CD40 Ligand, soluble C-X-C Motif chemokine 16, S100-A12, Eotaxin, soluble E-selectin, Fibronectin, Granulocyte colony-stimulating factor, Granulocyte-macrophage colony-stimulating factor, Heparin-binding growth factor 2, soluble Hepatocyte growth factor receptor, Interleukin-1 receptor antagonist, Interleukin-1 beta, Interleukin-10, Interleukin-15, Interleukin-3, Myeloperoxidase, Nidogen-1, soluble Oxidized low-density lipoprotein receptor 1, Pappalysin-1, soluble P-selectin glycoprotein ligand 1, Antileukoproteinase, soluble Kit ligand, Tissue inhibitor of metalloproteinase 1, Tissue inhibitor of metalloproteinase 2, soluble Tumor necrosis factor, soluble Vascular cell adhesion molecule 1, and Vascular endothelial growth factor A as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
Tumor targeting metal complex, synthetic method and application
ActiveCN106866743AImprove utilizationSmall toxicityRuthenium organic compoundsOrganic active ingredientsAbnormal tissue growthApoptosis
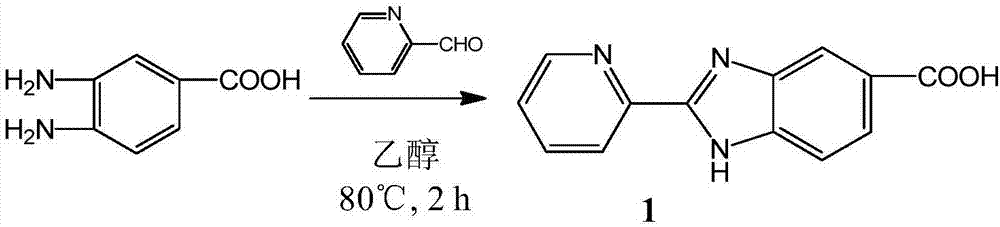
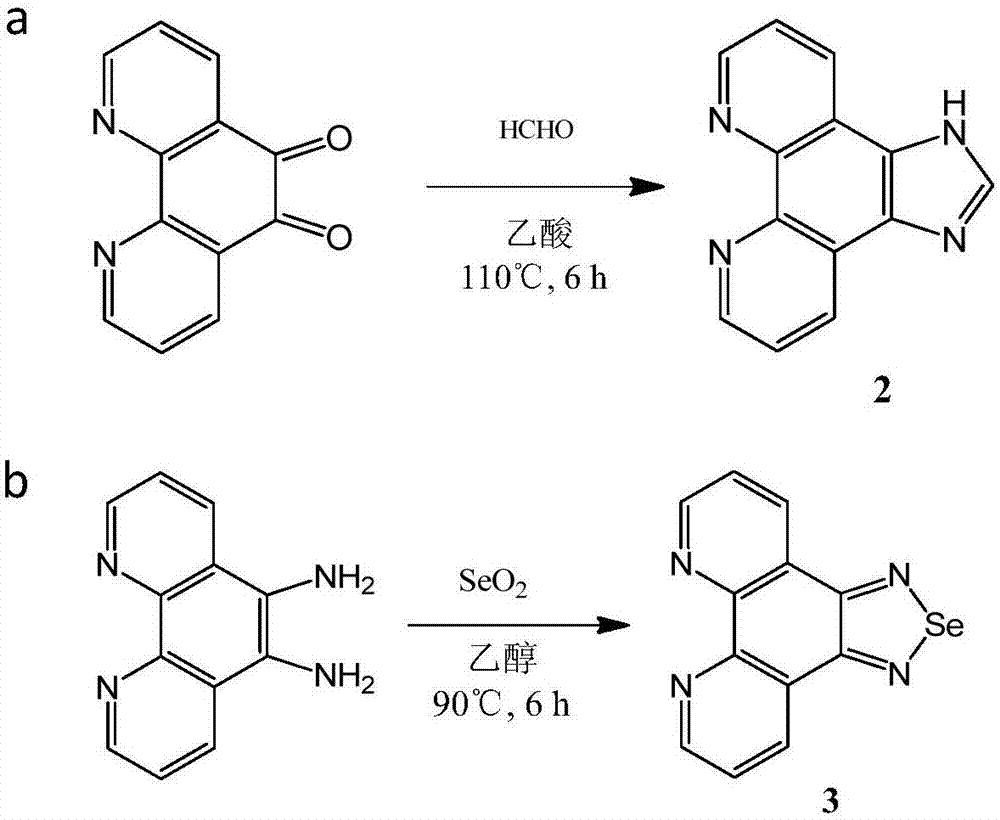
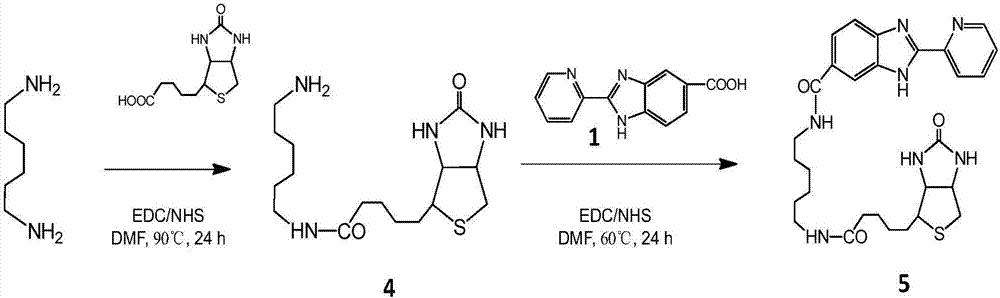
The invention discloses a tumor targeting metal complex, a synthetic method and application. The synthetic method comprises the steps of connecting a ligand with a substituent group and a targeting molecule, complexing the ligand and metal ions, and synthesizing to obtain the tumor targeting metal complex, wherein the ligand is one of a phenanthroline derivative, a dipyridyl derivative, a benzimidazole derivative, a phenyl-pyridine derivative and a thiophene-pyridine derivative; the targeting molecule is one of biotin, folic acid, integrin, transferrin, cell-penetrating peptide, octa-poly-L-arginine, MUC-1 attached membrane protein, galactosamine, neovascular targeting peptide and a granulocyte-macrophage colony-stimulating factor; the metal ions are ruthenium, osmium, rhodium or iridium ions. The complex obtained by the method provided by the invention can be selectively absorbed by tumor cells, and induces apoptosis of the tumor cells. In a nude mouse tumor-bearing model, the tumor targeting metal complex can image and treat tumors, and the toxicity of organs and tissues is reduced.
Owner:JINAN UNIVERSITY
Antibodies to magmas and uses thereof
InactiveUS7358041B2Peptide/protein ingredientsMicrobiological testing/measurementMitochondrial diseaseGranulocyte
The present invention relates to isolated and purified Magmas protein, isolated and purified nucleic acids encoding Magmas protein, and antibodies to Magmas protein, which interacts with granulocyte macrophage colony stimulating factor (GM-CSF). More specifically, the invention relates to uses of such anti-Magmas antibodies. The invention further relates to a method for diagnosis, prognosis and treatment of diseases, particularly, cancer, Alzheimer's disease and mitochondrial diseases using Magmas sequences and antibodies directed against the Magmas protein or fragments thereof.
Owner:SHORT MARY K +1
Compositions having means for targeting at least one antigen to dendritic cells
A composition that can be used as a vaccine containing means for targeting at least one antigen to dendritic cells and as adjuvants a granulocyte macrophage colony stimulating factor and a CpG oligodeoxynucleotide and / or a CpG-like oligodeoxynucleotide. This composition can used to treat cancers, infectious diseases caused by bacterial, viral, fungal, parasitic or protozoan infections, allergies and / or autoimmune diseases.
Owner:INSTITUT CURIE +4
Oral adhering piece for treating oral exulceratio, and its prepn. method
InactiveCN1718239AImprove efficacyPromote absorptionPeptide/protein ingredientsDigestive systemWater insolubleOral ulcers
An oral sticking sheet of the recombinant human granulocyte-macrophage colony stimulating factor for treating oral ulcer is composed of a water-insoluble protecting layer and an adhesive layer containing said active medicine, adhesive, the stabilizer for stabilizing the activity of protein and percutaneous absorption promoter.
Owner:深圳市孚沃德生物技术有限公司
Modified granulocyte macrophage colony stimulating factor (gm-csf) with reduced immunogenicity
InactiveUS20040092717A1Modify characteristicPeptide/protein ingredientsPeptide preparation methodsIn vivoImmunogenicity
The present invention relates to polypeptides to be administered especially to humans and in particular for therapeutic use. The polypeptides are modified polypeptides whereby the modification results in a reduced propensity for the polypeptide to elicit an immune response upon administration to the human subject. The invention in particular relates to the modification of human granulocyte macrophage colony stimulating factor (GM-CSF) to result in GM-CSF proteins that are substantially non-immunogenic or less immunogenic than any non-modified counterpart when used in vivo.
Owner:MERCK PATENT GMBH
Preparation method of large-scale culture dendritic cell vaccine and application thereof
InactiveCN103301449ABlood/immune system cellsAntibody medical ingredientsWhite blood cellTumor necrosis factor alpha
The invention provides a preparation method of a large-scale culture dendritic cell vaccine. The preparation method comprises the following process steps of: performing in-vitro separation and purification on collected white blood cells in human peripheral blood by adopting a blood cell separation machine to obtain a large number of mononuclear cells, wherein the order of magnitude can be above 10<9>; using a human granulocyte-macrophage colony stimulating factor and interleukin 4 to induce the culture to obtain a large number of immature dendritic cells, wherein the order of magnitude can be above 10<8>; and loading a tumor specific antigen in the immature dendritic cells, and then obtaining the mature dendritic cell vaccine with an anti-tumor effect under the induction culture conditions of a tumor necrosis factor alpha and lipopolysaccharides. The invention further provides a multi-time and multi-treatment course clinical application of the dendritic cell vaccine obtained through the method.
Owner:泰州市数康生物科技有限公司
Use of recombinant human granulocyte macrophagocyte colony stimulating factor in preparation of drug for preventing and treating hepatitis B
ActiveCN1990043APeptide/protein ingredientsDigestive systemColony-stimulating factorGenetic engineering
The invention discloses a use of recombinant human granulocyte macrophage colony stimulating factor in the preparation of treatment or prevention hepatitis B, while includes simultaneous or successive given recombinant human granulocyte macrophage colony stimulating factor and genetic engineering hepatitis B vaccine for the prevention or treatment of hepatitis B.
Owner:华北制药金坦生物技术股份有限公司
Site-specific modification of proteins through chemical modification enabling protein conjugates, protein dimer formation, and stapled peptides
ActiveUS20110263832A1Diminishment of extentAvoid spreadingHormone peptidesHybrid immunoglobulinsLupus erythematosusAdduct
The present invention generally provides methods for the site-specific modification of peptides, polypeptides, and proteins, e.g., granulocyte macrophage colony-stimulating factor, human superoxide dismutase, annexin, leptin, antibodies and the like, cytokines and chemokines, at their N-termini and at sites at which unnatural aminoacids have been introduced along the protein framework. The modifications described herein can be used for the synthesis and application of the adducts in radio-labeling, molecular imaging and protein therapeutic applications, and the treatment of disorders such as rheumatoid arthritis, lupus erythematosus, psoriasis, multiple sclerosis, type-1 diabetes, Crohn's disease, and systemic sclerosis, Alzheimer disease, cancer, liver disease (e.g., alcoholic liver disease), and cachexia.
Owner:ADVANCED PROTEOME THERAPEUTICS
Vaccination with immuno-isolated cells producing an immunomodulator
The present invention relates to immuno-protected encapsulated cells producing an immunomodulator, for example GM-CSF (granulocyte-macrophage colony stimulating factor). The cells of the invention are particularly well adapted for providing an active adjuvant or immunomodulator, for example in the context of immunisation in humans and animals. These cells can be used for vaccination where they provide the immunomodulator in an active form, in a continuous, non-immunogenic manner in the immediate vicinity of the vaccine antigen(s). The invention also relates to a vaccine composition comprising immuno-protected encapsulated cells producing an immunomodulator and an antigenic component. The invention also relates to a kit comprising a cell as described and an antigenic component. The strategy of the invention is perfectly suited for both cancer immunotherapy and vaccination against infectious agents.
Owner:MAXIVAX SA
Escherichia coli strain secreting human granulocyte colony stimulating factor (G-CSF)
The present invention provides a recombinant plasmid vector comprising a kanamycin resistance gene, a promoter, an endoxylanase signal sequence, a nucleotide sequence coding for an oligopeptide consisting of 13 amino acids including 6 consecutive histidine residues, and a human granulocyte colony stimulating factor(hG-CSF) gene; an E. coli transformed with the said vector; and, a process for producing complete hG-CSF protein with high purity from the protein pool secreted by the said microorganism. In accordance with the invention, the hG-CSF protein can be prepared with high purity through rather simple process facilitating secretion of large amount of hG-CSF fusion protein into the periplasm, which does not require complicated processes such as solubilization and subsequent refolding required for isolation of the hG-CSF protein produced in cytoplasm as insoluble inclusion bodies by conventional techniques, thus, the hG-CSF protein can be widely used as an active ingredient in the development of supplementary agents for anticancer therapy.
Owner:KOREA ADVANCED INST OF SCI & TECH
Method of isolating and culturing mesenchymal stem cell derived from umbilical cord blood
InactiveCN1878860AImprove the success rate of cultivationMicroorganismsSkeletal/connective tissue cellsInterleukin 6G-csf therapy
The present invention relates to a method of isolating and culturing mesenchymal stem cells using umbilical cord blood that is most ideal for cell therapy. The method comprises adding an anti-coagulant to umbilical cord blood having a volume of more than 45 ml per unit, which is obtained within 24 hours after parturition; diluting the resulting mixture of the anti-coagulant and umbilical cord blood with an alphaMEM (alpha-minimum essential medium), followed by centrifugation to harvest monocytes; and subjecting the obtained monocytes into suspension culture in the alphaMEM containing Stem Cell Factor, GM-CSF (granulocyte-macrophage colony-stimulating factor), G-CSF (granulocyte colony-stimulating factor), IL-3 (interleukin-3) and IL-6 (interleukin-6).
Owner:韩薰
Cosmetic Compositions
Disclosed is a method for preventing and / or soothing dry or sensitive skin against external stresses, comprising contacting the skin with a composition including an agent that decreases the release of granulocyte-macrophage colony-stimulating factor (GMCSF) from keratinocytes in the skin, wherein under conditions of external stress, dry or sensitive skin is soothed and / or prevented. Agents that decrease the release of GMCSF include silybin, piperine, tetrahydropiperine, nortrachelogenine, rhapontin, arctiin, artigenin and mixtures thereof. Also disclosed is a method of identifying an agent that decreases the level of GMCSF released from keratinocytes.
Owner:COGNIS IP MANAGEMENT GMBH
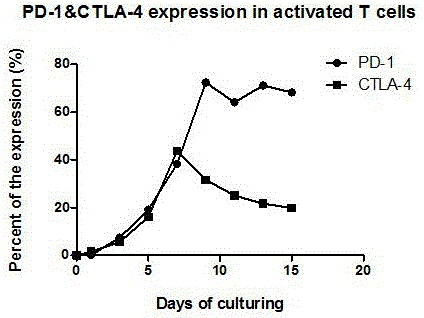
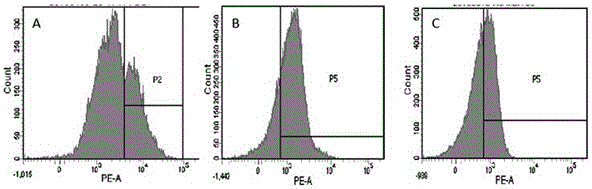
Preparation method of high-efficiency killer cell preparation adopting immunodetection point dual-block CTL (cytotoxic lymphocyte)
ActiveCN106177931AReduce exhaustEnhance tumor killing effectCancer antigen ingredientsBlood/immune system cellsTumor specificInterleukin 4
The invention discloses a preparation method of a high-efficiency killer cell preparation adopting an immunodetection point dual-block CTL (cytotoxic lymphocyte). The preparation method comprises steps as follows: step 1), collecting mononuclear cells in peripheral blood; step 2), performing DC (dendritic cell) separation and T cell separation on the separated mononuclear cells with an adherence method; step 3), adding adhering DCs to a serum-free culture medium containing GM-CSF (granulocyte-macrophage colony-stimulating factor) and IL-4 (interleukin-4) for preparation of a DC vaccine; step 4), adding suspending T cells to CD3 and IL-2 for T cell culture and amplification; step 5), adding antigen-supported DCs to the amplified T cells for preparation of the CTL, and adding PD-1 (Programmed death 1) and CTLA-4 (cytotoxic t lymphocyte associated antigen-4) antibodies for preparation of the cell preparation with the dual-block CTL effect. According to the novel technology and the novel method provided by the invention, an immune brake effect is effectively regulated and T cell depletion is retarded by blocking inhibitory molecules on the tumor specific CTL surfaces, so that the tumor cytotoxicity of CTL effector cells is substantially improved.
Owner:浓孚雨医药河北有限公司
Compositions to promote the healing of skin ulcers and wounds
InactiveUS20160346354A1Promote defense mechanismPromote wound healingPeptide/protein ingredientsHydroxy compound active ingredientsSkin ulcerationsGranulocyte macrophage colony-stimulating factor
The present invention provides compositions comprising as an essential feature granulocyte-macrophage colony-stimulating factor (GM-CSF) together with fosfomycin for the treatment of wounds, ulcers, sores, burns and other injuries to the skin or mucous membranes of the body.
Owner:REPONEX PHARMA APS
Dendritic cell precursors
A method of generating a population of dendritic cell (DC) precursors includes obtaining a population of progenitor cells from a subject and culturing the progenitor cells in a culture medium. The culture medium can include Flt3 ligand and interleukin-6 and be free of granulocyte-macrophage colony-stimulating factor.
Owner:THE CLEVELAND CLINIC FOUND +2
Composition for promoting physiologically regulative regeneration of damaged tissue as well as preparation method and use thereof
ActiveCN104056258AReturn to normal structureRestore physical functionPeptide/protein ingredientsDermatological disorderTissue repairDamages tissue
The invention discloses a composition for promoting physiologically regulative regeneration of a damaged tissue. The composition comprises such components as albumin, epidermal growth factor, transforming growth factor alpha, keratinocyte growth factor, basic fibroblast growth factor, platelet-derived growth factor, vascular endothelial growth factor, interleukin-8 and granulocyte-macrophage colony stimulating factor. The eight protein factors are combined for use to comprehensively develop the physiological tissue repair function; the eight protein factors functionally promote each other; based on the own physiological tissue recovery mechanism of the human body, the wound healing is accelerated and the wound healing quality is improved, including recovering the structure and the functions of the normal skin tissue. Each cell factor-containing component has clear mechanism of action without any potential toxic and side effect and objectionable odor. Such a combined recovery agent is capable of recovering the normal structure and the physiological functions of tissues more quickly under the physiological conditions of the human body.
Owner:高忠翔
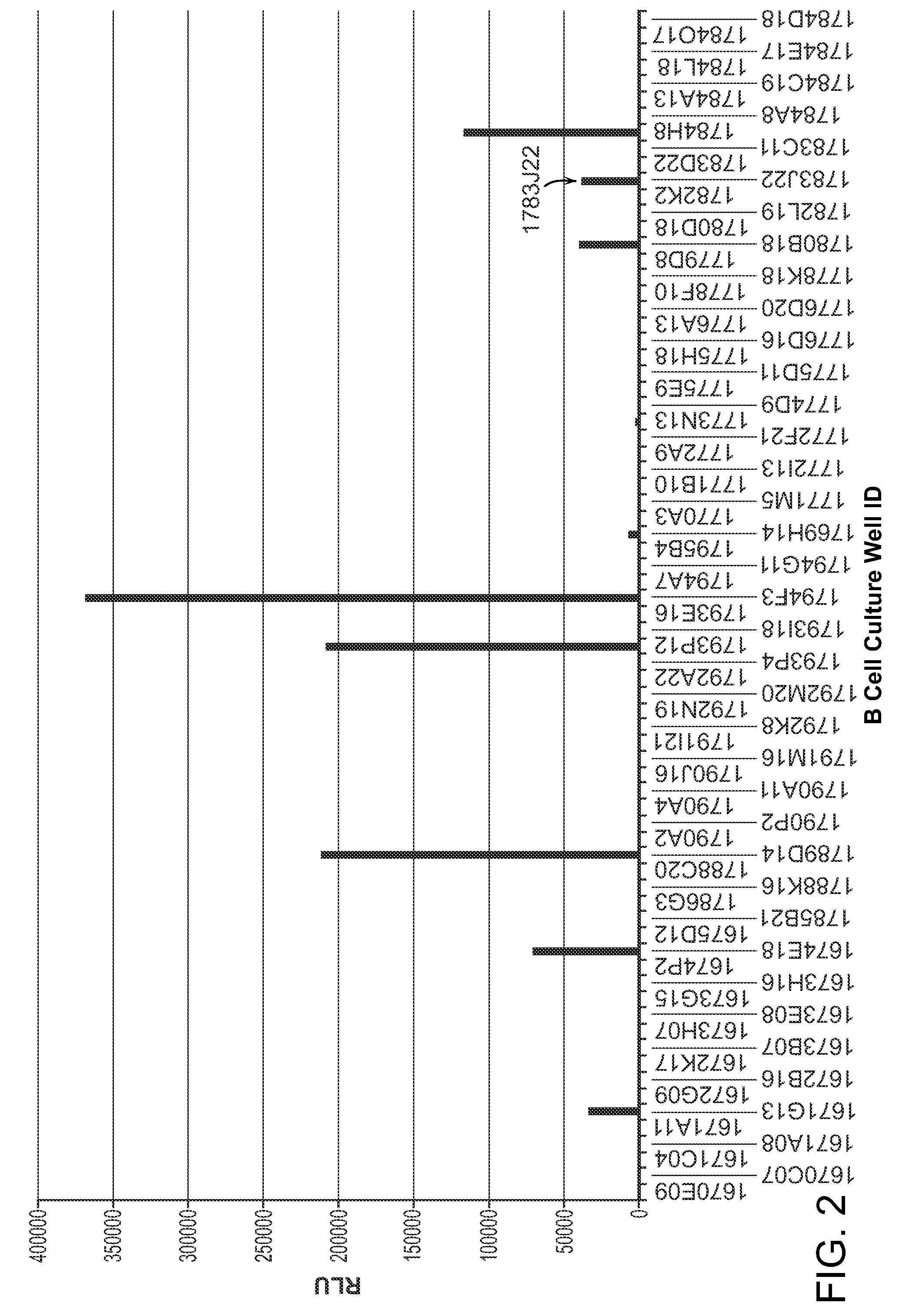
Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) Neutralizing Antibodies
InactiveUS20100291075A1Inhibit biological activityAntibacterial agentsNervous disorderEpitopeMonoclonal antibody
The invention provides a GM-CSF neutralizing human monoclonal antibody, 1783J22, as well as methods of making and use thereof. The monoclonal antibody is further characterized by its ability to bind epitopes from GM-CSF proteins of multiple species.
Owner:THERACLONE SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com