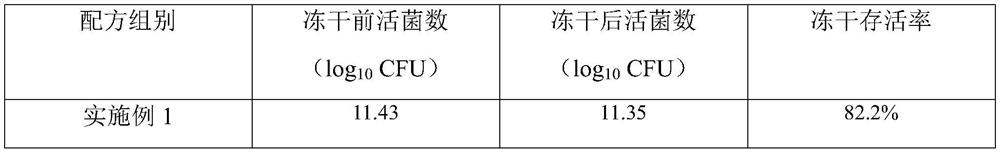
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
42 results about "Fecal microbiota" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
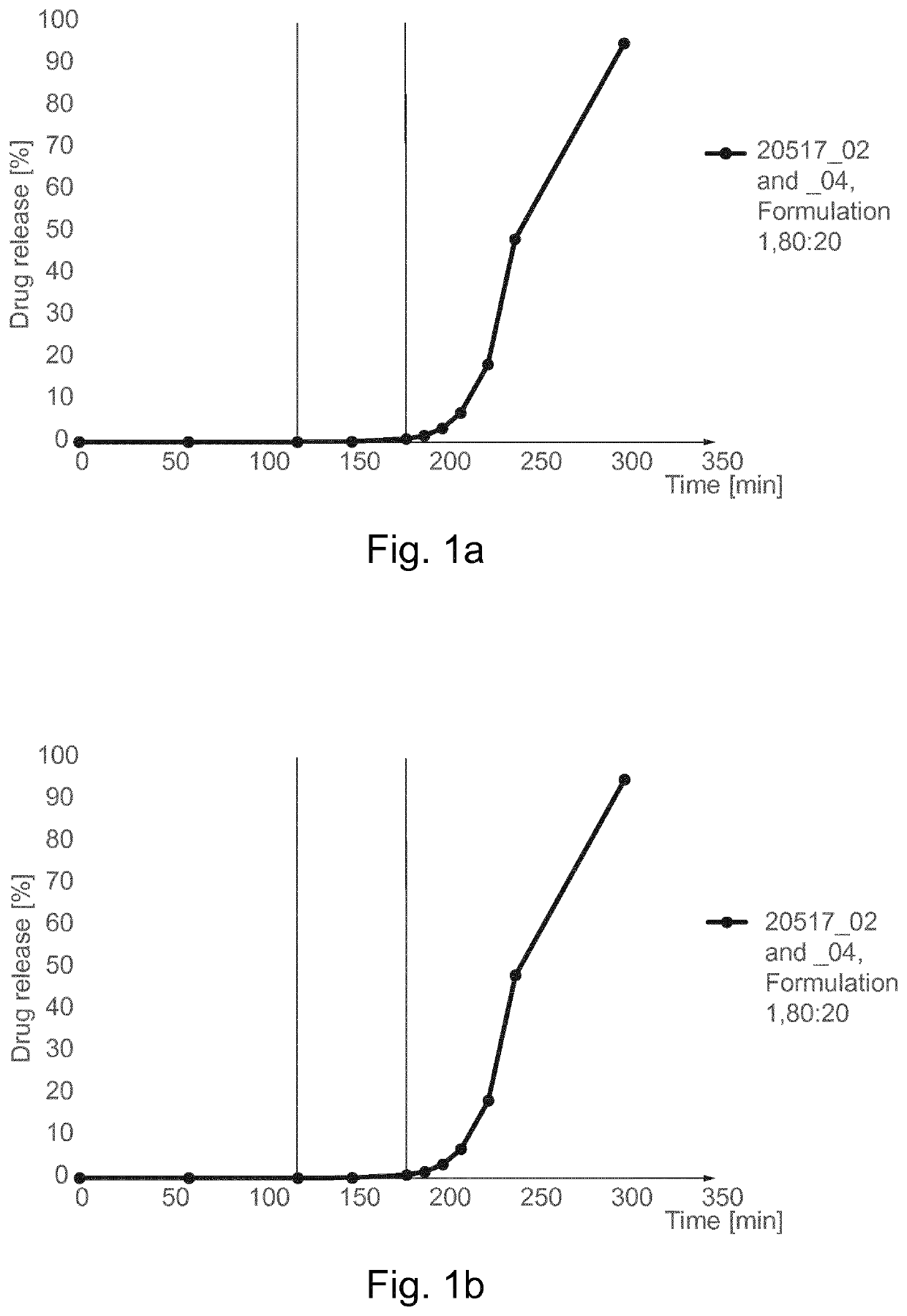
Freeze dried fecal microbiota for use in fecal microbial transplantation
The present invention provides freeze-dried compositions that include an extract of human feces and a cryoprotectant, and methods for making and using such compositions, including methods for replacing or supplementing or modifying a subject's colon microbiota, and methods for treating a disease, pathological condition, and / or iatrogenic condition of the colon.
Owner:RGT UNIV OF MINNESOTA
Fecal microbiota capsule, as well as preparation method and application thereof
The invention belongs to the technical field of fecal microbiota transplantation, and particularly relates to a fecal microbiota capsule, as well as a preparation method and application of the fecal microbiota capsule. The preparation method comprises the following steps: (1) a fecal microbiota solution is prepared; (2) the fecal microbiota solution, obtained in the step (1), is added with a freeze-drying protective additive, and freeze drying is performed to obtain fecal microbiota freeze-dried powder; (3) a capsule is filled with the fecal microbiota freeze-dried powder, obtained in the step (2), to obtain the fecal microbiota capsule. Through adoption of the fecal microbiota capsule, flora is enabled to be comparatively concentrated and can be preserved for a long time at a low temperature; implementation of flora transplantation is facilitated, and invasive implementation manners, such as a gastroscope, an enteroscope and a nasointestinal feeding tube, are not needed; when the fecal microbiota capsule is applied to weak patients, the safety is increased.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
Compositions for the restoration of a fecal microbiota and methods for making and using them
In alternative embodiments, the invention provides compositions and methods for treating various disorders and conditions in mammals, including chronic disorders in which there is a presence of an abnormal microbiota or an abnormal distribution of microflora in the gastrointestinal tract. In alternative embodiments, the invention provides liquid preparations or formulations derived from a human fecal material (e.g., a stool) processed, e.g., filtered and / or centrifuged, such that all bacteria, fungal spores and viruses are removed, but retaining the native biologically active molecules from the fecal material and bacteriophages. In alternative embodiments, the invention provides a "rough-", "incomplete-" or medium- filtered microbiota which still comprises native physiological components or nutritive agents for the bacteria, e.g., retains native biologically and nutritionally active components. In alternative embodiments, the invention provides a highly filtered or substantially purified microbiota in combination with, or having added back, a liquid preparation or formulation of the invention. In alternative embodiments, the invention provides compositions or formulations where the bacteria, or microbiota, component has been cultured, or cultured under anaerobic conditions, or harvested, stored and / or cultured under anaerobic conditions. In alternative embodiments, the invention provides various additives, compositions and donor restrictions for treating these disorders and conditions.
Owner:托马斯·朱利叶斯·波洛迪
Preparation method of high-activity fecal microbiota capsule
PendingCN109679877AHigh activityFor long-term storageBacteriaBacteria material medical ingredientsMedicineSolvent
The invention discloses a preparation method of a high-activity fecal microbiota capsule. The high-activity fecal microbiota can reach the intestinal tract of a patient to take effects under the protection of acid-resistant enteric capsules. The preparation method comprises the following steps: S1, preparing fecal microbiota liquid; S2, evaluating the activity and the flora structure of the fecalmicrobiota liquid; S3, adding the fecal microbiota liquid obtained in S2 into a freezing and drying protecting agent, and cryopreserving until the fecal microbiota capsule is transplanted to the patient; when a receptor is transplanted with the fecal microbiota capsule, thawing the fecal microbiota liquid and centrifuging to obtain a thallus; S4, dissolving the thallus in the S3 by using a fecal microbiota solvent with proper volume and loading the fecal microbiota thallus into a double-layer acid-resistant enteric capsule. The preparation method of the high-activity fecal microbiota capsule,disclosed by the invention, has the beneficial effects that the flora has high activity and can be preserved at low temperature for a long time; the method is convenient for implementation of flora transplantation and does not need an invasive implementation mode.
Owner:广州承葛生物科技有限公司 +1
Full fecal microbiota compound capsule as well as preparation method and application thereof
InactiveCN108567799AImprove function and effectIncrease the number ofNervous disorderMetabolism disorderUlcerative colitisConstipation
The invention relates to the field of medicines, and provides a full fecal microbiota compound capsule. The full fecal microbiota compound capsule comprises a wall material and a core material, wherein the wall material is an enteric capsule shell, and the core material comprises full fecal microbiota and prebiotics; the effective viable count in the full fecal microbiota compound capsule is 1*10<10>-2.88*10<10> / grain. The full fecal microbiota and the prebiotics can be taken as the core material, the characteristic that prebiotics selectivity is used for accelerating the growth and propagation of beneficial microorganisms is used for obviously improving the amount of beneficial bacteria floras in the full fecal microbiota, and the effective viable count is lowered. On one hand, the survival time of beneficial microorganisms with functions in the full fecal microbiota can be effectively improved, and on the other hand, the function effect of the full fecal microbiota compound capsule after intestinal tract planting can be improved. Therefore, the full fecal microbiota compound capsule has the obvious curative effects for patients who suffer from refractory difficult-distinguishingclostridium infection, constipation, diarrhea, Crohn's disease, ulcerative colitis, irritable bowel syndrome, food intolerance and the like.
Owner:GENERAL HOSPITAL OF PLA
Technology for preparing intestinal flora preparation in fecal microbiota transplantation clinic treatment
InactiveCN109010380AExtended storage timeExcellent formulation performanceDigestive systemUnknown materialsAnaerobic bacteriaFiltration
The invention discloses a technology for preparing an intestinal flora preparation in fecal microbiota transplantation clinic treatment. The technology comprises the following steps: collecting feces,preparing intestinal flora, and filling the intestinal flora preparation, wherein the step of collecting feces includes fecal collection and anaerobic transportation, the step of preparing the intestinal flora preparation includes stepwise filtration under aerobic and anaerobic conditions, centrifuging and mixing, and the step of filling the intestinal flora preparation adopts storage using a glycerol protection solution and a cryopreservation technology. A novel aerobic and anaerobic separation method is adopted in the separation process of an intestinal flora solution, the aerobic and anaerobic separation method selected according to the characteristics of the living environment of intestinal florae can maximally protect the flora diversity of aerobic bacteria and anaerobic bacteria inthe intestinal tract, and an added glycerol protection agent maintains the bacterial activity within a certain period of time and improves the effect of the intestinal flora preparation.
Owner:卓源健康科技有限公司
Methods for treating autism spectrum disorder and associated symptoms
InactiveCN108243608AReduce severityNervous disorderBacteria material medical ingredientsSymptom improvementIntestinal microorganisms
The present disclosure relates to compositions and methods for treating autism spectrum disorder (ASD) by restoring an ASD patient's gut microbiota. These methods can be used with ASD patient with orwithout ongoing gastrointestinal symptoms. Provided here is a method for ASD treatment in a subject in need thereof comprising or consisting essentially of administering a therapeutic composition comprising a fecal microbe or a fecal microbiota preparation to the subject. Also provided here is a method comprises administering an antibiotic to a human subject; subjecting the human subject to a bowel cleanse; and administering purified fecal microbiota to the human subject. Further provided are evaluation and quantitative characterization of patient symptom improvements upon treatment describedhere.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA +2
Freeze dried fecal microbiota for use in fecal microbial transplantation
InactiveUS20180110810A1Better long term storageEasy to transportBacteria material medical ingredientsViral/bacteriophage medical ingredientsDiseaseMicroorganism
The present invention provides freeze-dried compositions that include an extract of human feces and a cryoprotectant, and methods for making and using such compositions, including methods for replacing or supplementing or modifying a subject's colon microbiota, and methods for treating a disease, pathological condition, and / or iatrogenic condition of the colon.
Owner:RGT UNIV OF MINNESOTA
Experimental mouse model with fecal microbiota transplantation and construction method
PendingCN107349225AGood effectAuthentic and Reliable ResponseSaccharide peptide ingredientsUnknown materialsFecal microbiotaAntibiotics
The invention discloses an experimental mouse model with fecal microbiota transplantation and a construction method. The construction method comprises the following steps that intragastric injection with an antibiotic solution is conducted on a mouse, the number of times of intragastric injection is at least one time a day, and the number of days of intragastric injection is at least one day; after intragastric injection with the antibiotic solution is conducted on the mouse, a laxative solution is taken as a water resource for drinking, and the number of days of drinking the laxatives solution is at least one day; and after the mouse drinks the laxative solution, fecal microbiota transplantation is conducted on the mouse, specifically, the experimental mouse model with fecal microbiota transplantation is constructed. According to the experimental mouse model with fecal microbiota transplantation and the construction method, the construction method is simple, and situation can be truly and reliably reflected.
Owner:THE FIFTH AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV
Intestinal flora researching method based on feces filtering and transplanting
InactiveCN109008958AExclude direct effectsSimple methodDisease diagnosisDiagnostic recording/measuringDiseaseMicroorganism
The invention discloses a medicament and intestinal flora cooperation researching method based on feces filtering and transplanting. The method comprises the following steps of 1, preparing a fecal microbiota transplantation acceptor experiment animal, wherein a pseudo-germfree or a sterile animal is selected as the fecal microbiota transplantation acceptor experiment animal, thereby creating an environment without bacteria or little bacteria in an intestinal tract, and facilitating colonization of external microbes; 2, preparing a fecal microbiota transplantation denor experiment animal, wherein the denor animal receives disease modeling and medicament gavage processing, and establishing a positive and negative experiment contrast of the drug effect; and 3, making the acceptor experimentanimal receive filtering and non-filtering fecal microbiota transplantation of the donor experiment animal. The method according to the invention is based on a typical fecal microbiota transplantationintestinal flora researching method and further realizes advantages of simple operation and easy application. The method further has advantages of eliminating direct influence of drug to the intestinal tract, focusing a flora mediation medicament function and supplying a concise method for researching interacting between the medicament-modulated flora and a host.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
Methods and materials for using biomarkers which predict susceptibility to clostridium difficile infection
InactiveUS20190216861A1Remodel the gut microenvironmentAntibacterial agentsMicrobiological testing/measurementBacteroidesClostridium difficile infections
This document relates to biomarkers of gut microbiota dysbiosis. Bacteria that are increased or decreased in gut microbiota dysbiosis can be used as biomarkers to predict dysbiosis in patients with diarrhea and / or to predict susceptibility to Clostridium difficile infection (CDI). In addition, provided herein are compositions including at least three bacteria that are de creased in gut microbiota dysbiosis which can be used, for example, to restore heathy gut microbiota (e.g., by probiotic or by fecal microbiota transplant) to treat CDI.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Intestinal tract micro-ecological map of longevity family and application of intestinal tract micro-ecological map in field of aging health
PendingCN111455016AThe mechanism of treatment is clearMicrobiological testing/measurementDigestive systemBiotechnologyBacteroides
The invention discloses establishment of an intestinal tract micro-ecological map of a longevity family and an application of the intestinal tract micro-ecological map in the field of aging health. Itis discovered that the following microorganisms including at least one of bacteroides-prevotella, porphyromonadaceae, parabacillus, anaerobic cocci and bifidobacterium adolescentis can be used to more comprehensively observe the flora distribution of the intestinal tracts of longevity crowds, a new marker is provided for diagnosing the health condition of the body and predicting the life of the human body, and more reasonable diet and nutrition suggestions are provided for the healthy life of people. Meanwhile, a new method and thought are provided for research on a mechanism of interaction between metabolites of intestinal microorganisms of the longevity crowds and the bodies, an anti-aging treatment mechanism is clearer, and a solid theoretical basis is provided for research on novel conditioning products including microecological preparations, viable bacterial drugs, fecal microbiota transplantation, nutrient products and the like.
Owner:广州市华永睿健生物科技有限公司
Freeze-drying protective agent, fecal microbiota freeze-dried product and preparation method of freeze-drying protective agent
ActiveCN112195103AProtected SurvivalSpeed up the outflowMicroorganism preservationVitamin CFreeze-drying
The invention belongs to the field of microorganisms, and particularly relates to a freeze-drying protective agent, a fecal microbiota freeze-dried product and a preparation method of the freeze-drying protective agent. The freeze-drying protective agent comprises polydextrose, mannitol, glycine, vitamin C and Tween 80; the freeze-drying fecal microbiota product prepared from the freeze-drying protective agent is high in fecal microbiota total fungus survival rate and superior to trehalose in effect; and the potential risk caused by trehalose, maltodextrin and the like can be reduced, and thefinal curative effect of the fecal microbiota transplanting product can be potentially improved.
Owner:SHENZHEN XBIOME BIOTECH CO LTD
Compositions and methods for c. difficile treatment
InactiveUS20180000872A1CDI clearance rateAntibacterial agentsPowder deliveryClostridial infectionClostridium difficile
The present disclosure provides compositions and methods for treating Clostridium difficile infection (CDI) including primary and recurrent CDI. In particular, the compositions and methods described herein are capable of achieving a CDI clearance rate of at least 80% through a single oral dose of a pharmaceutical composition comprising a freeze-dried fecal microbiota preparation.
Owner:RGT UNIV OF MINNESOTA
Stable capsules with fecal microbiota or a culture of microorganisms
PendingUS20200188310A1Increase loadMinimizes risk of lossUnknown materialsCapsule deliveryHard CapsuleEdible oil
The present invention provides a hard capsule comprising an outer and one inner capsule, wherein the outer capsule comprises a hydrophobic liquid and the inner capsule which comprises a composition comprising uncoated microorganisms and optionally at least one desiccant. The composition may comprise a filtrate composed of viable fecal bacteria useful forfecal microbiota transplantation (FMT) or a mixture of one or more different types of microorganisms isolated from intestinal microbiome, such as bacteria, archaea, virus, or fungi such as yeast. The composition may comprise a desiccant, preferably a food approved component which is degradable in the gastrointestinal tract. The hydrophobic liquid, if present, is preferably at least one edible oil. Also provided are methods of treating or preventing a dysbiosis in a human subject, comprising administering to the human subject at least one capsule of the invention.
Owner:CHR HANSEN AS
Fecal bacteria capsule and its preparation and application
The invention belongs to the technical field of fecal microbiota transplantation, and particularly relates to a fecal microbiota capsule, as well as a preparation method and application of the fecal microbiota capsule. The preparation method comprises the following steps: (1) a fecal microbiota solution is prepared; (2) the fecal microbiota solution, obtained in the step (1), is added with a freeze-drying protective additive, and freeze drying is performed to obtain fecal microbiota freeze-dried powder; (3) a capsule is filled with the fecal microbiota freeze-dried powder, obtained in the step (2), to obtain the fecal microbiota capsule. Through adoption of the fecal microbiota capsule, flora is enabled to be comparatively concentrated and can be preserved for a long time at a low temperature; implementation of flora transplantation is facilitated, and invasive implementation manners, such as a gastroscope, an enteroscope and a nasointestinal feeding tube, are not needed; when the fecal microbiota capsule is applied to weak patients, the safety is increased.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
Fecal microbiota collection, cryopreservation and unfreezing method of fecal microbiota transplantation technology
ActiveCN111876331AReduce concentrationAvoid cytotoxicityBacteriaMicroorganism based processesAntifreeze proteinCytotoxicity
The invention relates to a fecal microbiota collection, cryopreservation and unfreezing method of a fecal microbiota transplantation technology. The method comprises the following steps: (1) preparingfecal microbiota; (2) carrying out vitrification freezing on the fecal microbiota prepared in the step (1) to obtain cryopreserved fecal microbiota; and (3) unfreezing and recovering the cryopreserved fecal microbiota obtained in the step (2). According to the invention, a vitrification freezing technology is adopted, so that the freezing rate (greater than 20000 DEG C / min) is greatly increased,cells rapidly form a 'glassy state' in a rapid cooling process, ice crystals are prevented from being formed inside and outside the cells, excessive damage to florae in the fecal microbiota is avoided, and an antifreezing protein is added into a cryoprotectant, so that the cytotoxicity of the cryoprotectant is avoided; and the survival rate of the flora in the unfrozen fecal microbiota liquid is increased to 90% or above.
Owner:山东省大健康精准医疗产业技术研究院
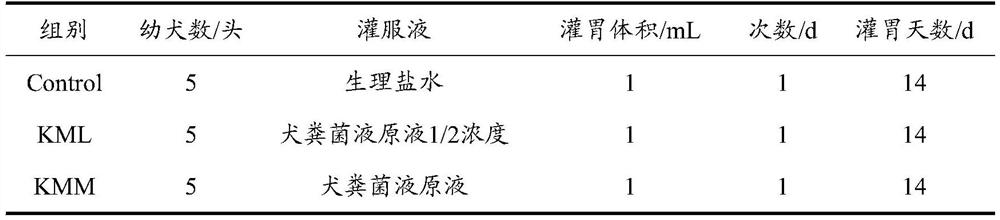
Method for improving working performance of puppies of Kunming dogs by fecal microbiota transplantation
The invention discloses a method for improving working performance of puppies of Kunming dogs by fecal microbiota transplantation, and belongs to the technical field of biology. The method comprises the following steps that excrement donors are screened; grandmother of male young dogs with same breeding conditions and excellent working performance is selected as microbiota source donor; a fecal microbiota stock solution is prepared, and storing is conducted at low temperature; and when transplantation is conducted, fecal microbiota transplantation liquid is prepared; the fecal microbiota transplantation liquid is administered to the different healthy male young dogs which are from the same healthy grandmother and are 45 age days-55 age days through gavage, and continuous gavage is conducted for 14 days or longer; and non-antibiotic feed is fed. According to the method, and the working quality behaviors of the puppies, namely the working performance of the puppies, can be obviously improved.
Owner:YUNNAN AGRICULTURAL UNIVERSITY +1
Fecal microbiota composition for use in reducing treatment-induced inflammation
PendingUS20210299188A1Raise the ratioReduce the ratioAntipyreticBacteria material medical ingredientsFecal microbiotaGastroenterology
The invention relates to the use of fecal microbiota transplant for preventing and / or reducing systemic and gut treatment-induced inflammation in an individual in need thereof.
Owner:MAAT PHARMA
Fecal microbiota for treating patients undergoing a hematopoietic stem cell transplant
InactiveUS20200188450A1Reduce riskAntibacterial agentsBacteria material medical ingredientsHsc transplantationAllogeneic hematopoietic stem cell transplant
The invention relates to a fecal microbiota sample for use in the prevention and / or treatment of infectious or non-infectious complications resulting from allogeneic hematopoietic stem cell (HSC) transplantation, or for the treatment of cancer in a recipient patient, said fecal microbiota sample and said hematopoietic stem cells originating from the same donor subject, or said fecal microbiota sample being administered prior to HSC transplantation.
Owner:CENT NAT DE LA RECHERCHE SCI +3
Method for preparing fecal microbiota transplantation capsule containing benzoylaconine by utilizing human fecal microbiota fermented aconite roots
InactiveCN110742929AMaximum releaseGood heartAntipyreticAnalgesicsIntestinal microorganismsCurative effect
The invention relates to a method for preparing a fecal microbiota transplantation capsule containing benzoylaconine by utilizing human fecal microbiota fermented aconite roots. Fecal microbiota transplantation (FMT) means that a functional flora in healthy human feces is transplanted into gastrointestinal tracts of a patient to re-establish a new intestinal flora so as to realize treatment on intestinal and parenteral diseases. Clinical application discovers that a required curative effect cannot be achieved after certain patients take the prepared aconite roots, one of main reasons causing individual differences is incomplete intestinal flora of the patient, and toxic substances in the prepared aconite roots cannot be normally metabolized into non-toxic active ingredients, the active ingredients have low utilization, and the required curative effect of the prepared aconite roots cannot be achieved. Healthy high-quality feces of a fecal microbiota transplant donor are utilized, are used for simulating intestinal microbes in vitro to achieve an effect of transforming the prepared aconite roots, aconitine and other toxic ingredients in the prepared aconite roots can be transformed into nontoxic metabolitic products through proper fermentation conditions, and the content of benzoylaconine and other active ingredients is increased. The fecal microbiota capsules prepared from fecalmicrobiota fermentation liquid can be used in fecal microbiota transplantation treatment in clinical application.
Owner:广东南芯医疗科技有限公司
Fecal virome and therapeutic efficacy of fecal microbiota transplantation
ActiveUS20190321421A1Effective treatmentIncrease diversityHealth-index calculationMicrobiological testing/measurementPhysiologyEfficacy
The present invention resides in the discovery that abundance and diversity of viral species in the fecal matter from a donor used in fecal microbiota transplantation (FMT) treatment can influence the outcome of the FMT treatment. Thus, novel methods are provided for identifying subjects as suitable donors for FMT, for assessing the likelihood of FMT treatment success, and for enhancing FMT treatment efficacy. Also provided are kits and compositions for FMT with enhanced efficacy.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Pharmaceutical oral formulation comprising bacteria
ActiveUS20200345790A1Viability is conservedTaxonomic (diversity) profile preservedPowder deliveryBacteria material medical ingredientsMethacrylate methylPharmaceutical Substances
The present invention relates to a pharmaceutical oral formulation for use in the administration of at least two bacteria derived from fecal microbiota to mammals for the treatment and / or prevention of dysbiosis and associated pathologies. The pharmaceutical oral formulation is encapsulated mixture of at least two bacteria derived from fecal microbiota wherein the capsules are coated in a pH responsive polymer composition comprising:a. 50-70% poly(methyl acrylate-co-methyl methacrylate-co-methacrylic acid) 7:3:1 by weight of dry polymer,b. 10-30% poly(methacrylic acid-co-ethyl acrylate) 1:1 by weight of dry polymer,c. at least one fatty acid mono-, di- or tri-glyceride ester, or mixtures thereof,d. at least one plasticizer,e. at least one non-ionic emulsifier.The pharmaceutical oral formulation is especially designed to be delivered principally in the ileum and colon and to maintain the sample bacterial viability and diversity.
Owner:MAAT PHARMA +1
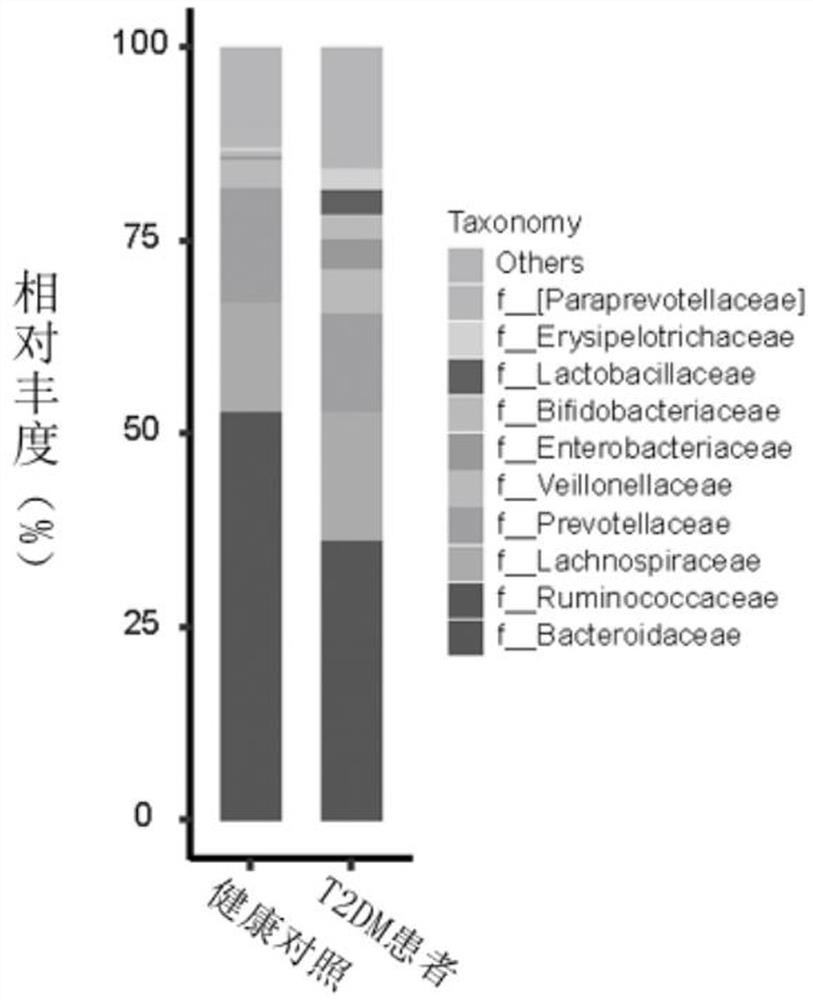
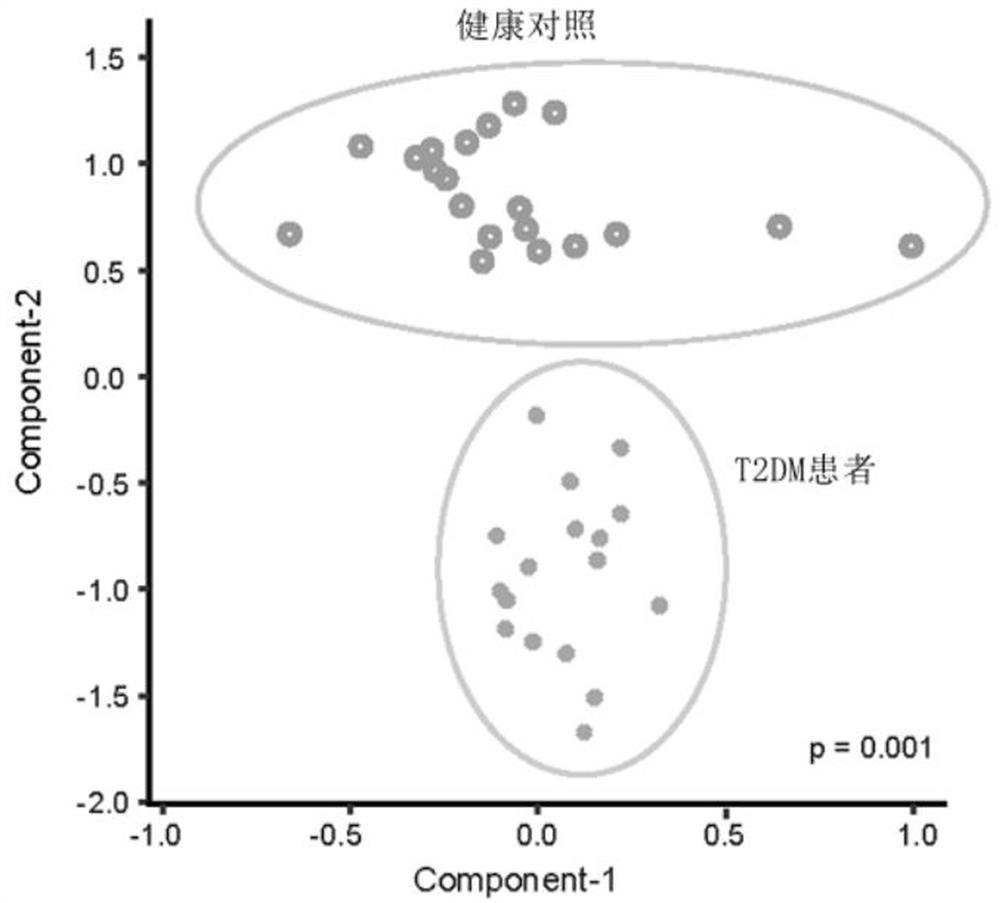
Microbial marker for evaluating fecal microbiota transplantation curative effect of type 2 diabetes mellitus patients and application thereof
InactiveCN113337630AAvoid over-medicationAchieve the goal of personalized medicineMicrobiological testing/measurementDisease diagnosisIntestinal microorganismsDiabetic patient
The invention discloses an intestinal metagenomic feature used as a fecal microbiota transplantation curative effect screening marker for type 2 diabetes mellitus patients. The intestinal microbe metagenomic feature is Rinkenellaceae family and Anerotrunk family intestinal flora, and the type 2 diabetes mellitus patient suitable for fecal microbiota transplantation treatment are selected according to the expression levels of the specific intestinal flora markers. Corresponding detection kits are designed and developed according to the intestinal flora biomarkers and different detection technology platforms. The system and the product can be used for precise treatment of type 2 diabetes mellitus based on individualization.
Owner:BERKELEY NANJING MEDICAL RES CO LTD +1
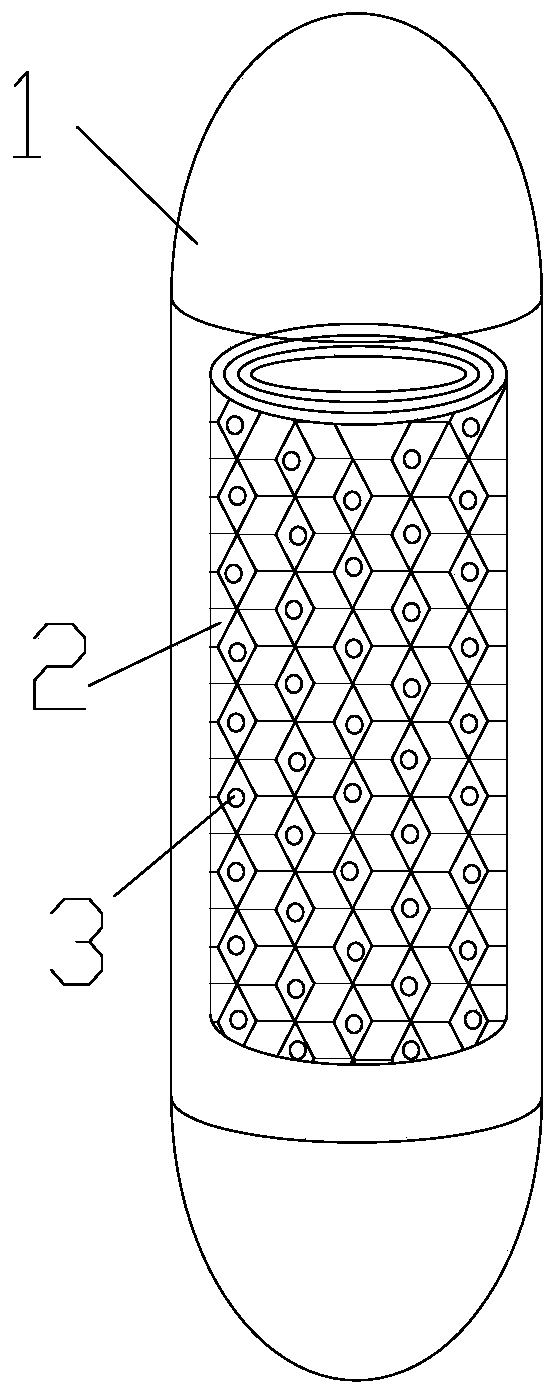
Fecal microbiota capsule and preparation method thereof
ActiveCN110721202AExtended durationIncrease the number of transplantsDigestive systemUnknown materialsIntestinal wallsFecal microbiota
The invention discloses a fecal microbiota capsule which comprises an enteric capsule case and a core material, wherein the core material consists of a core material carrier and fecal microbiota freeze-dried powder; the core material carrier is of a porous round tubular structure; the fecal microbiota freeze-dried powder is adsorbed in pores of the core material carrier; the core material carrieris formed by pressing natural and porous loofah sponge into layers and rolling the layers; and the fecal microbiota freeze-dried powder is prepared from fecal microbiota liquid and freeze-drying protecting agents through freeze-drying. The invention also discloses a preparation method of the fecal microbiota capsule. The preparation method comprises the steps of preparing the fecal microbiota liquid; adding the freeze-drying protection agents into the fecal microbiota liquid; adding the core material carrier; and then performing soaking; and charging the core material into the enteric capsulecase to obtain the fecal microbiota capsule. The fecal microbiota capsule has the advantages that the release speed of the fecal microbiota freeze-dried powder can be reduced; the fecal microbiota inthe fecal microbiota freeze-dried powder is further prevented from being discharged out of the intestinal tract too early; the release duration of the fecal microbiota freeze-dried powder in the intestinal tract is prolonged; the contact with more intestinal walls is realized to increase the fecal microbiota transplantation quantity; and the fecal microbiota transplantation effect is improved.
Owner:THE THIRD PEOPLES HOSPITAL OF SHENZHEN
Preparation method and application of fecal microbiota liquid and preparation method and application of fecal microbiota freeze-dried powder
InactiveCN112458019AFor long-term storageLow toxicityPowder deliveryBacteriaUlcerative colitisMicrobiology
The invention discloses a preparation method and application of fecal microbiota liquid and a preparation method and application of fecal microbiota freeze-dried powder, and belongs to the technical field of fecal microbiota transplantation. The preparation method of the fecal microbiota liquid and the preparation method of the fecal microbiota freeze-dried powder comprise the following steps: preparing original microbiota liquid; performing centrifuging and washing; preparing the fecal microbiota liquid; and preparing the fecal microbiota liquid freeze-dried powder. The fecal microbiota liquid and the freeze-dried powder thereof prepared by the preparation method disclosed by the invention are used for preparing medicines for treating ulcerative colitis. The fecal microbiota freeze-driedpowder prepared by the invention is concentrated in flora and can be stored for a long time at low temperature. Through washing treatment, the toxicity of fecal microbiota is reduced or removed. A freeze-dried powder technology is adopted, so that implementation of flora transplantation is facilitated, and the operation is convenient.
Owner:广东南芯医疗科技有限公司 +1
Method for gut mucosa preparation to enhance microbial engraftment
In alternative embodiments, provided are products of manufacture for microbiome transplantation and engraftment, including fecal microbiota transplantation (FMT) delivery devices, and methods for using them, including methods for replacing an individual's gastrointestinal (GI), e.g., colonic, microbiome, and methods for the treatment, amelioration or prevention of an in situ microbiome space, or a gastrointestinal (GI) disease, infection or condition or a disease or condition caused by, initiated by or exacerbated by a pathological microbiome, e.g., pathological GI or colonic microbiome. In particular a method for microbiome transplantation and engraftment in an individual in need thereof, the method comprising: removing of some or all colonic fecal material from the colon of the individual in need thereof by washing out colonic fecal material from the colon, wherein said washing out comprises administering a formulation comprising a biofilm dissolving or disrupting agent to the colon, and administering a FMT material, liquid, formulation or solution, or a normal microbiome, to the individual in need thereof.
Owner:MILIS ANTONY +1
Tumor microbiome signature and therapeutic use of fecal microbiota transplantation on pancreatic cancer patients
PendingUS20220196667A1Modulate immune systemAffect tumor growthBacteria material medical ingredientsDigestive systemMicroorganismPancreas Cancers
Provided herein are methods of predicting whether a pancreatic cancer patient will be a short-term or long-term survivor based on their intra-tumoral microbiota. Also provided are methods of treating pancreatic cancer patients using fecal microbial transfer from long-term pancreatic cancer survivors as well as pharmaceutical compositions comprising fecal microbiota obtained from long-term pancreatic cancer survivors.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Long-term stable live fecal microbiota composition
PendingUS20220143107A1Minimizes risk of lossRisk minimizationBacteria material medical ingredientsCapsule deliveryMicroorganismPharmaceutical medicine
The present invention provides a solid oral pharmaceutical composition comprising a pharmaceutically effective amount of living microorganisms and one or more pharmaceutically acceptable water absorbing excipient(s), wherein the composition has a water content, determined according to European Pharmacopoeia 9.4, section 2.5.12., from 0.5 to 30% with respect the total weight of the composition. The invention also provides processes for its preparation as well as it use in therapy. The live-cell based composition of the invention is stable at mild conditions.
Owner:INST DINVESTIGACIONS BIOMEDIQUES AUGUST PI I SUNYER IDIBAPS +3
Optimized Fecal Microbiota Transplantation Carrier Medium
InactiveUS20180133323A1Maximize the effectPharmaceutical delivery mechanismPharmaceutical non-active ingredientsMicroorganismActive agent
The present invention is a microbially inert, optimized carrier medium for fresh Fecal Microbiota Transplantation samples, intended for same-day transplantation, in which the carrier material is gastrointestinally active while remaining predominantly microbially inert. The key active agent in the present optimized collection and transplantation medium is polyethylene glycol, and in one embodiment of the invention the 1,000,000 to 10,000,000 grams per mole molecular weight of the polyethylene glycol provides a useful high viscosity to the present collection / carrier medium.
Owner:KEITH ALEC D
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com