Preparation method of high-activity fecal microbiota capsule
A high-activity, fecal bacteria technology, applied in the field of preparation of high-activity fecal bacteria capsules, can solve the problems of patients' clinical improvement, failure to analyze the structure of the bacterial group, etc., and achieve good clinical results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] A preparation method for highly active fecal bacteria capsules, under the protection of acid-resistant enteric-coated capsules, highly active fecal bacteria can reach the patient's intestinal tract to play a role, comprising the following steps:
[0033] S1, preparation of feces liquid;
[0034] S2. Evaluation of fecal liquid activity and flora structure;
[0035] S3. The fecal bacteria liquid obtained in step S2 is added with a freeze-drying protective agent and frozen until the fecal bacteria capsule is transplanted to the patient. When the fecal bacteria capsule is transplanted to the recipient, the fecal bacteria liquid is thawed and centrifuged to obtain the bacteria;
[0036] S4. Dissolving the bacterial cells in step S3 with an appropriate volume of fecal bacteria solvent, and packing the fecal bacterial cells into double-layer acid-resistant enteric-coated capsules.
[0037] In step S1, the preparation method of fecal bacteria liquid is:
[0038] S10. Instrume...
Embodiment 1
[0050] Isolation of fecal bacteria
[0051] S10. Instrument preparation: Seal the separation tank containing the donated feces with a lid with a helical blade stirring rod, and connect the stirring rod to a feces separation machine specially made by Nanjing Famet Company. Connect 5 aseptic and airtight filter tanks to discharge and install them in the separator in sequence. The initial tank is connected to the separation tank with a conduit, and the final tank is connected to the collection tank to form a complete closed system consisting of stirring, filtering and collecting. For separation equipment, the safety cabinet is sterilized by ultraviolet light for 30 minutes before use;
[0052] S11. Start the machine: use the filling pump to fill 500ml of 0.9% normal saline into the separation tank, turn on the mixer at 1000rpm for 5min, then inject 500ml of normal saline, and stir at 1000rpm for 5min; this process completely mixes the feces with the normal saline , so that the i...
Embodiment 2
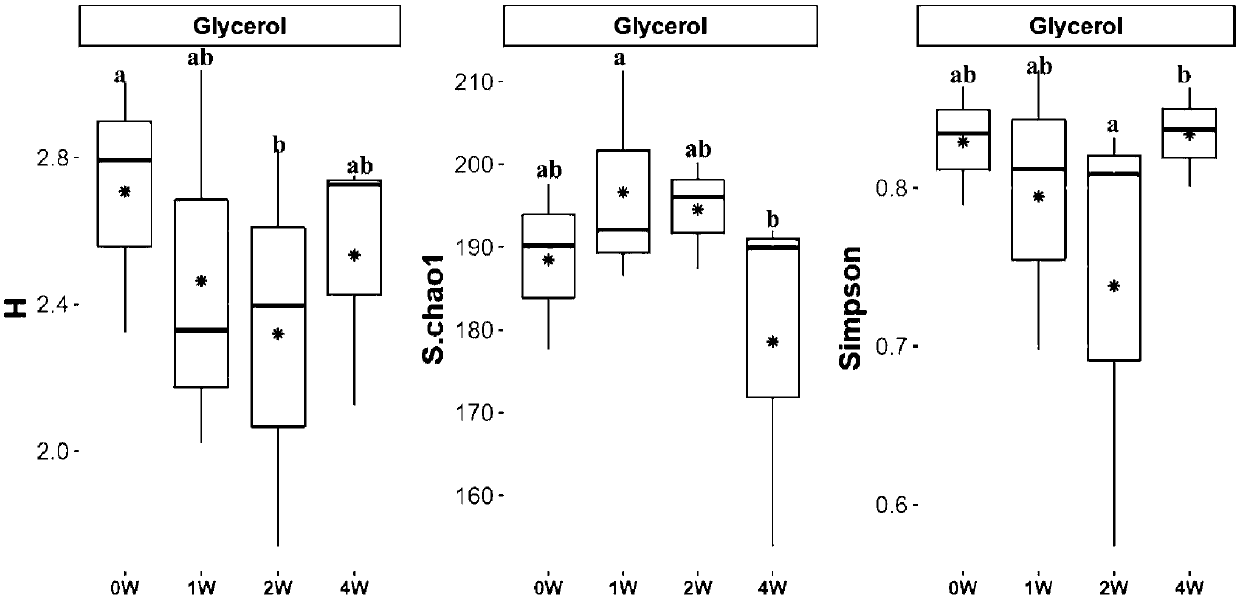
[0056] Evaluation of Fecal Bacteria Activity During Cryopreservation
[0057] 1) For the bacterial cells obtained in step S13 in Example 1, divide 1 mL of bacterial liquid at five time points of day 0, 1 week, 2 weeks, 3 weeks and 1 month, and place them at -80°C Preservation (except for fresh fecal bacteria);
[0058] 2) Take 10 μl of bacterial solution and mix with 990 μl of 0.9% NaCl solution to obtain a dilution of fecal bacteria;
[0059] 3) Take out the frozen bacterial liquid at different time points, and prepare samples according to the kit Live / Dead BacLightTM BacterialViability and Counting Kit;
[0060] 3) Add 987 μl of 0.9% NaCl solution, 1.5 μl of SYTO9 and 1.5 μl of PI dye to a 2 ml sterilized centrifuge tube, and finally add 10 μl of the fecal bacteria dilution in step 4), mix well and place in the dark Incubation for 15 minutes;
[0061] 4) Setting parameters, counting the ratio of live bacteria by flow cytometry.
[0062] Proportion of viable bacteria duri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com