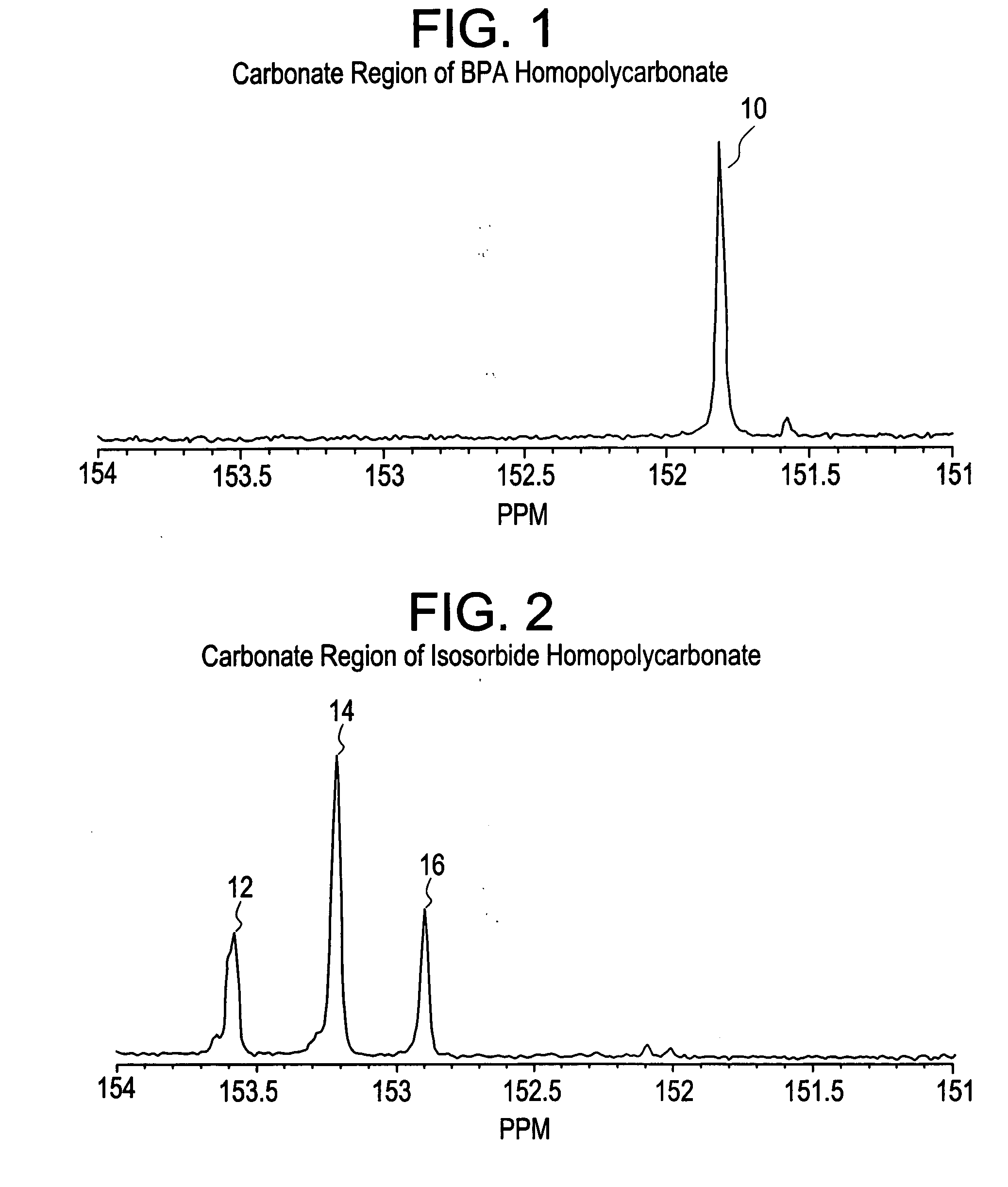
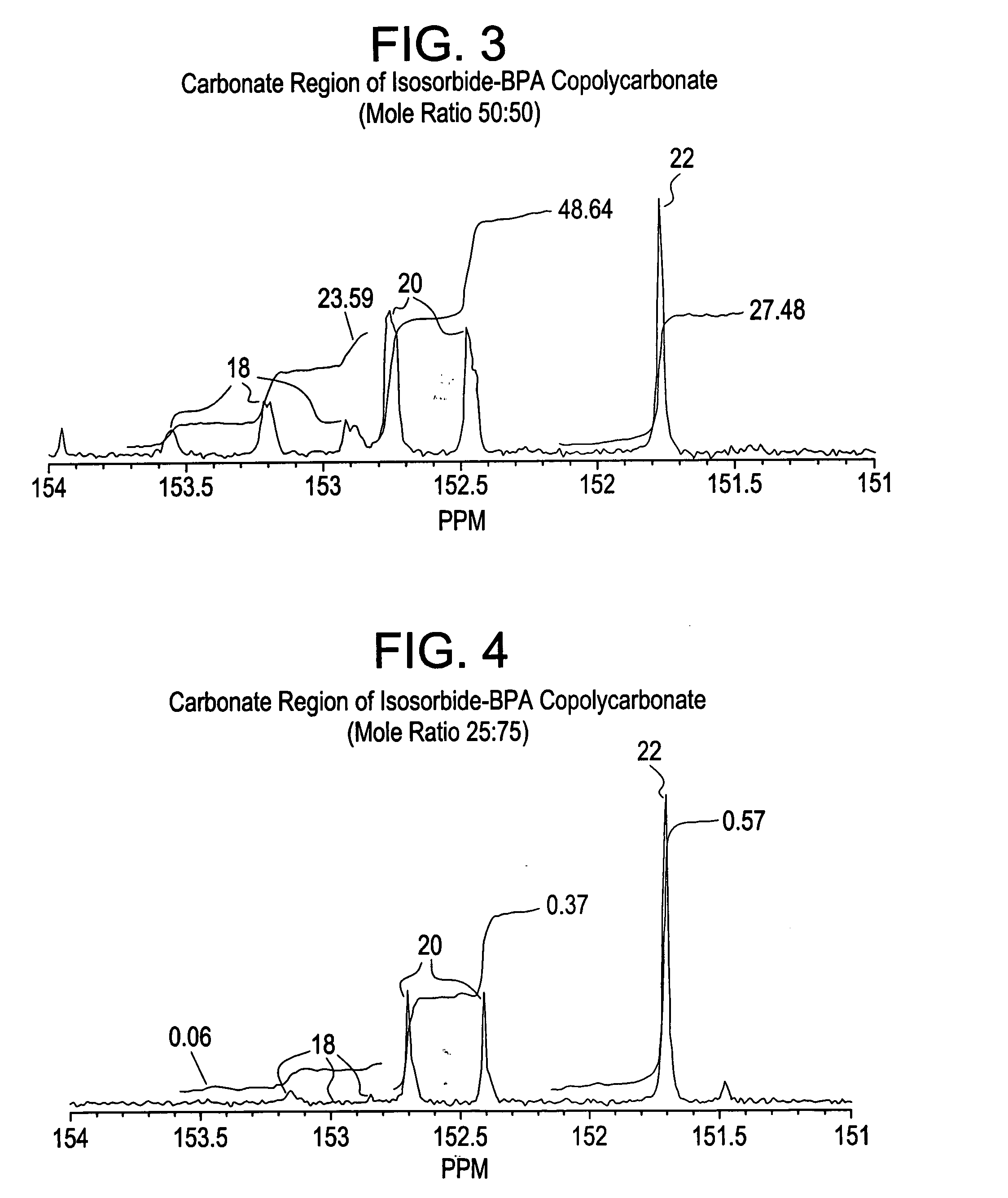
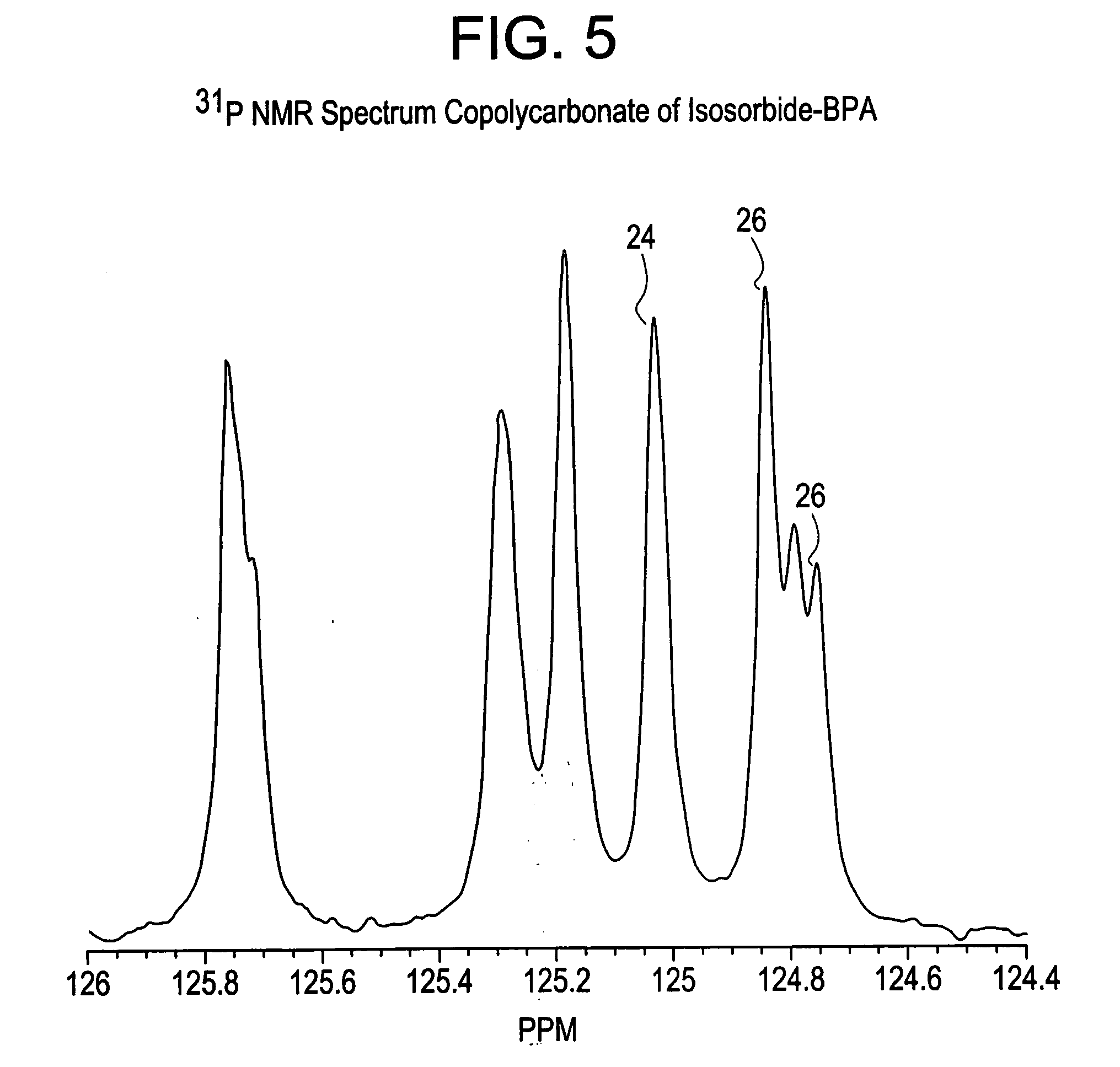
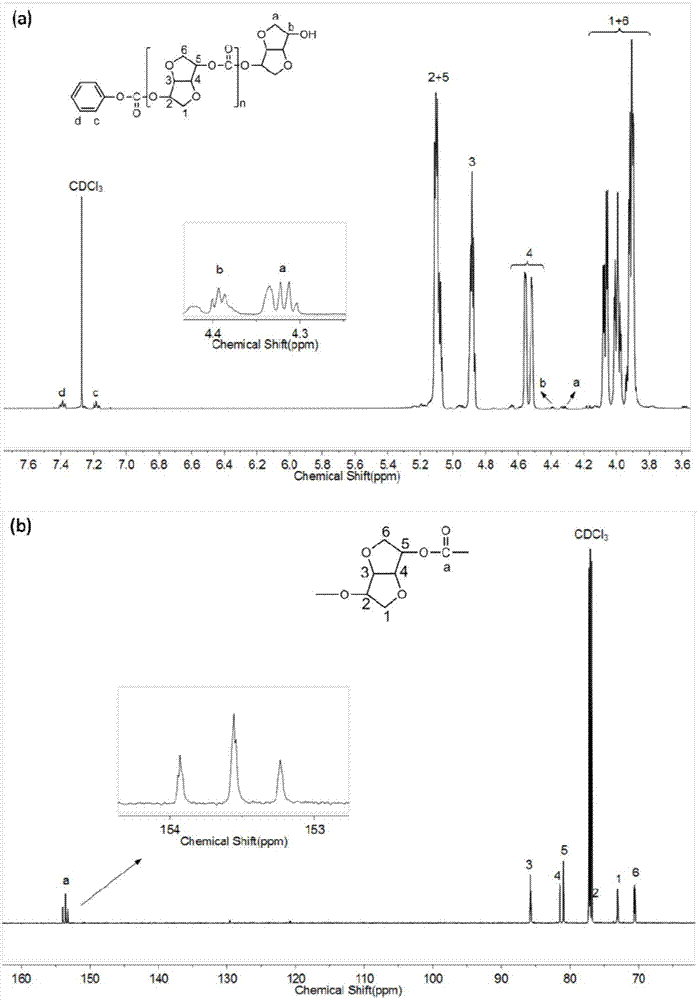
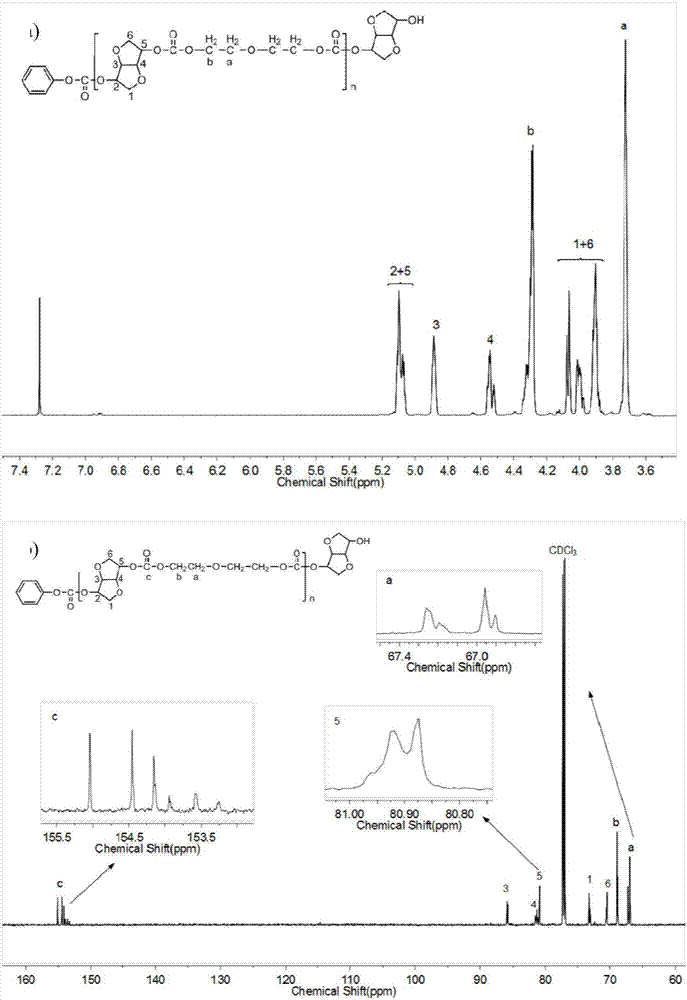
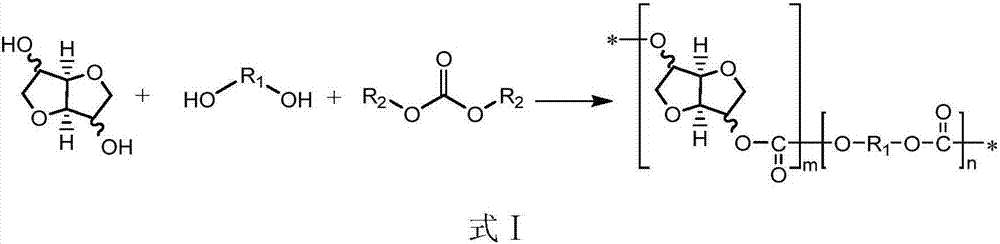
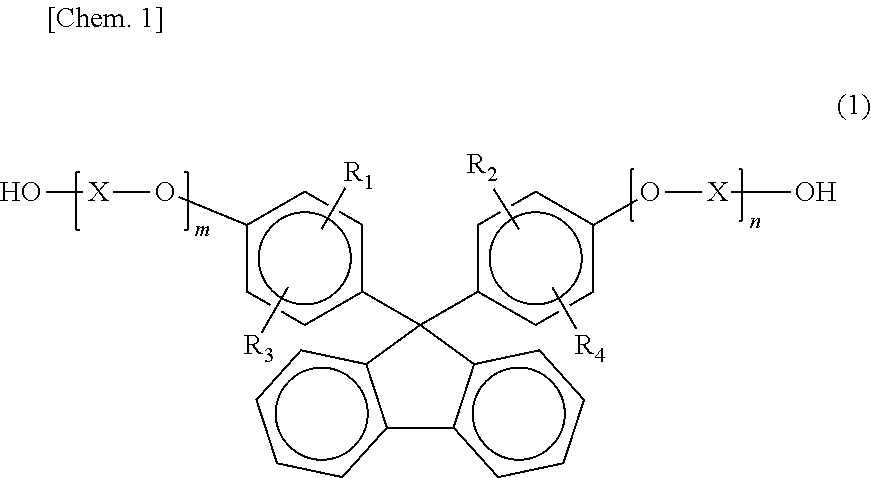
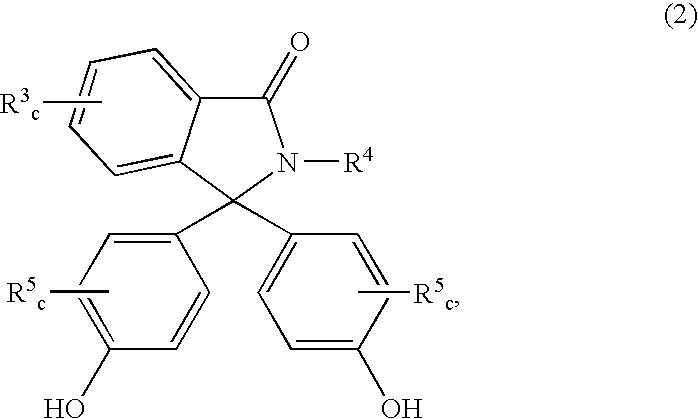
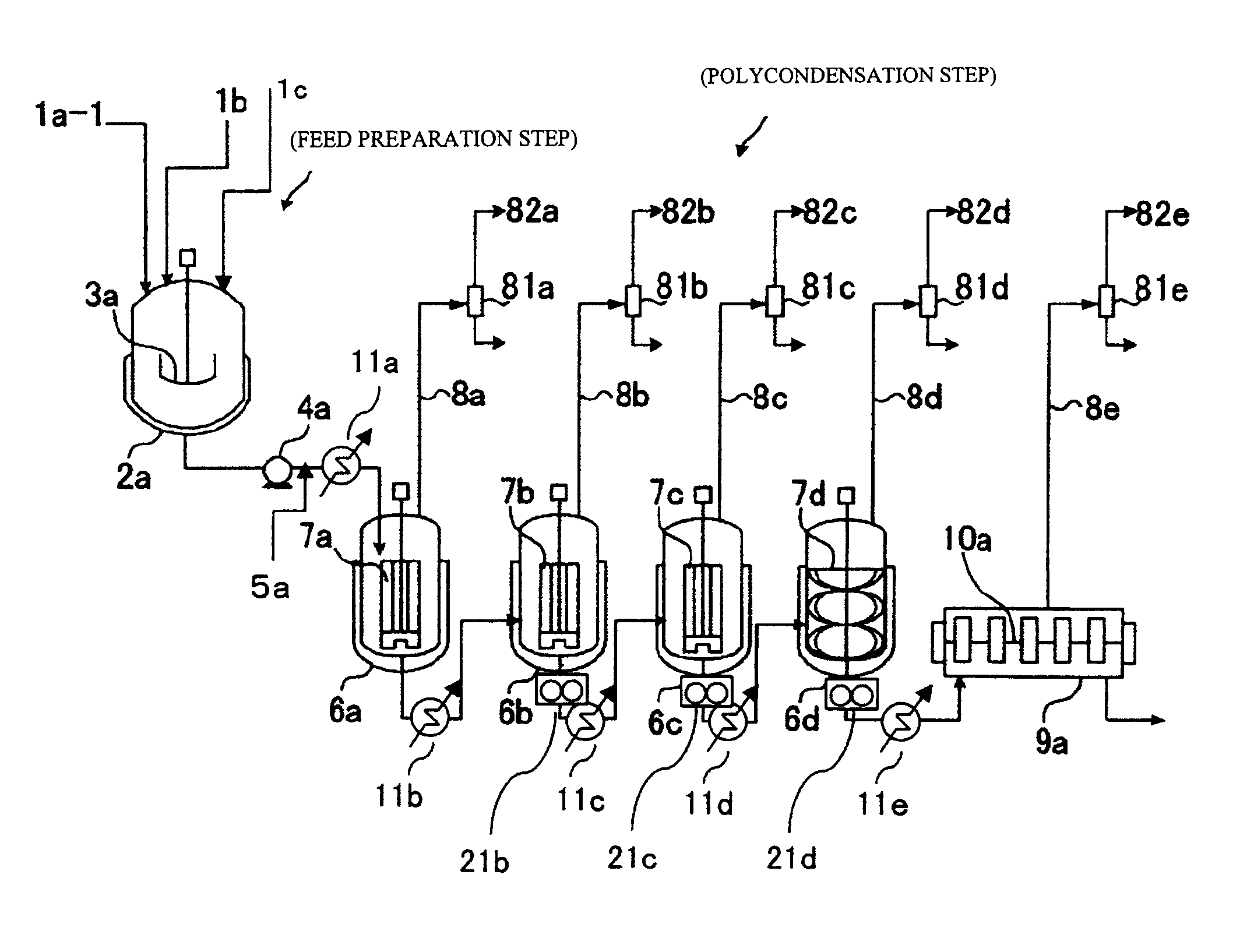
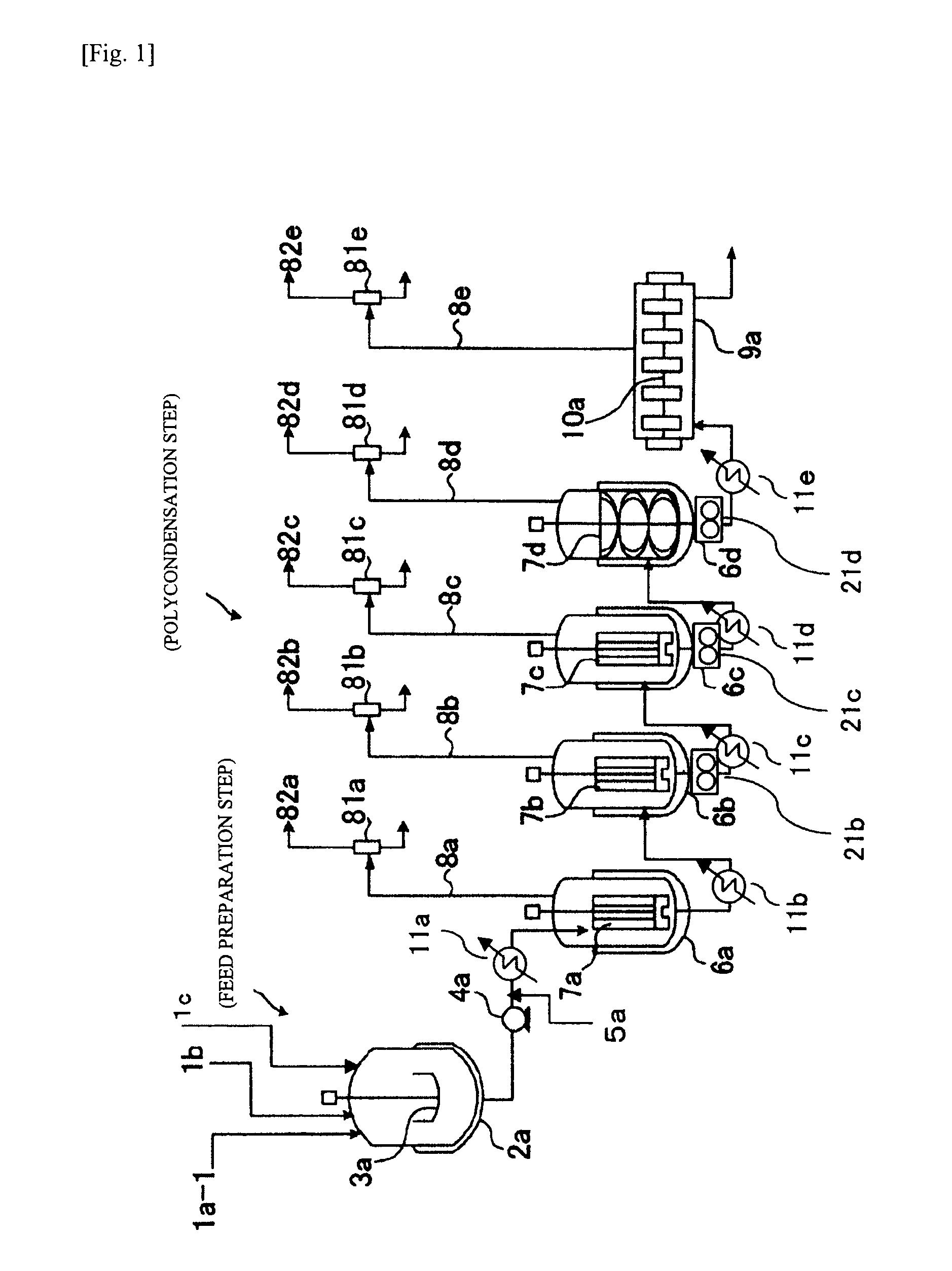
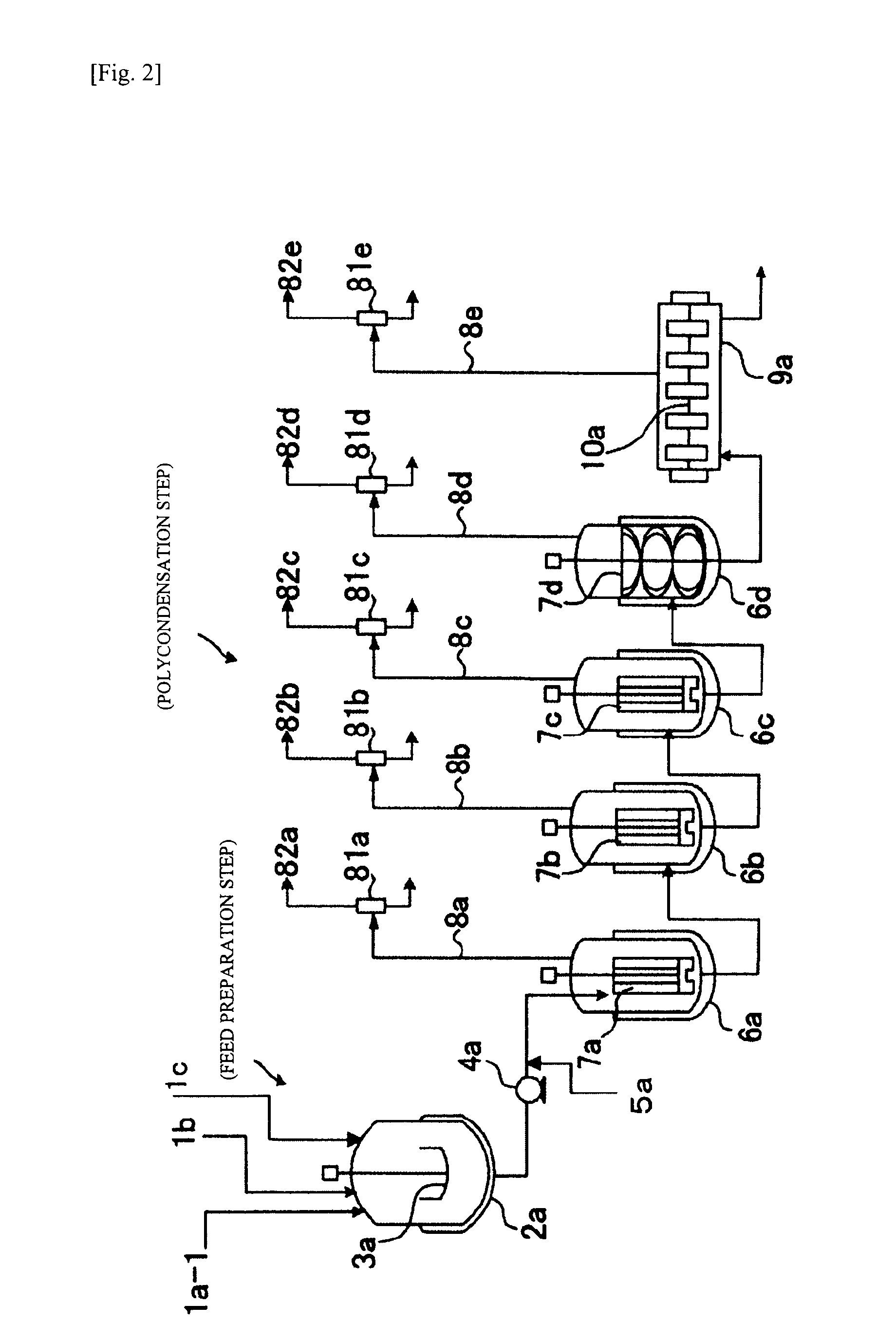
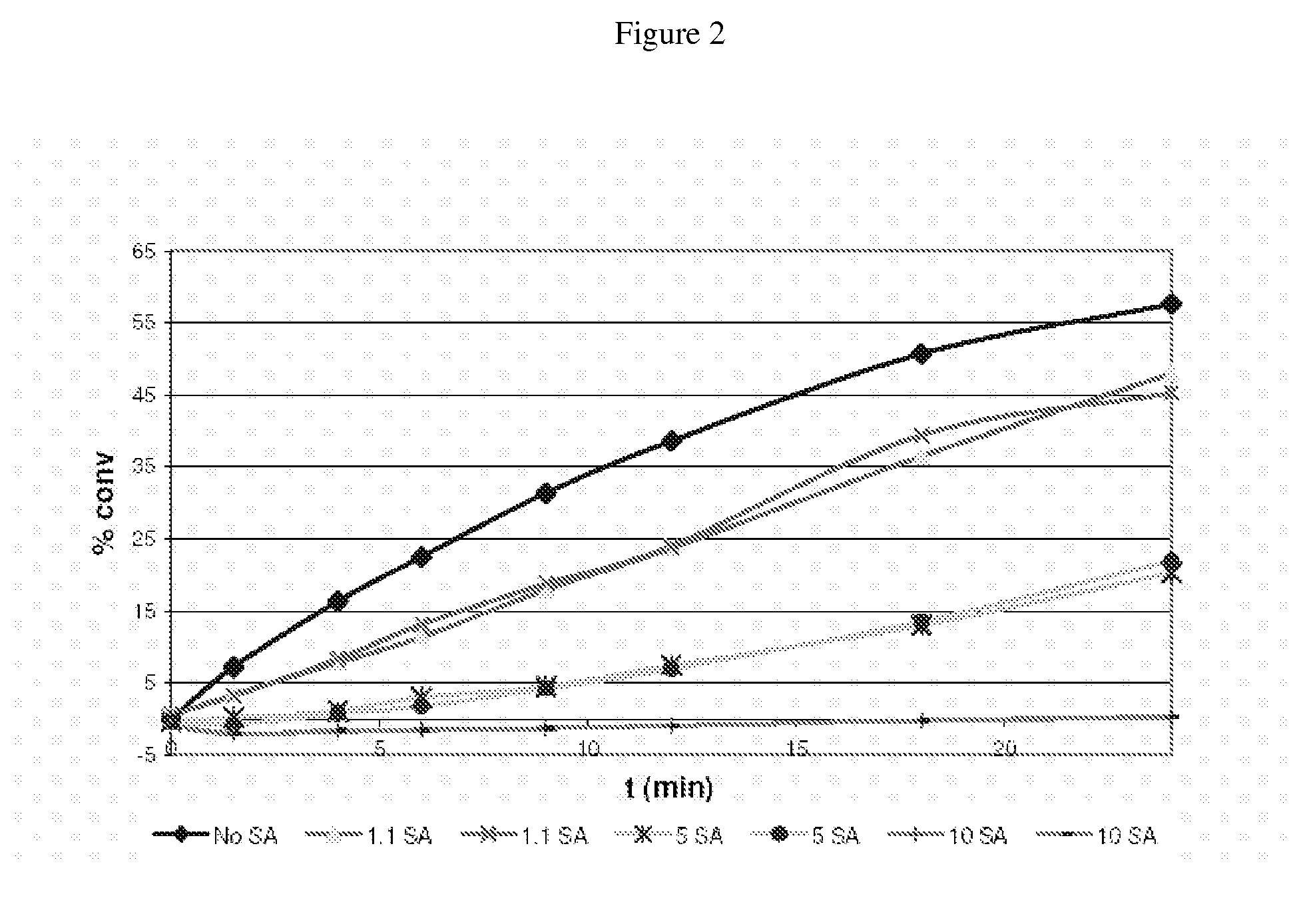
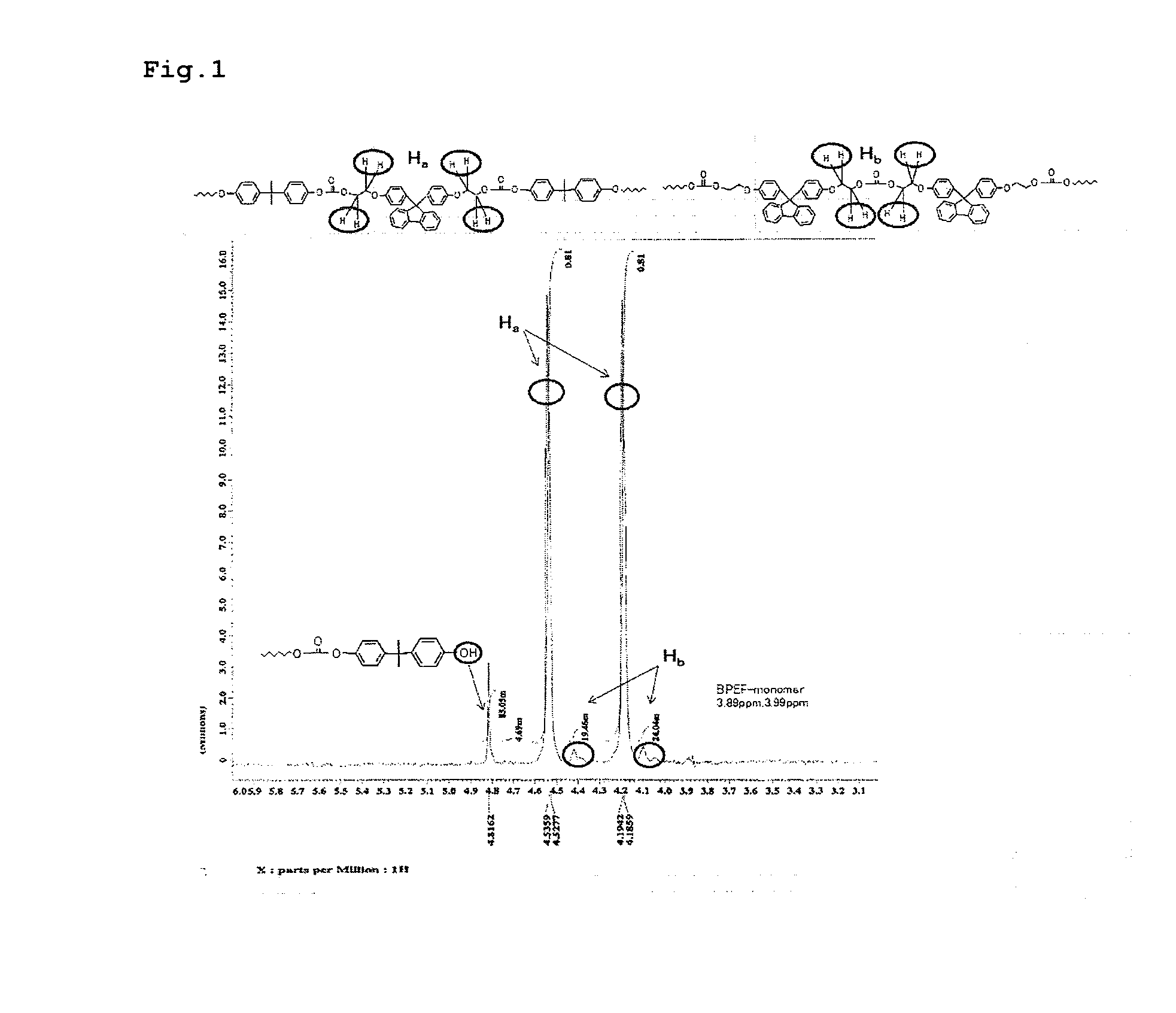
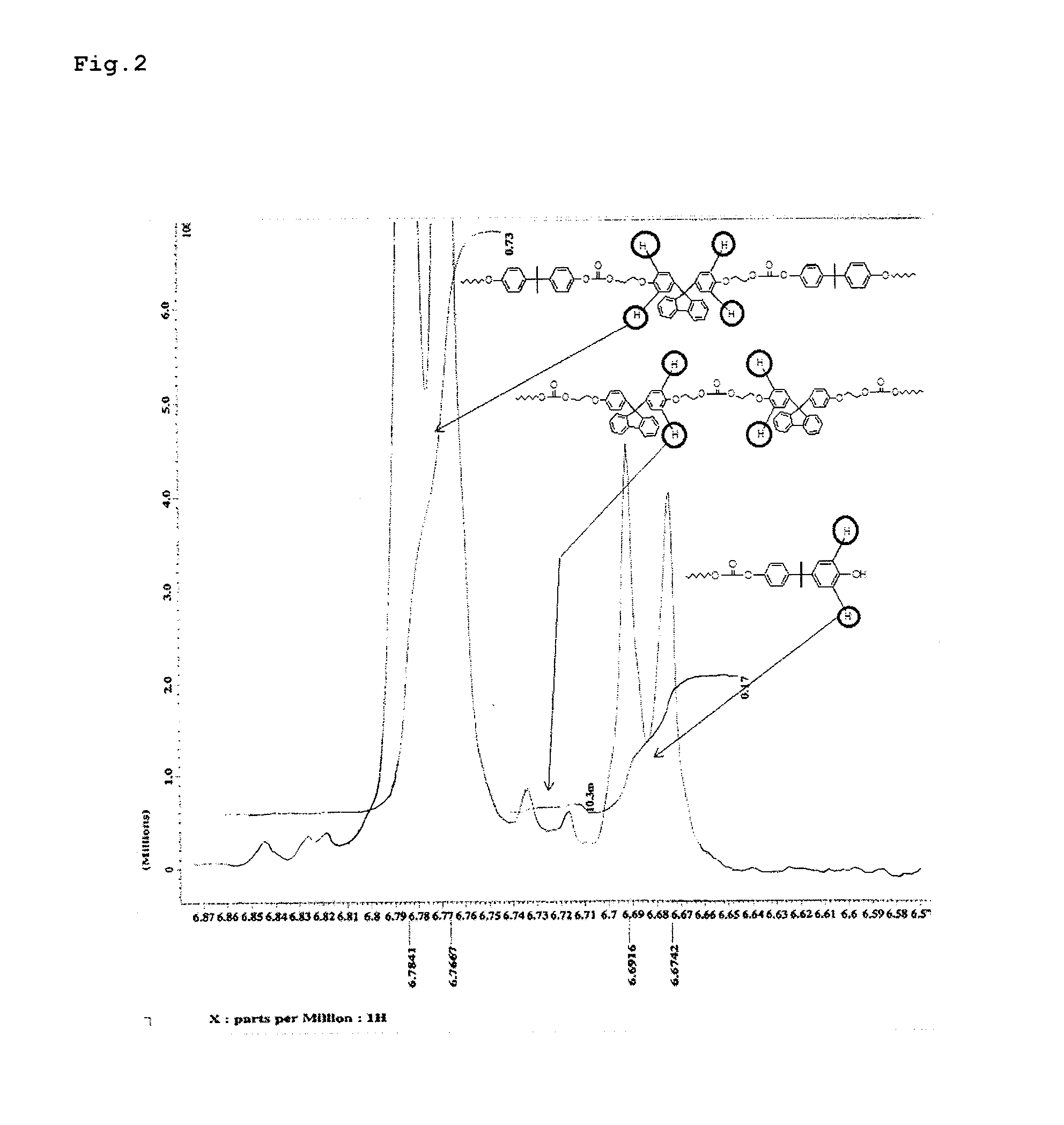
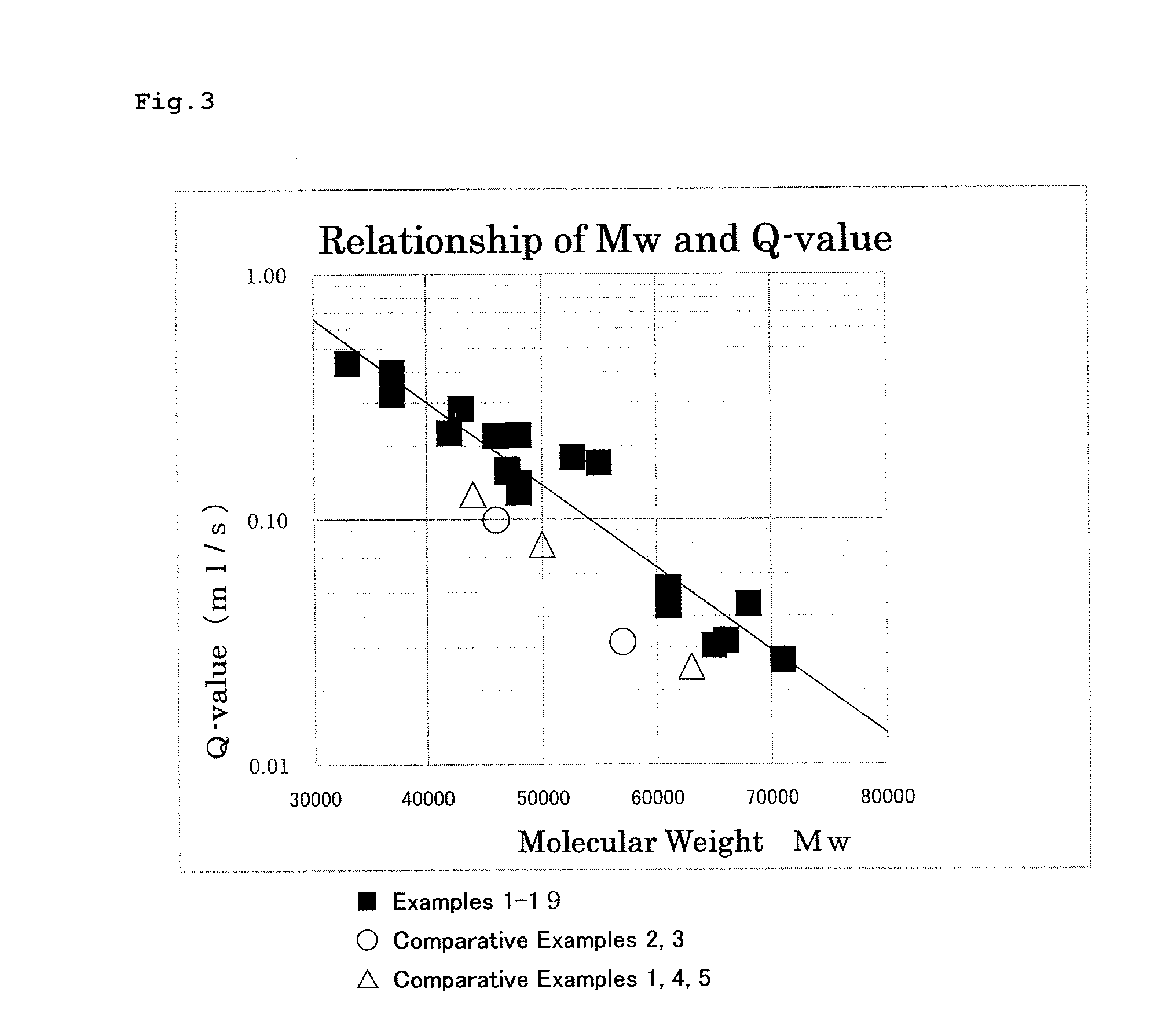
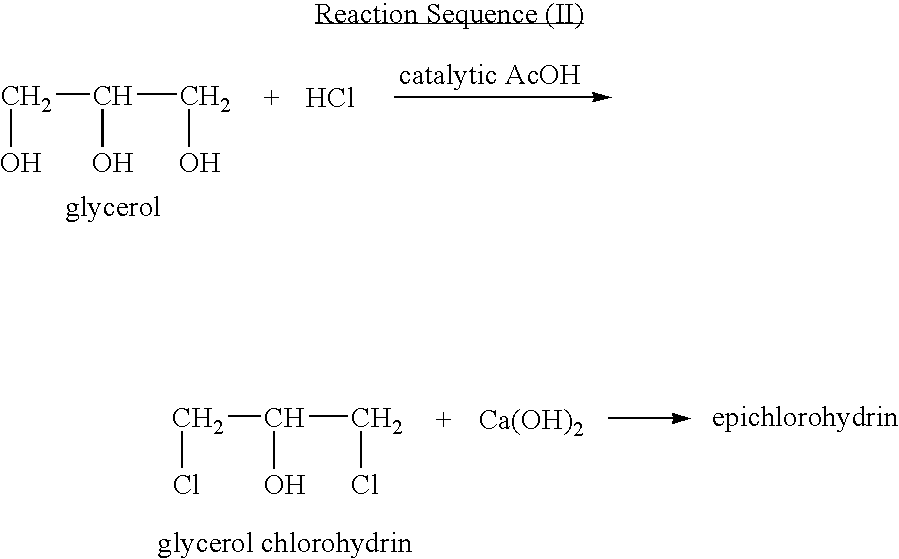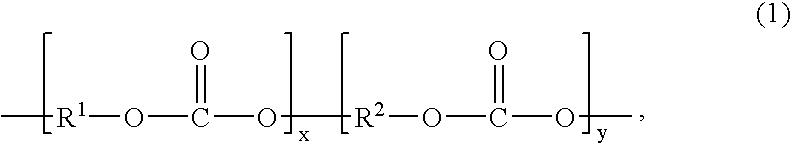Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
363 results about "Dihydroxylation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
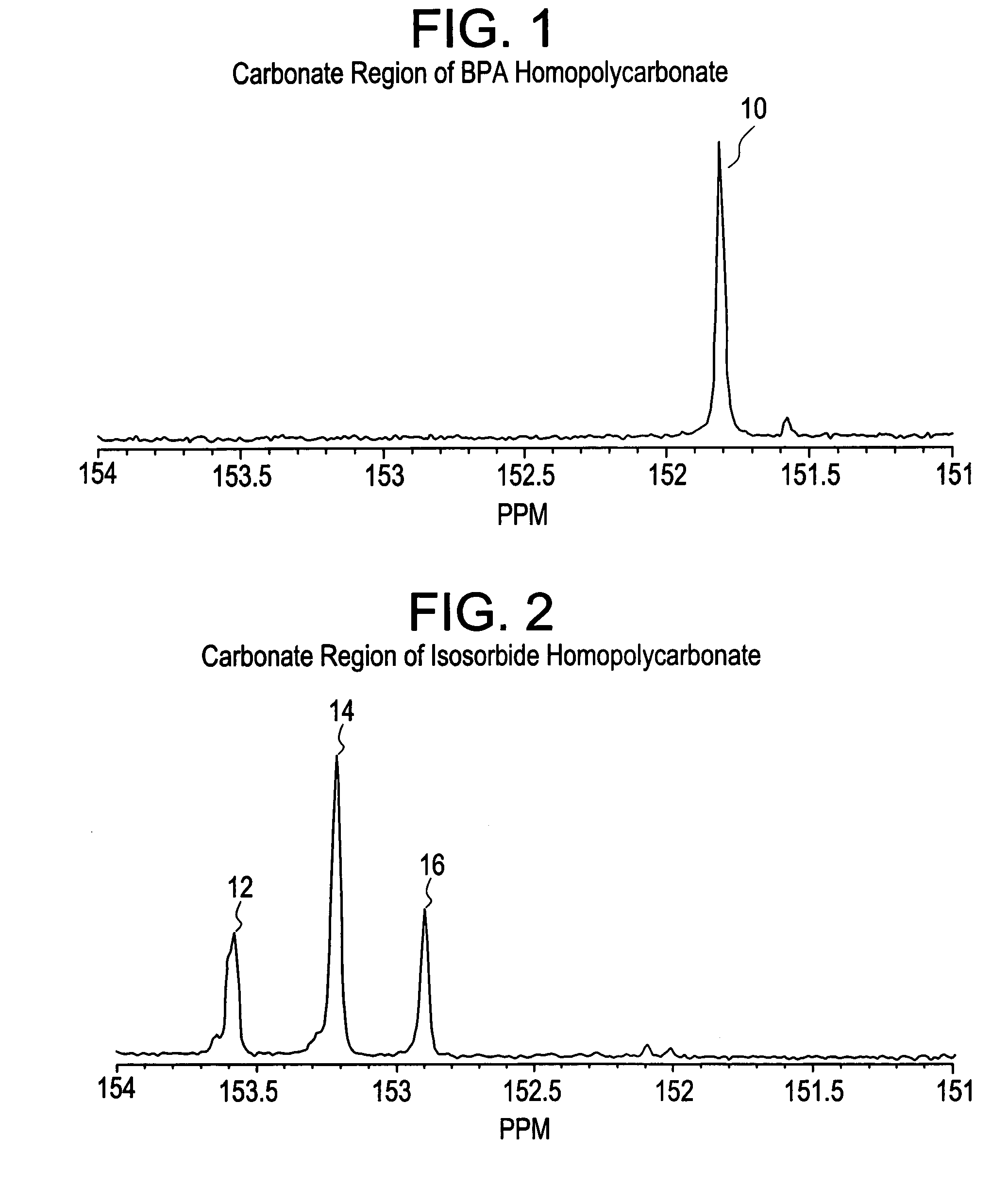
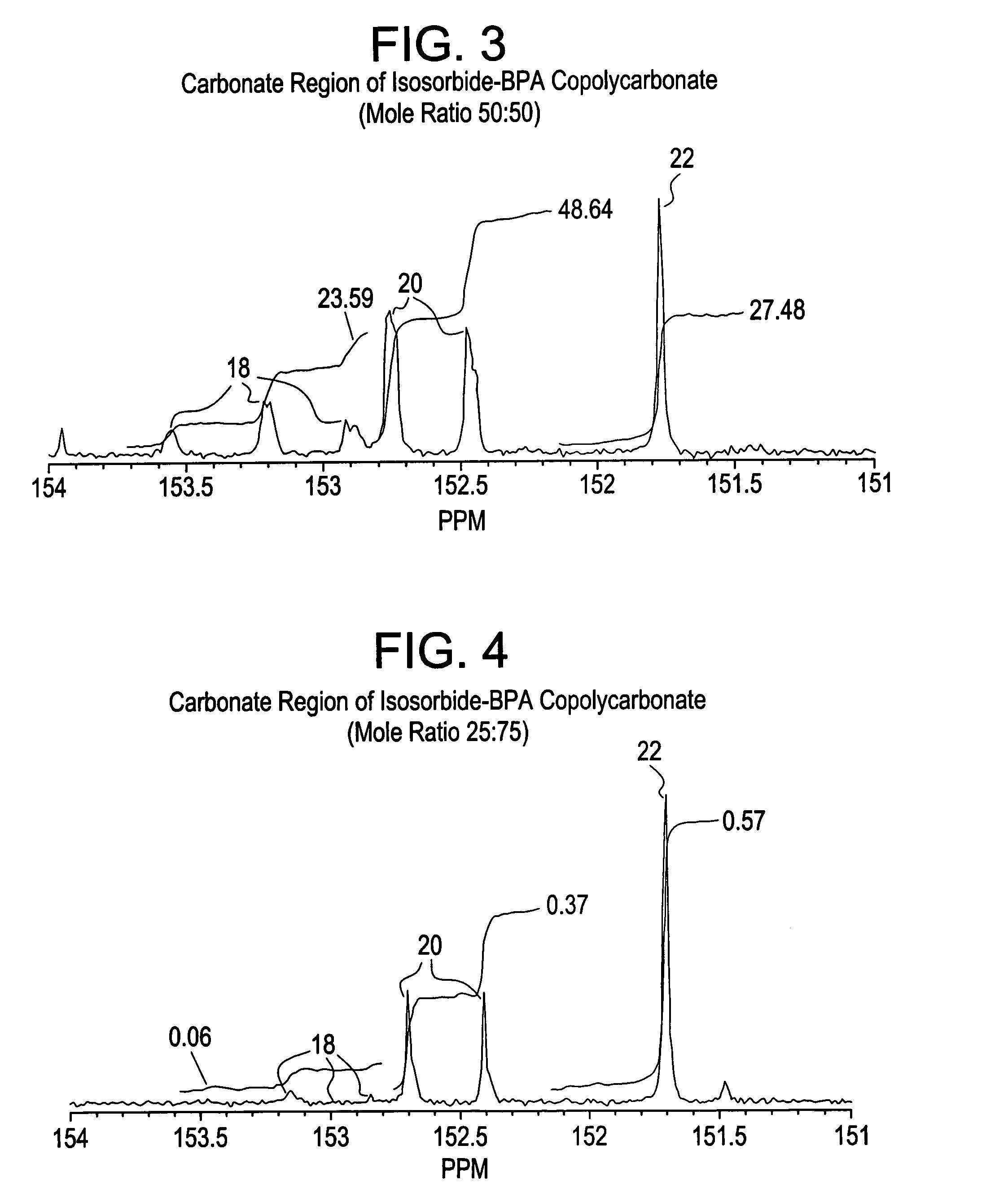
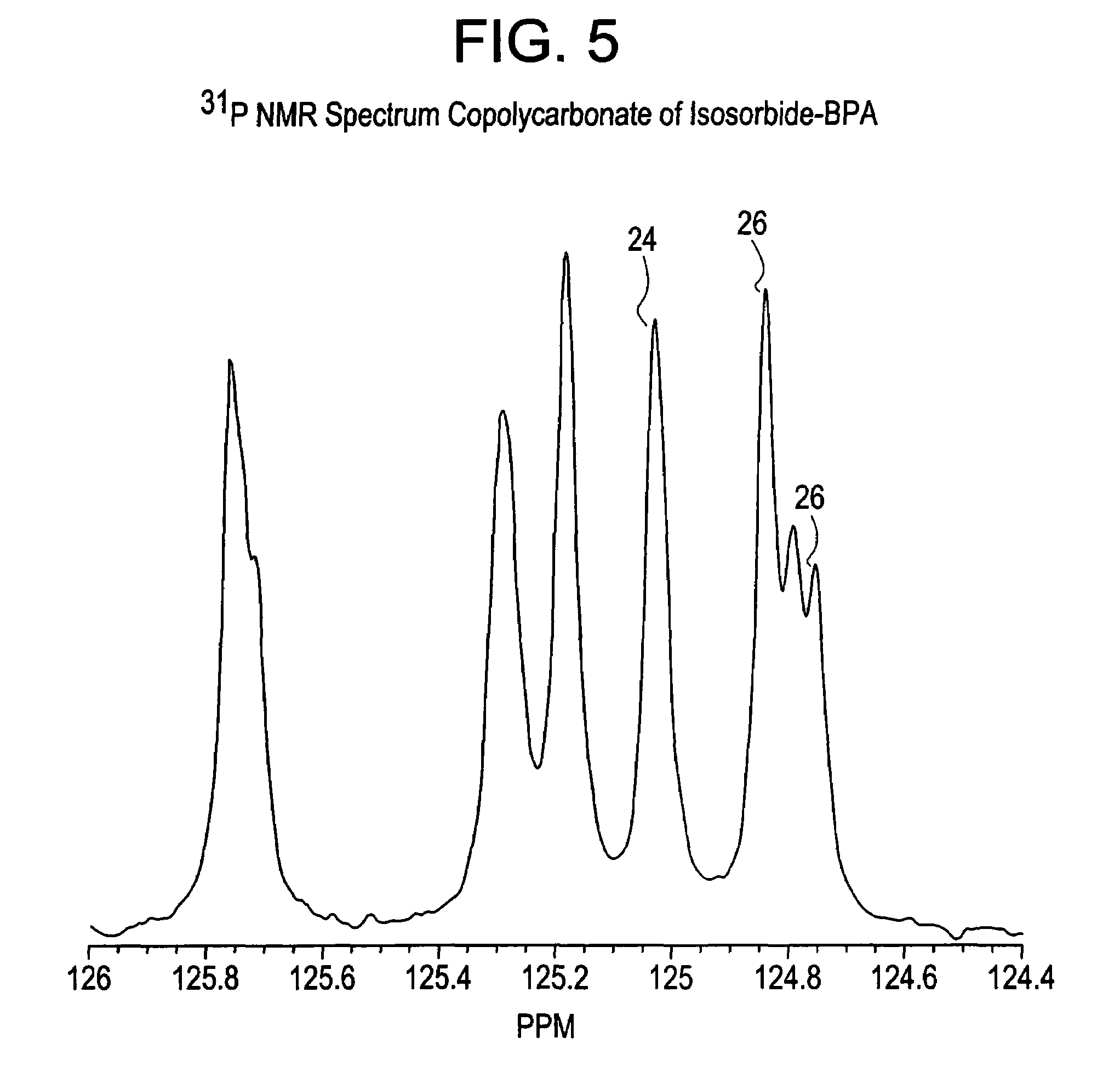
Dihydroxylation is the process by which an alkene is converted into a vicinal diol. Although there are many routes to accomplish this oxidation, the most common and direct processes use a high-oxidation-state transition metal (typically osmium or manganese). The metal is often used as a catalyst, with some other stoichiometric oxidant present. In addition, other transition metals and non-transition metal methods have been developed and used to catalyze the reaction.
Aliphatic diol polycarbonates and their preparation
The disclosure provides high quality, low yellowness index copolycarbonates comprising structural units derived from at least one aliphatic diol, at least an aromatic dihydroxy compound and a diaryl carbonate. Also disclosed herein is a method of making such copolycarbonates in presence of one or more catalysts.
Owner:SHPP GLOBAL TECH BV
Stabilized aromatic polycarbonate
The relative intensity of fluorescent light of a melt-polycondensed aromatic polycarbonate produced by the transesterification process (melt polymerization process) of an aromatic dihydroxy compound and a carbonic acid diester in the presence of a catalyst comprising a basic nitrogen compound and / or a basic phosphorus compound in combination with an alkali metal compound is suppressed to 4.0x10-3 or below at a wavelength of 465 nm based on a standard substance in a fluorescent light spectrum obtained by the excitation wavelength of 320 nm. An aromatic polycarbonate having good color stability, especially good stability to the deterioration of the chip color during storage of the chip in air at room temperature can be produced by the present invention.
Owner:TEIJIN LTD
Aliphatic diol polycarbonates and their preparation
The disclosure provides high quality, low yellowness index copolycarbonates comprising structural units derived from at least one aliphatic diol, at least an aromatic dihydroxy compound and a diaryl carbonate. Also disclosed herein is a method of making such copolycarbonates in presence of one or more catalysts.
Owner:SHPP GLOBAL TECH BV
Polycarbonate copolymer and method of producing the same
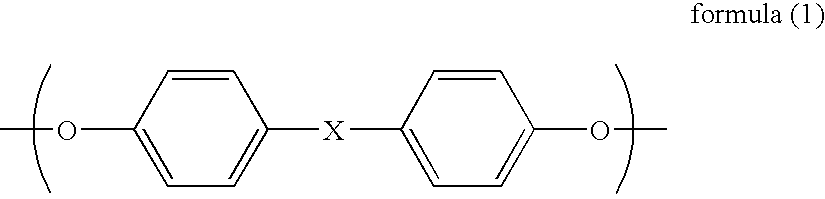
The object is to provide a polycarbonate copolymer having excellent mechanical strength, good heat resistance, a low refractive index, a large Abbe number, a low berefringence and excellent transparency and containing a plant-derived raw material. Disclosed is a polycarbonate copolymer which comprises a constituent unit derived from a dihydroxy compound represented by the general formula (1) and a constituent unit derived from an alicyclic dihydroxy compound and has an Abbe number of 50 or greater and “a 5% heating weight loss temperature” (a temperature at which the 5% weight loss under heating is observed) of 340° C. or higher. Also disclosed is a process for producing the polycarbonate copolymer by reacting a dihydroxy compound represented by the general formula (1) and an alicyclic dihydroxy compound with a carbonate diester in the presence of a polymerization catalyst.
Owner:MITSUBISHI CHEM CORP
Aliphatic diol-based polycarbonates, method of making, and articles formed therefrom
Owner:SABIC INNOVATIVE PLASTICS IPBV
Branched polycarbonate-polysiloxane copolymers and processes for producing the same
Methods for making a branched polycarbonate-polysiloxane copolymer are provided. An interfacial mixture comprising water, an organic solvent, a polyhydric branching agent, a non-siloxane-containing dihydroxy compound, an endcapping agent, a phase transfer catalyst, and a base is formed. The base and the branching agent are dissolved in the mixture before the non-siloxane-containing dihydroxy compound is added and the interfacial mixture has a basic pH. A first carbonate precursor is added to the interfacial mixture while maintaining the pH at from about 3 to about 9 to form a branched polycarbonate mixture. Next, the pH is increased to from about 8 to about 13 and a siloxane oligomer is added to the branched polycarbonate mixture. The branched polycarbonate mixture is then reacted to form the branched polycarbonate-polysiloxane copolymer. The resulting branched copolymer contains 20 ppm or less of residual chloride, is transparent, has improved flow properties, and has good flame retardance at thin wall thicknesses.
Owner:SABIC INNOVATIVE PLASTICS IP BV
Polycarbonates and method of preparing same
InactiveUS20080004417A1Unfavorable propertyReduce in quantityCeramic shaping apparatusTransesterificationEnd-group
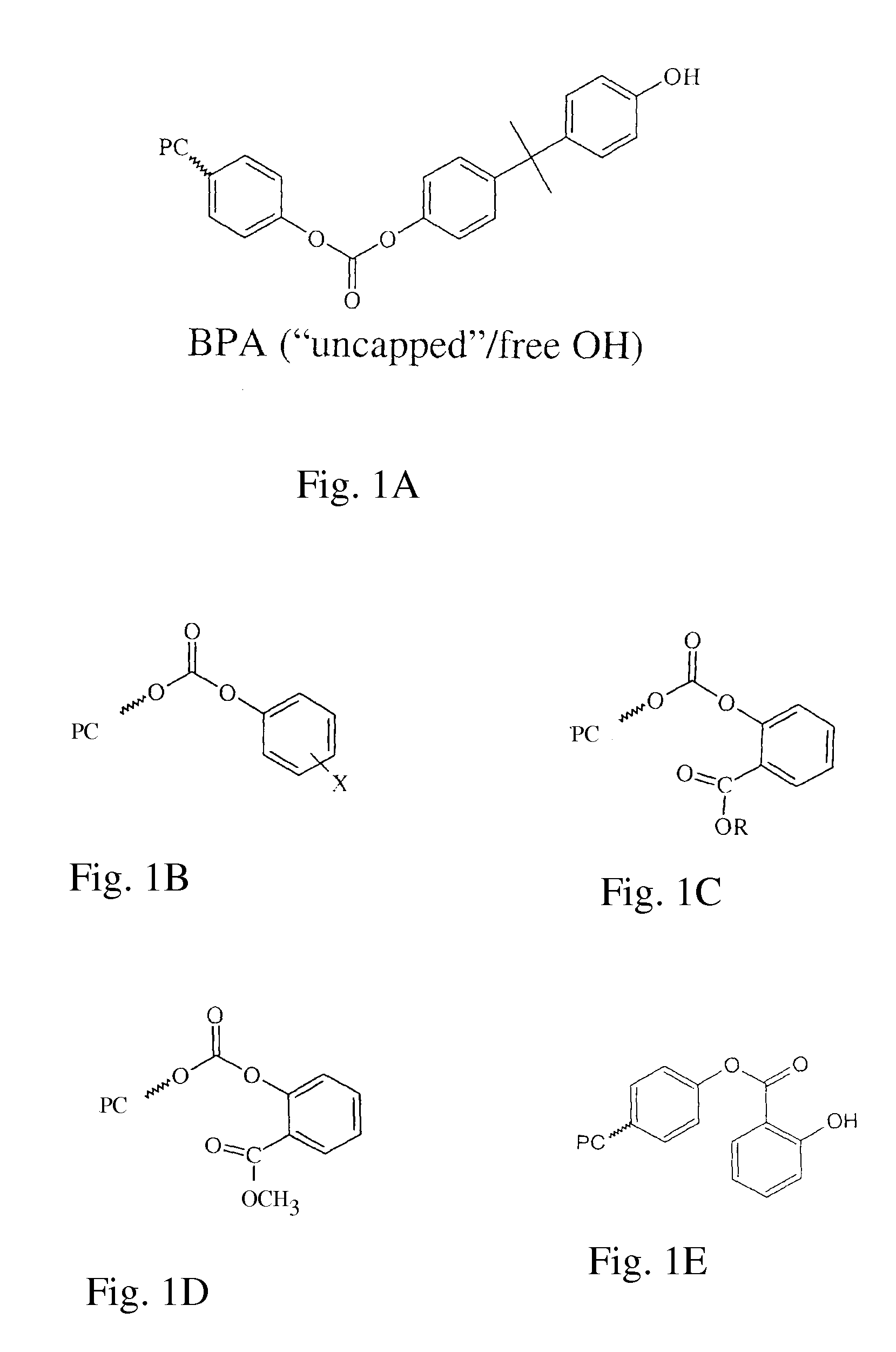
Polycarbonates incorporating terminal carbonate groups derived from ester-substituted activated carbonates, for example terminal methyl salicyl carbonate (TMSC) derived from the use of BMSC as the activated carbonate in a transesterification process, have unfavorable properties with respect to color, hydrolytic stability and thermal stability, particularly when the polycarbonate containing such end groups is molded. The number of activated carbonate end groups formed during the melt transesterification formation of polycarbonate can be reduced, however, without sacrificing the benefits of using an activated diaryl carbonate, and without requiring a separate reaction or additional additives by reacting a dihydroxy compound with an activated diaryl carbonate in the presence of an esterification catalyst to produce a polycarbonate, wherein the molar ratio of activated diaryl carbonate to dihydroxy compound is less than 1 when expressed to at least three decimal places, for example 0.996 or less.
Owner:SABIC GLOBAL TECH BV
Method of Preparing Polycarbonate
ActiveUS20080004418A1Unfavorable propertyReduce in quantityCeramic shaping apparatusPolyesterEnd-group
Polycarbonates incorporating terminal carbonate groups derived from ester-substituted activated carbonates in a transesterification process have unfavorable properties with respect to color, hydrolytic stability and thermal stability, particularly when the polycarbonate containing such end groups is molded. The number of activated carbonate end groups formed during the melt transesterification formation of polycarbonate can be reduced by reacting a dihydroxy compound with an activated diaryl carbonate in the presence of an esterification catalyst to produce a polycarbonate, in the presence of a monohydroxy chainstopper such as para-cumyl phenol in an amount that results in 35 to 65 mol % of the end groups being derived from the monohydroxy chainstopper. Suitably, the reactants are provided such that the molar ratio of activated diaryl carbonate to the total of dihydroxy compound plus ½ the chainstopping reagent that is less than 1.
Owner:SHPP GLOBAL TECH BV
Processes for producing polycarbonate and molded polycarbonate articles
ActiveUS20110003101A1Efficient and stable productionLow refractive indexFlexible coversWrappersHeat resistanceRefractive index
A subject for the invention relates to processes for producing a polycarbonate containing a plant-derived starting material and to molded articles thereof, the polycarbonate having excellent mechanical strength, heat resistance, a low refractive index, a large Abbe number, low birefringence, and excellent transparency. The invention relates to a process for producing a polycarbonate which includes a step in which one or more dihydroxy compounds including a dihydroxy compound having at least one linking group —CH2—O— in the molecule thereof are reacted with a carbonic acid diester in the presence of a polymerization catalyst, wherein the dihydroxy compound having at least one linking group —CH2—O— in the molecule thereof has a formic acid content lower than 20 ppm. The invention further relates to a molded article constituted of a polycarbonate or a composition of the polycarbonate, the polycarbonate being a polycarbonate which contains constituent units derived from a dihydroxy compound having at least one linking group —CH2—O— in the molecule thereof and has an Abbe number of 50 or larger and a 5% weight loss temperature of 340° C. or higher.
Owner:MITSUBISHI CHEM CORP
Aromatic polycarbonate, production method and molded products thereof
InactiveUS6410678B1Reduce in quantityStability is not achievedRecord information storageOptical record carriersHigh humidityShell molding
An aromatic polycarbonate which has high durability and is excellent in color tone, transparency and mechanical strength, especially an aromatic polycarbonate which has high durability and stability when it is used under high temperature and high humidity conditions for a long time, and production method and use thereof. In the aromatic polycarbonate which has a sodium element content of 100 ppb or less and a content of each of Ni, Pb, Cr, Mn and Fe of 70 ppb or less and the method of producing a polycarbonate by polycondensing an aromatic dihydroxy compound and a carbonic acid diester, the above aromatic polycarbonate is produced by controlling 1) the content of sodium element contained in each of the above raw materials to 52 ppb or less, 2) the content of each of Fe, Cr, Mn, Ni and Pb to 40 ppb or less and 3) the amount of a specific catalyst to a specific value based on the content of Fe contained in the raw materials.
Owner:TEIJIN LTD
Production of poly(carbonate-co-ester) copolymers
A poly(carbonate-co-ester) block copolymer is synthesized using synthetic strategies that can be incorporated into conventional melt facilities that are commonly used in the production of polycarbonate polymers. The polycarbonate block of the poly(carbonate-co-ester) copolymer is derived from a polycarbonate reaction mixture comprising an aromatic dihydroxy compound and a carbonic acid diester, such as bisphenol A and diphenyl carbonate, respectively. The second block of the copolymer is derived from a polyester prepolymer, the polyester prepolymer comprising a diol, diacid or diester, and at least one monomer that is selected to advantageously incorporate desired properties into the poly(carbonate-co-ester) copolymer. The polyester prepolymer is introduced to the polycarbonate reaction mixture to form the poly(carbonate-co-ester) copolymer. Properties of the copolymer can be altered by varying numerous conditions of the reaction.
Owner:SABIC INNOVATIVE PLASTICS IP BV
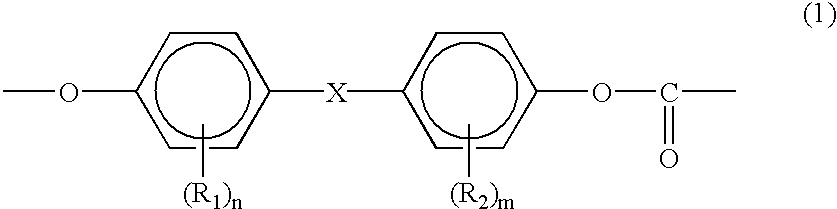
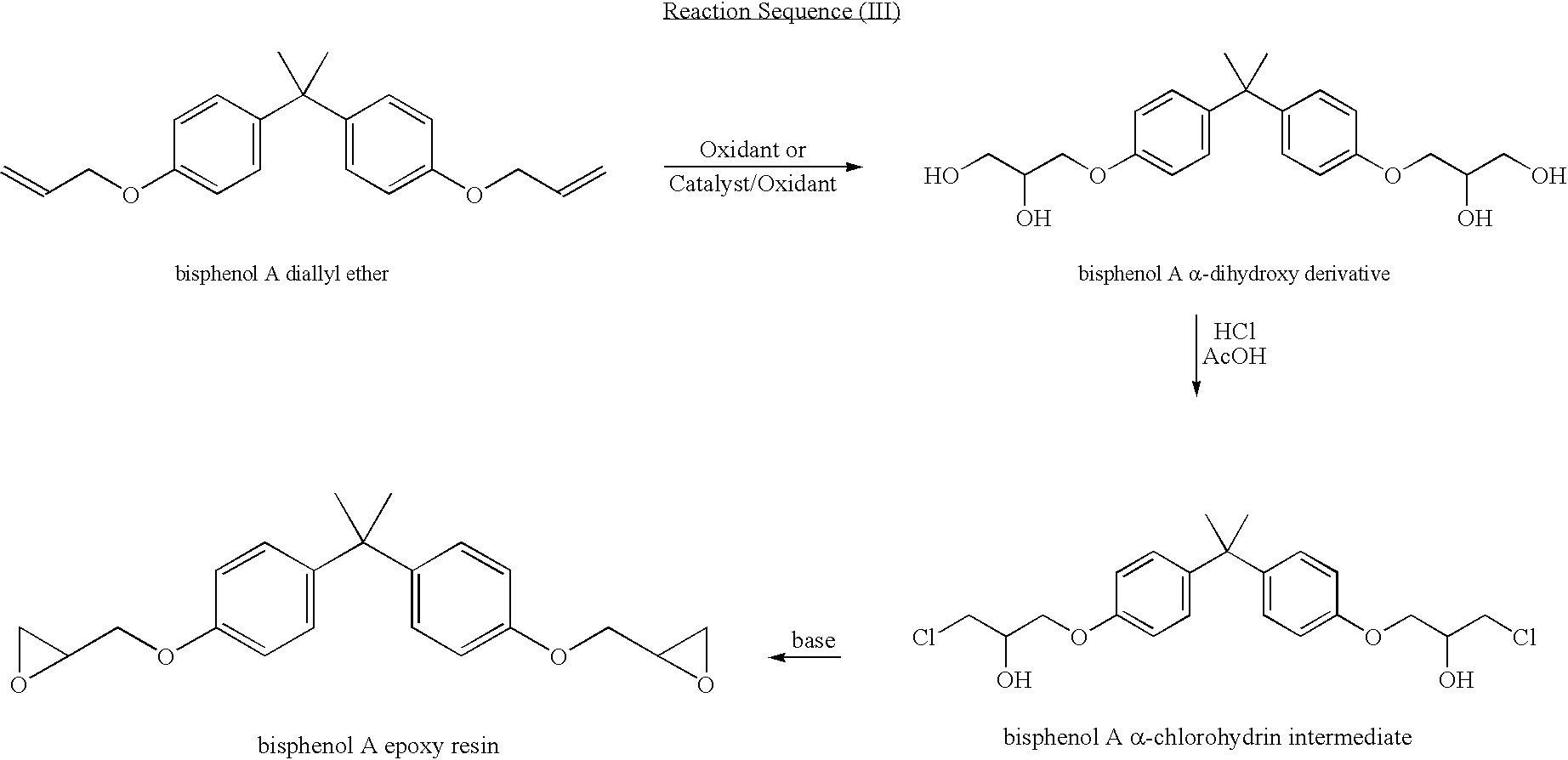
Process for manufacturing an alpha-dihydroxy derivative and epoxy resins prepared therefrom
A process for manufacturing an α-dihydroxy derivative from an aryl allyl ether wherein such α-dihydroxy derivative can be used to prepare an α-halohydrin intermediate and an epoxy resin prepared therefrom including epoxidizing an α-halohydrin intermediate produced from a halide substitution of an α-dihydroxy derivative which has been obtained by a dihydroxylation reaction of an aryl allyl ether in the presence of an oxidant or in the presence of an oxidant and a catalyst.
Owner:DOW GLOBAL TECH LLC
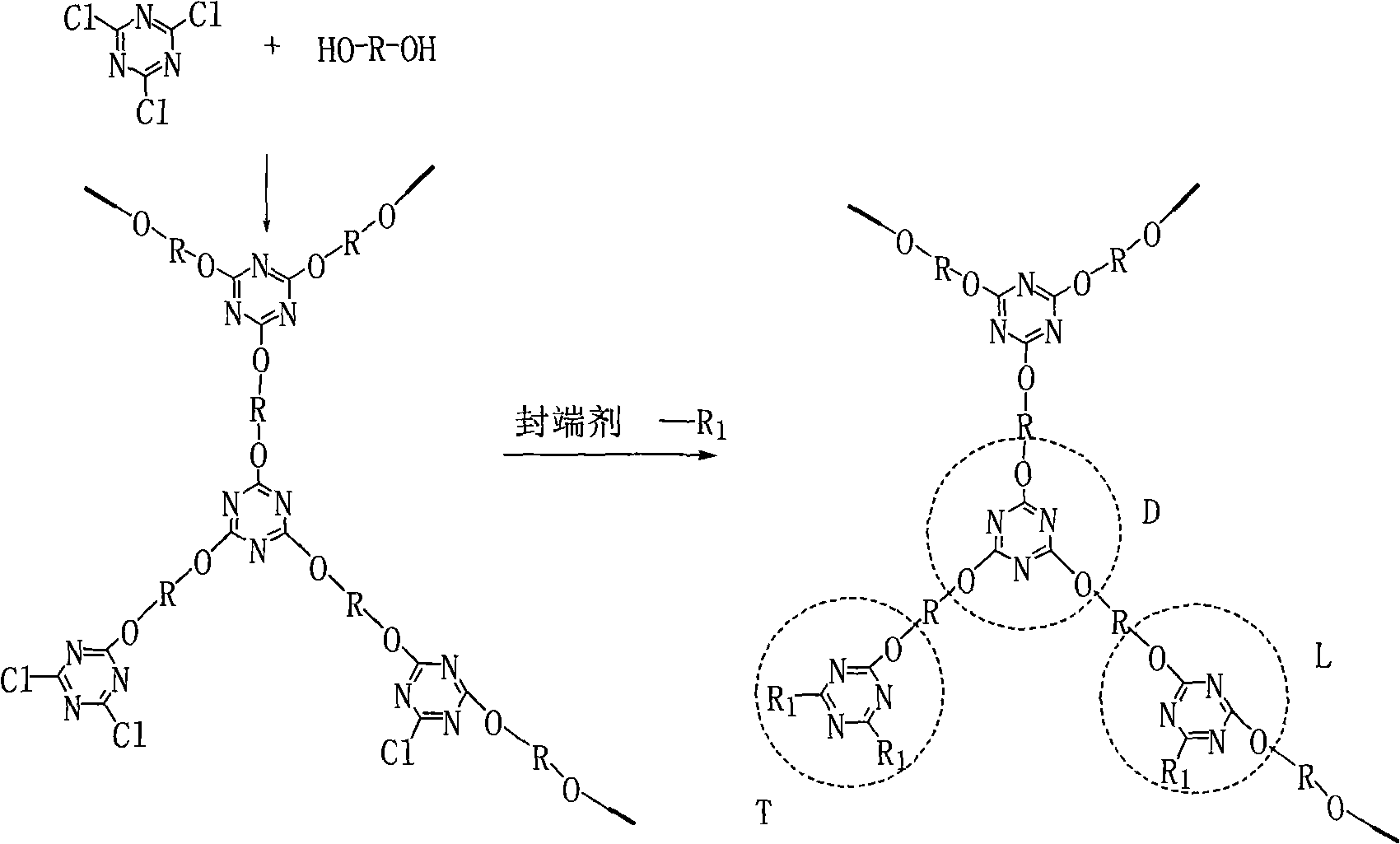
Preparation method for triazine hyperbranched macromolecular carbon forming agent
The invention discloses a preparation method for a triazine hyperbranched macromolecular carbon forming agent. The method is characterized by comprising the following steps: 1), mixing diamino or hydroxyl compound with non-protonic organic solvent, and adding catalyst in the atmosphere of inert gases; 2), dropwise adding cyanuric chloride non-protonic organic solution, and continuing the reaction at the temperature after finishing dropwise adding the cyanuric chloride non-protonic organic solution; 3), adding blocking agent, and carrying out reaction again; and 4), precipitating, filtering, cleaning the organic solvent, separating, and drying to obtain the triazine hyperbranched macromolecular carbon forming agent. Compared with the prior art, the invention has the advantages that the 'one-pot' method is adopted, the reaction process is continuous, the reaction time is short, the product has favorable heat stability, the carbon forming rate is high, and the processability is good.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
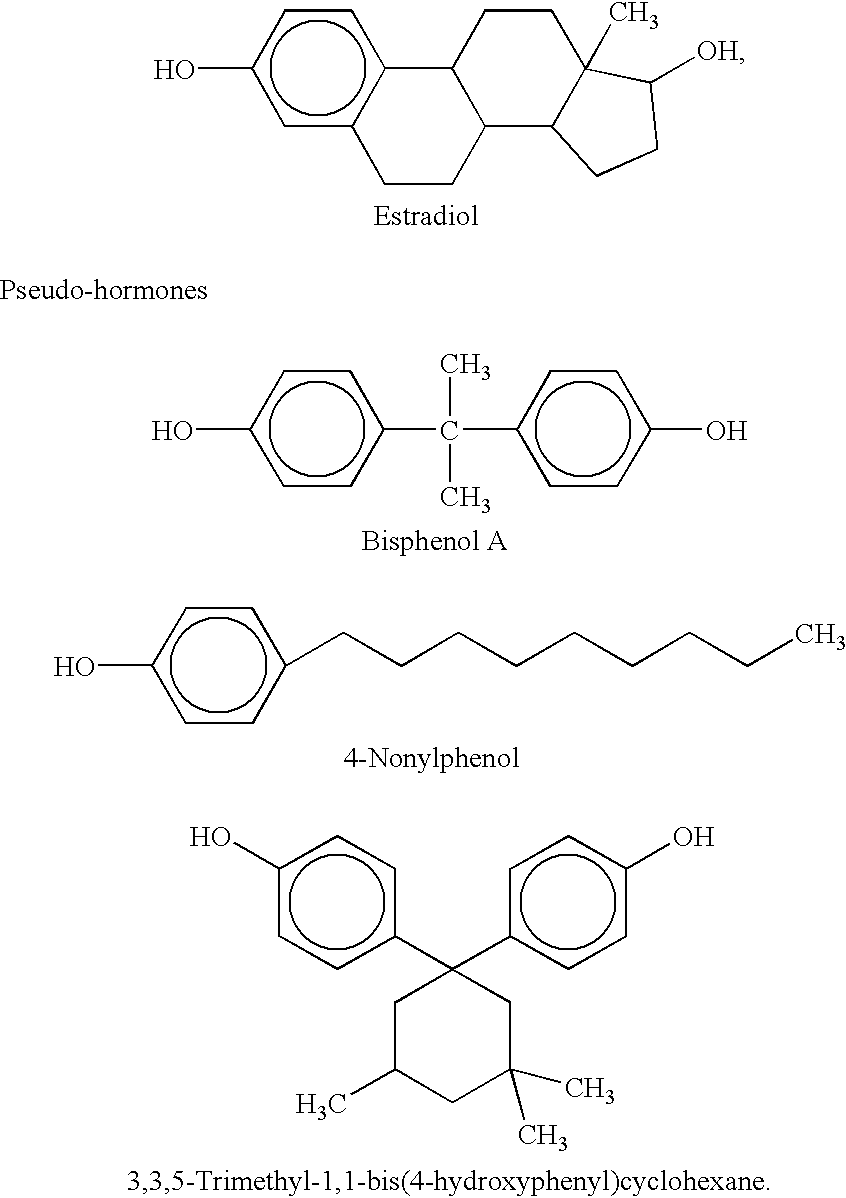
Polycarbonate resin having low tendency of releasing environmental endocrine disruptors
A polycarbonate resin reduced in the release of environmental hormones which comprises structural units represented by general formula (A) and structural units represented by general formula (B), the structural units represented by general formula (A) accounting for 5 to 90 mol % of all the structural units, and which has an intrinsic viscosity of 0.30 to 0.60 dl / g. The polycarbonate resin is produced through the copolymerization of a bispenol whose ability to link to estrogen acceptors is low with an aliphatic dihydroxy compound. The resin hence has the basic properties inherent in polycarbonates, i.e. mechanical strength and heat resistance, and is reduced in the release of environmental hormones.
Owner:MITSUBISHI GAS CHEM CO INC
Polyester-polycarbonate blends
A composition of matter comprising a thermoplastic resin composition derived from (i) about 20 weight percent to about 80 weight percent of a polyester derived from a diol comprising cyclohexane dimethanol and a diacid; (ii) about 5 weight percent to about 80 weight percent of a copolycarbonate derived from about 20 weight percent to about 70 weight percent of a 2-hydrocarbyl-3,3-bis(hydroxyaryl)phthalimidine and from about 30 weight percent to about 80 weight percent of a second aromatic dihydroxy compound; (iii) about 0 weight percent to about 70 weight percent of a thermoplastic resin, C wherein the thermoplastic resin C is selected from the group consisting of a homopolycarbonate, a poly(estercarbonate), a poly(arylatecarbonate) and combinations thereof, and wherein the resin composition is transparent is disclosed. Also disclosed is a process to prepare this composition and articles therefrom.
Owner:SABIC INNOVATIVE PLASTICS IP BV
Polycarbonate diol and polyurethane using same
ActiveCN104884499AChemical resistantHas low temperature propertiesPolyureas/polyurethane adhesivesFibre treatmentDiolHydroxyl value
The present invention relates to: a polycarbonate diol which contains a structural unit derived from a compound represented by formula (A) and a structural unit derived from a compound represented by formula (B), and which has a hydroxyl number of 20-450 mg-KOH / g; and a polycarbonate diol which has a hydroxyl number of 20-45 mg-KOH / g and a glass transition temperature of -30°C or less as measured by a differential scanning calorimeter, and which provides, by hydrolysis, a dihydroxy compound that has an average number of carbon atoms of 3-5.5.
Owner:MITSUBISHI RAYON CO LTD
Aromatic polycarbonate, process for producing the same, polycarbonate composition, and hollow container obtained from the same
InactiveUS7084233B2Satisfactory color toneRetains intact melt characteristicEnvelopes/bags making machinerySynthetic resin layered productsTransesterificationPolystyrene
A subject for the invention is to provide a branched aromatic polycarbonate which is excellent in hue and in melt characteristics such as melt strength.The invention provides a branched aromatic polycarbonate having a viscosity-average molecular weight of 16,000 or higher obtained by the transesterification method, characterized in that the ratio of the weight-average molecular weight (Mw) to number-average molecular weight (Mn) as measured by gel permeation chromatography and calculated for standard polystyrene (Mw / Mn) is in the range of from 2.8 to 4.5 and that the proportion of the number of moles of all structural units yielded by a rearrangement reaction in the course of melt polymerization reaction to 1 mol of structural units having the framework of an aromatic dihydroxy compound used as a starting material is higher than 0.3 mol % and not higher than 0.95 mol.
Owner:MITSUBISHI ENG PLASTICS CORP +1
Biaxially stretched polylactic acid multilayer film and the use thereof
Disclosed is a biaxially stretched polylactic acid multilayer film and the use thereof as packaging film. The gist of the biaxially stretched polylactic acid multilayer film resides in the construction wherein a substrate layer (1) of the biaxially stretched film comprised of polylactic acid (A) has been laminated on at least one surface thereof with a coating layer (II) of an aliphatic polyester composition (D) comprised of 97˜5% by weight of an aliphatic polyester copolymer (B) having a melting point (Tm) of 80˜120° C., a crystallizing temperature (Tc) of 35˜75° C. and a difference of (Tm)−(Tc) within the range of 35˜55° C. and comprising an aliphatic or alicyclic dicarboxylic acid component (b1), an aliphatic or alicyclic dihydroxyl compound component (b2) and a difunctional aliphatic hydroxycarboxylic acid component (b3) and 3˜95% by weight of a polylactic acid copolymer (C) containing 7˜30% by weight of D-lactic acid, a total of the ingredients (B) and (C) being 100% by weight.
Owner:TOHCELLO CO LTD (JP)
Method of forming polycarbonate
InactiveUS7671165B2Easy transferCeramic shaping apparatusChemical/physical/physico-chemical stationary reactorsPHENOL LIQUIDTransesterification
A method of forming polycarbonate includes the steps of introducing a plurality of reaction components to a reactor operating under melt polymerization conditions and removing ester-substituted phenol from the reactor. The plurality of reaction components include a dihydroxy compound, an ester-substituted diaryl carbonate, and a melt transesterification catalyst. The reaction components are introduced in a plurality of reaction component streams. A first reaction component streams includes a melt transesterification catalyst dissolved or suspended in a liquid carrier containing an ester-substituted phenol. The composition of the first reaction component stream is selected such that ester-substituted phenol is not generated as a reaction product in the first reaction component stream.
Owner:SHPP GLOBAL TECH BV
Method for preparing polycarbonate based on high-efficiency catalysis by ionic liquid
The invention discloses a method by using imidazole type ionic liquid to catalyze an ester exchange of carbonic ester and dihydroxyl compound melting so as to synthesize polycarbonate at high efficiency. The method comprises the following steps of under an inert gas atmosphere, mixing the carbonic ester and the dihydroxyl compound, adding a catalyst, and performing the ester exchange and condensation, so as to obtain the product. The molecular weight of the obtained polymer is 1.0*10<4> to 2.0*10<5>g / mol, and the glass transition temperature is 50 to 200 DEG C. Compared with the ester exchangecatalysts such as the traditional alkaline metal salt, alkaline metal salt or quaternary ammonium and quaternary phosphor salt,, the polycarbonate with higher molecular weight can be catalyzed and synthesized by the imidazole type ionic liquid.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Preparation of new layered double hydroxides exchanged with osmate for asymmetric dihydroxylation of olefins to vicinal diols
InactiveUS6387033B1Facilitates oxygen transferHigh activityPreparation by oxidation reactionsCarboxylic acid esters preparationDiolAlkene
LDH-osmate of the formula [MII(1-x)MIIIx(OH)2][OsO42-]x / 2.zH2O wherein MII is a divalent cation selected from the group consisting of Mg2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+ and Ca2+ and MIII is a trivalent ion selected from the group consisting of Al3+, Cr3+, Mn3+, Fe3+ and Co3+, and x is the mole fraction having integral value ranging from 0.2 to 0.33, and z is the number of water molecules and ranges from 1 to 4, useful as, a catalyst, and a process for the preparation thereof and use thereof to manufacture vicinal diols.
Owner:COUNCIL OF SCI & IND RES
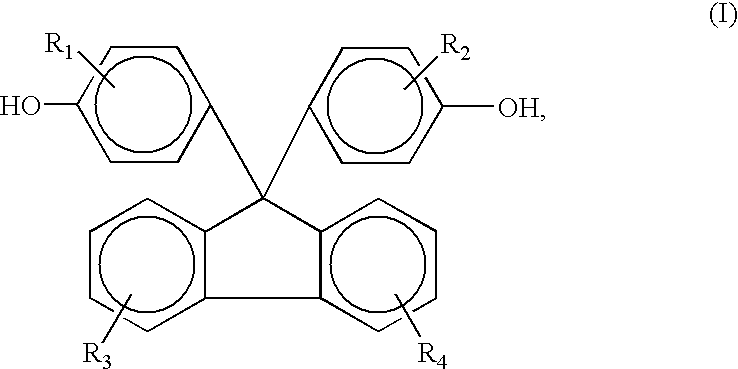
Polycarbonate resin and transparent film formed therefrom
ActiveUS20120308796A1Low photoelastic coefficientModerate glass transition temperatureSynthetic resin layered productsPolarising elementsStructural unitPhotochemistry
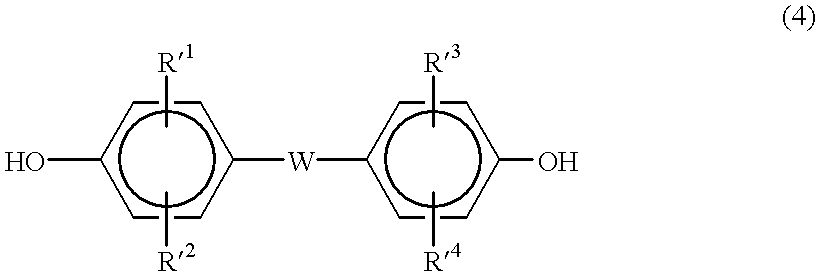
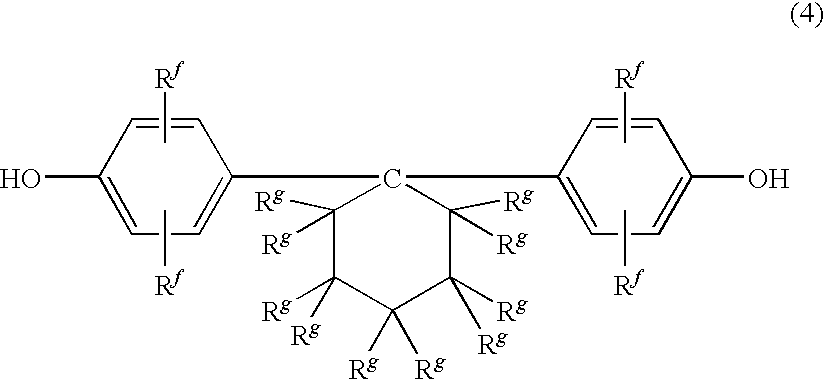
The polycarbonate resin of the invention includes a first structural unit derived from a dihydroxy compound represented by a general formula (1), a second structural unit derived from a dihydroxy compound represented by a general formula (2), and a third structural unit derived from at least one dihydroxy compound selected from the group consisting of a dihydroxy compound represented by a general formula (3), a dihydroxy compound represented by a general formula (4), a dihydroxy compound represented by a general formula (5), and a dihydroxy compound represented by a general formula (6), and in which the first structural unit derived from a dihydroxy compound represented by the general formula (1) accounts for 18% by mole or more of the polycarbonate resin. The above general formulae (1) to (6) are described in the specification of the present application.
Owner:MITSUBISHI CHEM CORP +1
Method for preparing polycarbonate through basic ionic liquid catalysis
The invention relates to a method for preparing polycarbonate through basic ionic liquid catalysis. The method is characterized in that quaternary ammonium and quaternary phosphonium basic ionic liquid serves as a catalyst, the dosage of the catalyst is 5*10<-3>%-5% that of a dihydroxy compound, the dihydroxy compound and dialkyl carbonate serve as the raw materials, the feeding molar ratio of the dihydroxy compound to dialkyl carbonate is 1:0.8 to 1:10, and polycarbonate is synthesized through melting ester exchange. The synthetic processes of polycarbonate comprise the two stages of ester exchange and polycondensation, wherein for the ester exchange stage, on the condition that the reaction temperature ranges from 98 DEG C to 150 DEG C, the pressure is atmospheric pressure, and the reaction time ranges from 3 h to 6 h, prepolymer is obtained, and for the polycondensation stage, polycarbonate is obtained by synthesizing the prepolymer on the condition that the temperature ranges from 210 DEG C to 260 DEG C, the vacuum degree ranges from 4.0*10<-3> MPa to 1.0*10<-5> MPa, and the reaction time ranges from 1 h to 7 h. The synthetic method has the following advantages that the catalyst is simple in component and high in activity, a by-product phenol can be recycled, and the cost is reduced; toxic phosgene is cleared off, and the method is environmentally friendly; zero emission is almost achieved, and the method completely conforms to the concept of cleaning production.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Method of making polycarbonate nanocomposites
A method of preparing a polycarbonate nanocomposite comprising forming a reactant mixture comprising a nanomaterial, a solvent, a dihydroxy compound and an activated carbonate; and polymerizing the dihydroxy compound and the activated carbonate in the presence of the solvent to form the polycarbonate nanocomposite is disclosed. Also disclosed are polycarbonate nanocomposites prepared in accordance with this method, and thermoplastic compositions comprising the polycarbonate nanocomposites.
Owner:SABIC INNOVATIVE PLASTICS IP BV
High heat polycarbonate compositions, methods for the preparation thereof, and articles derived therefrom
Melt blended compositions, comprising up to 20 wt % of an optional additive, and 80-100 wt % of a copolycarbonate having a Tg of 200° C. or more of (1)wherein the mole ratio of x:y is 35:65 to 90:10, R1 is derived from a dihydroxy compound (2)wherein R3 and R5 are each independently a halogen or a C1-6 alkyl group, R4 is a C1-6 alkyl, phenyl, or phenyl substituted with up to five halogens or C1-6 alkyl groups, and each c is independently 0 to 4; R2 is derived from a dihydroxy compound (6):wherein Ra and Rb are each independently a halogen atom or a monovalent C1-6 alkyl group; p and q are each independently integers of 0 to 4; and Xa is a divalent group; and 95 to 5 wt % of a polycarbonate having a Tg of less than 200° C. of formula (9)wherein R9 derived from a dihydroxy compound of formula (6); and 0.001 to 0.1 wt % of a transesterification catalyst.
Owner:SHPP GLOBAL TECH BV
Bisphenol-A free polyether resins based on phenol stearic acid and coating compositions formed therefrom
Coating compositions can be prepared from a polyether resin, wherein the smallest difunctional hydroxyl phenyl segment used to form the polyether resin has a molecular weight greater than about 500, and wherein the smallest difunctional hydroxyl phenyl segment used to form the polyether resin does not comprise two or more non-impaired hydroxyl groups attached to two or more different five-membered or six-membered carbon atom rings in a segment having a molecular weight less than about 500. The polyether resin can be prepared by reacting a dihydroxyl compound and / or a diamine compound with a phenol stearic acid compound to produce a diphenol, and reacting the diphenol with a diglycidyl ether compound to form the polyether resin.
Owner:SI GROUP INC +1
Process for producing polycarbonate
ActiveUS20110034646A1Efficient and stable productionLow refractive indexSynthetic resin layered productsChemical recyclingPolycarbonateFormic acid
A subject for the invention is to provide a polycarbonate having excellent mechanical strength, heat resistance, a low refractive index, a large Abbe number, reduced birefringence, and excellent transparency. The invention relates to a polycarbonate characterized by being obtained by subjecting one or more dihydroxy compounds including a dihydroxy compound having at least one linking group —CH2—O— in the molecule thereof to melt polycondensation with a carbonic acid diester, and by having a reduced viscosity of from 0.40 dL / g to 1.70 dL / g and a formic acid content lower than 5 ppm by weight.
Owner:MITSUBISHI CHEM CORP
Method for preparing polycarbonate by fusing ester exchange method and catalyst used for the same
The invention relates to a method of preparing polycarbonate with a fusing ester exchange method and catalyst used thereof, pertaining to the polycarbonate preparation technical field. The composite catalyst of the invention comprises acetylacetone metallic composition and alkaline compound containing nitrogen; the polycarbonate is prepared by fusing ester exchange between aromatic dicarboxylic compound and di-phosphate ester and the catalyst used is composite catalyst; the invention uses one mol dihydroxy compound, 10-8-10-4 mol acetylacetone metallic composite and 10-5-10-2 alkaline compound containing nitrogen under the temperature of 100 DEG C - 320 DEG C. The exchange reaction is gradually and fragmentally depressurized with unceasingly evaporating monohydric phenol from 133 millibar to 1 millibar below. The invention can produce the relatively small branching and cross linking polycarbonate with good color shade, and is good for preservation and postprocessing of the polycarbonate products.
Owner:TIANJIN UNIV
Process for the production of polycarbonate using an ester substituted diaryl carbonate
A method of preparing polycarbonate includes a steps of providing a melt reaction mixture and allowing the melt reaction mixture to react to build molecular weight, thereby preparing the polycarbonate. The melt reaction mixture has a dihydroxy compound, an ester substituted diaryl carbonate mixture, and a melt transesterification catalyst where the ester substituted diaryl carbonate mixture may contain acid-substituted phenol. The method also includes the step of adjusting the molar ratio of acid-substituted phenol, if present, to melt transesterification catalyst (acid-substituted phenol / catalyst) in the melt reaction mixture to an amount of less than 10.
Owner:SABIC INNOVATIVE PLASTICS IP BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com