Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "Dihydroxyaluminum aminoacetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dihydroxyaluminum aminoacetate. di·hy·drox·y·a·lu·mi·num am·i·no·ac·e·tate. Basic aluminum glycinate, a basic aluminum salt of aminoacetic acid containing small amounts of aluminum hydroxide and aminoacetic acid; used as an antacid. dihydroxyaluminum aminoacetate. An antacid used in treating gastric hyperacidity.
Medical defervescence patch and preparation method thereof
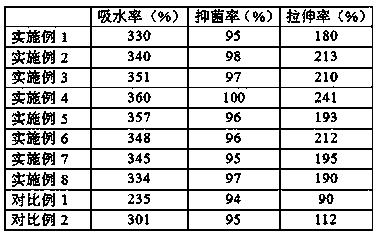
ActiveCN104688408AIncrease elasticitySlightly highAntipyreticAnalgesicsCross-linkDihydroxyaluminum aminoacetate
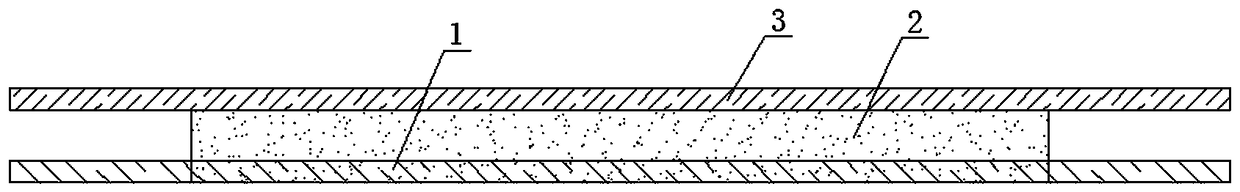
The invention discloses a medical defervescence patch and a preparation method thereof. The medical defervescence patch comprises a medical defervescence patch body composed of a backing layer, an adhesive layer and a screen membrane, wherein the screen membrane layer is arranged above the adhesive layer, the backing layer is arranged below adhesive layer, and the backing layer is made of a PU film. The invention also discloses a preparation method of the medical defervescence patch. The method includes the steps of uniformly mixing and stirring dihydroxyaluminum aminoacetate with a dispersant and a humectant, adding a cross-linking agent, an adhesion agent, a thickener, a temperature-sensitive color changing microcapsule, medical ethanol and the balance of purified water, adding peppermint essential oil, lemon essential oil and folium eucalypti essential oil in a cross-linking polymerization process of the above materials, uniformly dispersing and mixing into a polymer delivery material containing active ingredients, applying the material to an anti-adhesion layer to be made into attached preparation, and adhering the attached preparation to the PU film to obtain the medical defervescence patch. The PU film is non-toxic and harmless and has high water resistance and good moisture permeability. The medical defervescence patch is provided with temperature-sensitive ink for enabling a user to judge whether a fever is abated or not according to the color change on the medical defervescence patch, thereby being easy to operate.
Owner:施敬东
Cold compress paster and preparation method thereof
ActiveCN107050048AReduce energy consumptionLow costAntipyreticAnalgesicsDihydroxyaluminum aminoacetateTreatment effect
The invention discloses cold compress paster and a preparation method thereof. The cold compress paster comprises a backing layer as well as a hydrogel layer and an anti-adhesion layer which are adhered to the upper surface of the backing layer sequentially; the hydrogel layer is prepared from a hydrogel matrix and comprises the following oil phase and aqueous phase in parts by weight: oil phase: 18 to 22 parts of glycerinum, 4 to 7 parts of polyacrylic acid, 0.13 to 0.17 part of dihydroxyaluminum aminoacetate, and 1 to 5 parts of crosslinked polyvinylpolypyrrolidone (PVPP); aqueous phase: 65 to 69 parts of purified water, 0.1 to 0.5 part of ethylene diamine tetraacetic acid, 0.5 to 20 parts of anhydrous magnesium sulfate, 1 to 3 parts of polyvinylpyrrolidone (PVP) and 0.2 to 0.5 part of tartaric acid. According to the cold compress paster provided by the invention, the hydrogel layer integrates the traditional hydrogel cold compress and magnesium sulfate cold compress, so the cold compress paster has good auxiliary treatment effect, does not have any side effects and is convenient to use.
Owner:浙江崇山生物制品有限公司
Analgesic cold patch and preparation technology thereof
ActiveCN107375250ARapidly fully cross-linkedEvenly filledAntipyreticAnalgesicsDihydroxyaluminum aminoacetateGlycerol
The invention provides an analgesic cold patch and a preparation technology thereof. The specific method comprises the steps of firstly adding a first part of sodium polyacrylate, dihydroxyaluminum aminoacetate, polyvinylpolypyrrolidone, polymethylacrylic acid and iron oxide black to glycerin to prepare an oil phase; mixing tartaric acid, a preservative, a second part of sodium polyacrylate, a traditional Chinese medical extract, honey and water, and supplementing a penetration enhancer, a stabilizer, wintergreen oil, menthol and a heat sensitizer to prepare an aqueous phase; and finally mixing the oil phase with the aqueous phase in vacuum to obtain a hydrogel matrix, carrying out coating, cutting, packaging and curing in a bag to obtain the analgesic cold patch. The prepared cold patch has an excellent analgesic effect and has relatively good caking property with human skin.
Owner:贵州梵天苗成医疗器械科技有限公司
Hyperostosis cold compress paste and preparation method thereof
InactiveCN107296852AGuaranteed respiratory metabolismIncrease contact surfaceHydroxy compound active ingredientsSkeletal disorderDihydroxyaluminum aminoacetateIrritation
The invention discloses a hyperostosis cold compress paste which comprises a backing layer, a gel drug layer and an anti-bonding layer, wherein the gel drug layer contains the following raw materials: sodium polyacrylate, carbomer, glycerol, dihydroxyaluminum aminoacetate, tartaric acid, purified water, medical borneol, camphor, diclofenac sodium, menthol, atropa belladonna fluid extract, clove oil, propylene glycol, glycerol, medical azone, diphenhydramine, oleum menthae and capsaicin. The invention also discloses a preparation method for the hyperostosis cold compress paste. The method comprises the following steps: preparing a gel drug and then coating the backing layer with the gel drug by machine so as to form a gel drug layer; covering the gel drug layer with the anti-bonding layer; cutting, puncturing and slicing, thereby acquiring the hyperostosis cold compress paste. No organic solvent is added into the bruise cold compress paste disclosed by the invention; the cold compress paste can be directly coated and does not need to be heated; the technology is simple and no chemical residue exists; the cold compress paste is free from irritability and stimulation, is high in drug loading capacity and moisture retention, has high compatibility with skin, is anti-ageing, has excellent weather fastness and lasting viscidity and can be repeatedly uncovered and pasted.
Owner:四川利佰生物科技有限公司
Heat-sensitive temperature-sensing hydrogel and double-sided transparent film change-color fever cooling patch
ActiveCN108410105ASensitive to detect temperatureAchieve color transitionDiagnostic recording/measuringSensorsDihydroxyaluminum aminoacetateChange color
The invention discloses a heat-sensitive temperature-sensing hydrogel and a double-sided transparent film change-color fever cooling patch. The heat-sensitive temperature-sensing hydrogel comprises the following raw materials in parts by weight: 5 to 50 parts of glycerin, 0.5 to 30 parts of sodium polyacrylate, 0.05 to 10 parts of dihydroxyaluminum aminoacetate, 0.01 to 10 parts of titanium dioxide, 0.01 to 20 parts of SupGuard GM-BP, 0.01 to 30 parts of thermochromic microcapsule resin, 0.01 to 15 parts of mint and 20 to 100 parts of water. The heat-sensitive temperature-sensing hydrogel is sensitive in color change within the temperature range of the human body, the double-sided transparent film change-color fever cooling patch prepared by the heat-sensitive temperature-sensing hydrogelis sensitive in color change within the temperature range of the human body, and has good air breathability, and fever cooling conditions can be visually observed.
Owner:武汉南雪药业有限公司
Rheumatism cold compress paste and preparation method thereof
InactiveCN107296951AGuaranteed respiratory metabolismIncrease contact surfaceAnthropod material medical ingredientsHydroxy compound active ingredientsRheumatismGlycerol
The invention discloses a rheumatism cold compress paste which comprises a backing layer, a gel drug layer and an anti-bonding layer, wherein the gel drug layer contains the following raw materials: sodium polyacrylate, carbomer, glycerol, dihydroxyaluminum aminoacetate, tartaric acid, purified water, medical borneol, camphor, diclofenac sodium, menthol, atropa belladonna liquid extract, ginger oil, propylene glycol, glycerol, medical azone, diphenhydramine, oleum menthae and capsaicin. The invention also discloses a preparation method for the rheumatism cold compress paste. The method comprises the following steps: firstly, preparing a gel drug, and then coating the backing layer with the gel drug by machine so as to form the gel drug layer, coating the gel drug layer with the anti-bonding layer, cutting, puncturing and slicing, thereby acquiring the bruise cold compress paste. No organic solvent is added into the rheumatism cold compress paste disclosed by the invention; the rheumatism cold compress paste can be directly used for coating and does not need to be heated; the technology is simple and no chemical residue exists; the rheumatism cold compress paste is free from irritability and stimulation, is high in drug loading capacity and moisture retention, has high compatibility with skin, is anti-ageing, has excellent weather fastness and lasting viscidity and can be repeatedly uncovered and pasted; the production cost is lower.
Owner:四川利佰生物科技有限公司
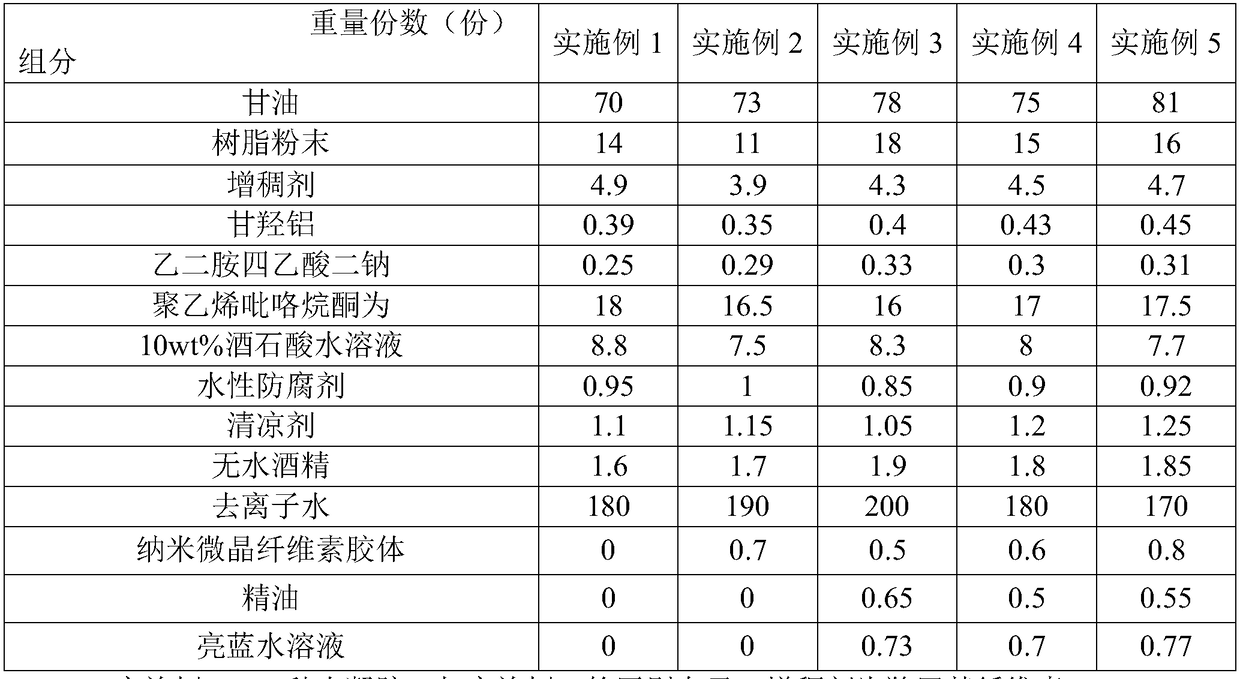
Hydrogel and process using hydrogel to produce cooling plaster
ActiveCN108403622ANot prone to precipitationModerate adhesivenessHydroxy compound active ingredientsAntipyreticDihydroxyaluminum aminoacetateAcetic acid
The invention discloses hydrogel and a process using the hydrogel to produce a cooling plaster and belongs to the technical field of cooling plaster production. The hydrogel comprises 70-81 parts of glycerin, 11-18 parts of resin powder, 3.9-4.9 parts of thickening agent, 0.35-0.45 part of dihydroxyaluminum aminoacetate, 0.25-0.33 part of ethylenediamine tetraacetic acid disodium, 16-18 parts of polyvinyl pyrrolidone, 7.5-8.8 parts of 10wt% tartaric acid aqueous solution, 0.85-1 part of waterborne preservative, 1.0-1.25 parts of cooling agent, 1.6-1.9 parts of anhydrous alcohol and 170-200 parts of deionized water, wherein the resin powder comprises at least one of H106 and HQ841. The hydrogel is good in adhesiveness and stripping strength, is mild and non-irritant, and cannot easily damage the skin.
Owner:杭州求是医学科技有限公司
Fever abatement patch
InactiveCN107362155AStickyNo stimulationAntipyreticAnalgesicsDihydroxyaluminum aminoacetateAdditive ingredient
The invention discloses a fever abatement patch. The patch comprises a bonding layer, a hydrogel layer and a protection layer in sequence, wherein the hydrogel layer is prepared from the following ingredients in percentage by weight: 2-3% of water-soluble mint oil, 0.5-8% of borneol, 5-6% of xanthan gum, 1-5% of polyvinylpyrrolidone, 0.3-0.5% of dihydroxyaluminum aminoacetate, 10-20% of glycerine, 0.2-0.3% of tartaric acid and the balance of deionized water. The adopted xanthan gum is single-spore polysaccharide generated by fermentation of pseudo xanthomonas, has multiple functions, integrates emulsification, suspension thickening, moisturizing and dipping, is bionic and lubricated, and has excellent performance, as the xanthan gum per se has a thickening characteristic, various thickening agents do not need to be added in the fever abatement patch, a solution per se has certain stickiness, the various ingredients in the fever abatement patch can distribute very well for a long time, give play to effects uniformly, and have no stimulation and toxicity to a human body.
Owner:苏州幼儿师范高等专科学校
A lidocaine gel emplastrum
InactiveCN109200033ARapid drug releaseControlled release rateOrganic active ingredientsAntipyreticDihydroxyaluminum aminoacetateOil phase
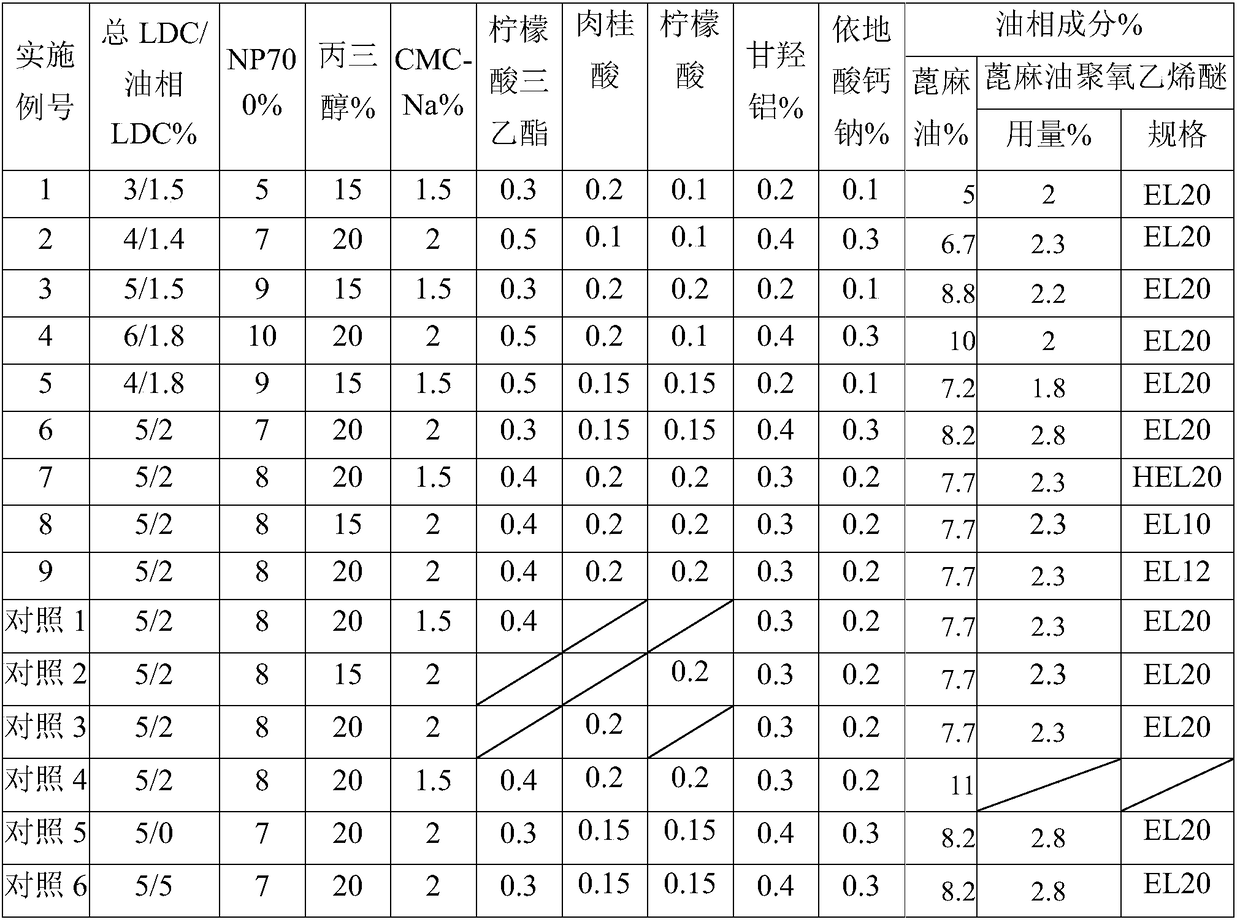
A lidocaine gel emplastrum comprise a backing layer, a drug store and a protective layer, and is characterized in that that drug store consists of the following components in percentage by weight: 3 to 6 % of lidocaine as an active ingredient; 7 to 12 % of oil phase component consisting of castor oil and castor oil polyoxyethylene ether with the ratio of 1:0.2-0.4; 5-10% of partially neutralized sodium polyacrylate as a water phase component, 15-20 % of glycerin, 1.5 to 2 % of sodium carboxymethyl cellulose, 0.3 to 0.5 % of triethyl citrate, 0.1 to 0.2 % of cinnamic acid, 0.1 to 0.2 % of citric acid, 0.2 to 0.4 % of dihydroxyaluminum aminoacetate, 0.1 to 0.3 % of calcium sodium edetate, and 1 to 3 % of filler, with the balance being water. The partially neutralized sodium polyacrylate is NP700, 30 to 50 % of the lidocaine is dispersed in the oil phase, and the rest of the lidocaine is dispersed in the aqueous phase.
Owner:北京茗泽中和药物研究有限公司
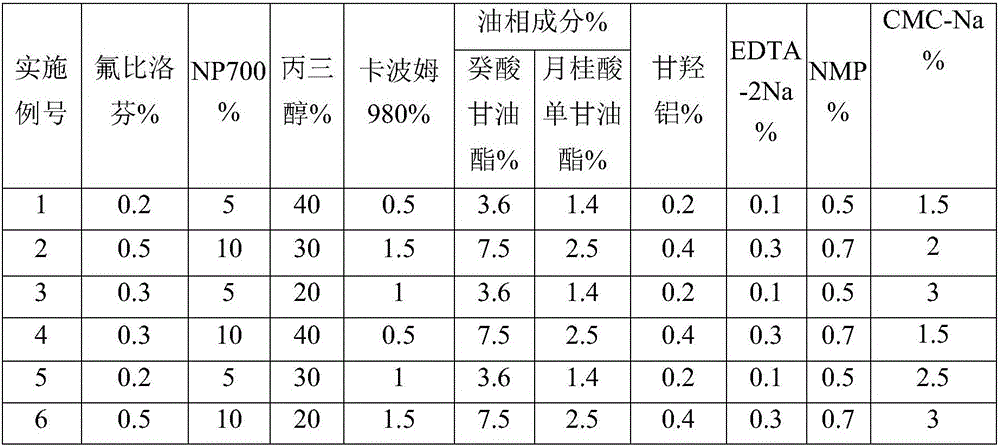
Flurbiprofen cataplasm preparation
InactiveCN106822065AFast transdermal absorptionImprove the speed of transdermal absorptionOrganic active ingredientsAntipyreticDihydroxyaluminum aminoacetateMedicine
The invention relates to a flurbiprofen cataplasm preparation. The flurbiprofen cataplasm preparation consists of a back lining layer, a medicine depot and a protective layer, and is characterized in that the medicine depot is prepared from the following components in percentage by weight: 0.2% to 0.5% of flurbiprofen as an active ingredient and 5% to 10% of oil phase component; the oil phase component is prepared from caprin and glycerol monolaurate according to a mass ratio of 1:(0.3-0.4); the flurbiprofen is dispersed into the oil phase, and the part using as the water phase component is used for neutralizing with 5% to 10% of sodium polyacrylate as a water phase component, 20% to 40% of moisturizing agent, 0.5% to 1.5% of Carbomer 980, 1.5% to 3% of CMC-Na (sodium carboxymethylcellulose), 0.1% to 0.2% of NMP (N-methyl pyrrolidone), 0.2% to 0.4% of dihydroxyaluminum aminoacetate, 0.1% to 0.3% of EDTA-2Na, 1% to 3% of filling agent, and the balance of water.
Owner:北京茗泽中和药物研究有限公司
Neonatal shading dressing and preparation method thereof
PendingCN109701068AKeep moistEasy to useAdhesive dressingsEye-masksDihydroxyaluminum aminoacetateProtecting eye
The invention relates to a neonatal shading dressing for eye protection of neonatal phototherapy, which consists of a light shielding layer, a supporting layer, a hydrogel layer and a separating layer, a preparation process comprises the following steps: 1) weighing 4 to 10% polyacrylic resin, 0.03 to 0.3% of dihydroxyaluminum aminoacetate, 0 to 0.3% of EDTA, and 15 to 30% of glycerin according tothe formula, and uniformly mixing the materials to obtain a glycerin phase; 2) dividing deionized water into three equal parts, weighing 0.1-1% of tartaric acid according to the formula, dissolving the material in 1 part of deionized water, and spreading 1-3% of carbomer on the liquid surface for overnight use to obtain a water phase 1; uniformly dispersing 0-2% of sodium carboxymethyl cellulosein another portion of deionized water, and swelling the material overnight to obtain a water phase 2; taking 1 part of water, adding 0.4-10% of polyvinylpyrrolidone to the water to dissolve completelyto obtain a water phase 3; and uniformly mixing the water phase 1 and the water phase 2 to obtain a water phase 4; and 3) adding the glycerin phase to the water phase 4, and after vacuum stirring for2 to 4 minutes, adding the water phase 3, uniformly mixed hydrogel, and obtaining the product after coating, cutting, packaging and the like.
Owner:HANGZHOU JUJIU SCI & BIOTECHNOLOGY CO
Gel patch with heat clearing, swelling removing and pain stopping functions and preparation method of gel patch
PendingCN109966366AReduce churnImprove complianceHydroxy compound active ingredientsAntipyreticDihydroxyaluminum aminoacetatePatient compliance
The invention relates to a gel patch with heat clearing, swelling removing and pain stopping functions and a preparation method of the gel patch. According to the method, amur corktree barks, Chinesegoldthread rhizomes, medicinal rhubarb roots and rhizomes, raw gypsum, purslane herbs, herba siegesbeckiae, lobedleaf pharbitis seeds, fourstamen stephania roots, calabash shells, Indian bread exodermis, Chinese clematis roots and yanhusuo rhizomes are extracted by ethyl alcohol or water, extracts are dried to obtain powdery alcohol extracts or water extracts, or original medicinal materials are directly grinded into powder and screened to obtain fine medicinal powder, the powdery alcohol extracts, the water extracts and / or the fine medicinal powder, borneol, mirabilite and substrates are crosslinked to prepare the gel patch, and the substrates comprise polyoxyethylene hydrogenated castor oil, sodium polyacrylate, dihydroxyaluminum aminoacetate, tartaric acid, glycerin, povidone and oilyazone. The prepared gel patch can remarkably relieve arthritis and has good functions of clearing heat and removing swelling. Compared with a traditional preparation, the gel patch has the advantagesthat medicine using modes are diverse, drug loading capacity is high, moist maintenance performances are good, residues are omitted, gastrointestinal reaction is omitted, using and carrying are convenient, and compliance of a patient can be remarkably improved.
Owner:上海市光华中西医结合医院
Rivastigmine gel paste and preparation method thereof
PendingCN108926549APromote softeningGood skin affinityNervous disorderPharmaceutical non-active ingredientsDihydroxyaluminum aminoacetateIrritation
The invention belongs to the technical field of pharmacy, and particularly relates to rivastigmine gel paste and a preparation method thereof. The rivastigmine gel paste comprises a backing film, a drug-loaded gel layer and an anti sticking layer. The drug-loaded gel layer comprises a pharmaceutically active ingredient and a gel matrix, and the pharmaceutically active ingredient is rivastigmine ora pharmaceutically acceptable substance thereof. The drug-loaded gel layer is characterized in that the weight ratio of the pharmaceutically active ingredient to the matrix is 1 to 5:50 to 300, and the matrix is prepared from the following auxiliary materials in parts by weight: 0.5-2 parts of sodium carboxymethylcellulose, 10-100 parts of partially-neutralized sodium polyacrylate, 0.1-2 parts ofdisodium edetate, 5-60 parts of povidone K30, 3-30 parts of carbomer, 30-80 parts of glycerol, 0.1-0.5 parts of ethylparaben, 0.01-2 parts of dihydroxyaluminum aminoacetate, 0.05-0.5 part of tartaricacid, 1-10 parts of vitamin E and 120-200 parts of water. The preparation method of the the rivastigmine gel paste has the advantages of simple process, stable quality, comfortable application, highclinical compliance, little irritation to skin, and large-scale production.
Owner:ANHUI ANKE YULIANGQING PHARMA
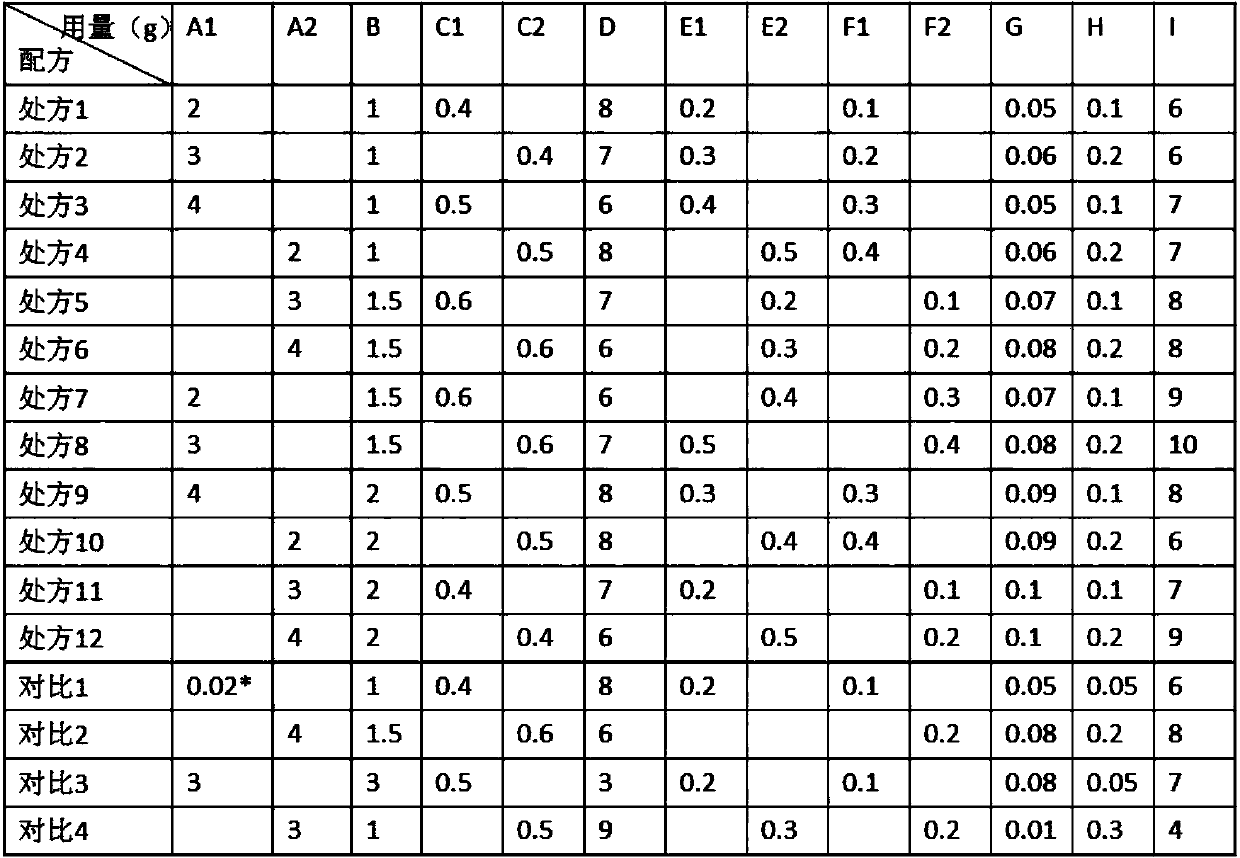
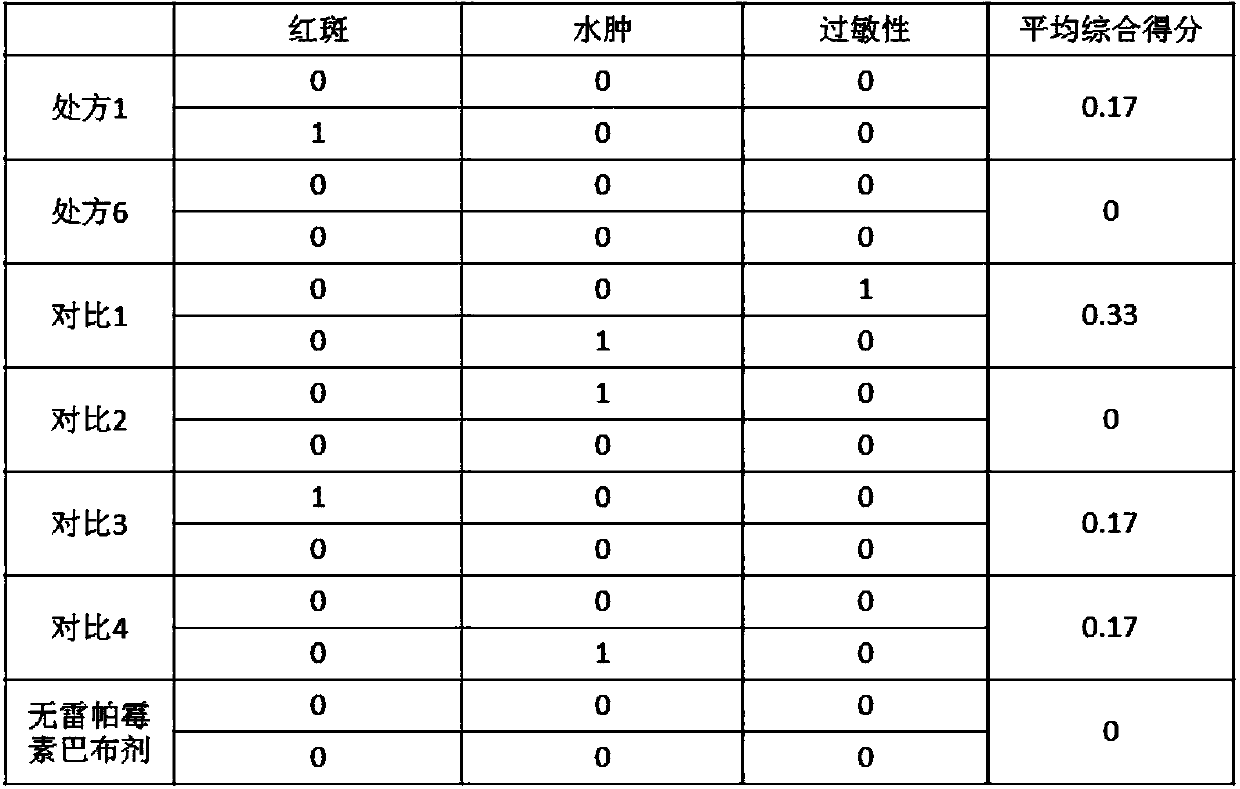
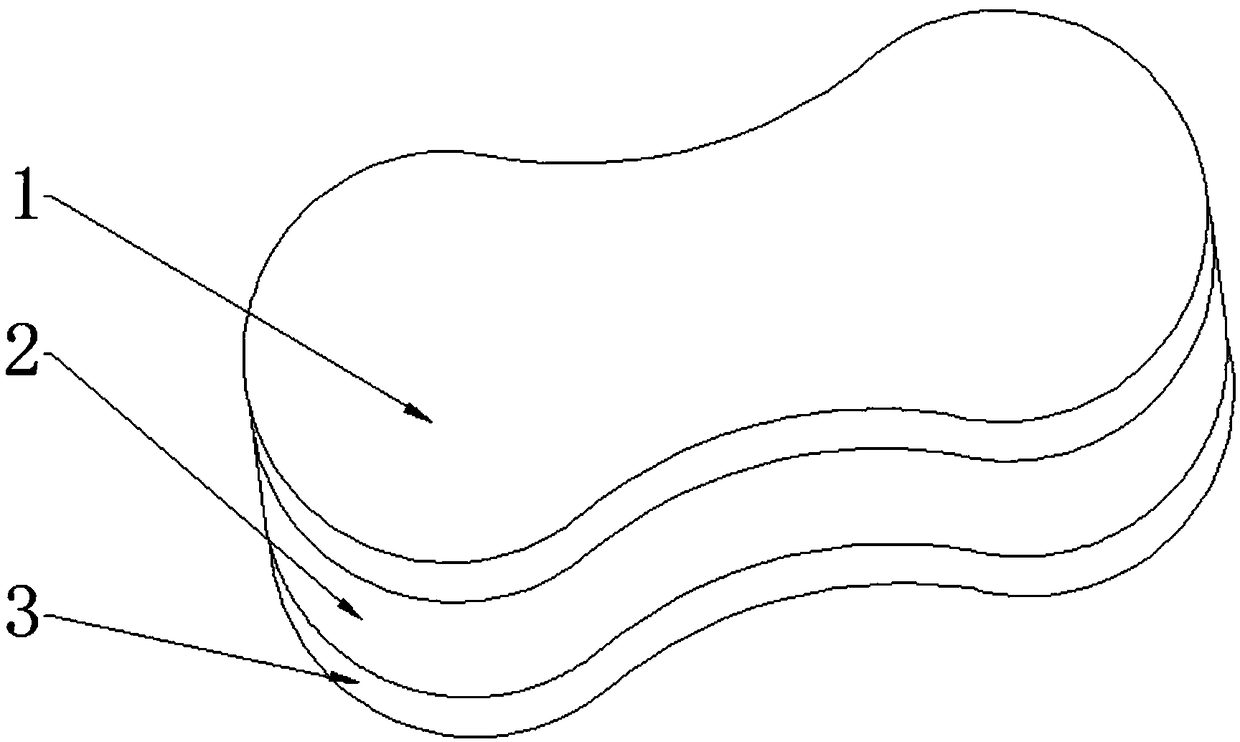
Rapamycin cataplasms and preparation method thereof
ActiveCN109528693AImprove targetingRegular shapeOrganic active ingredientsPharmaceutical non-active ingredientsDihydroxyaluminum aminoacetateOil phase
The invention discloses rapamycin cataplasms and a preparation method thereof. The cataplasms comprise traditional Chinese medicine components and a back lining layer, wherein the traditional Chinesemedicine components comprise 2-4g of rapamycin micelles, 1-2g of a hydrophilic polymer, 0.4-0.6g of a thickening agent, 6-8g of a hydrating agent, 0.2-0.5g of an oil phase substrate, 0.1-0.4g of a transdermal penetration enhancer, 0.05-0.1g of dihydroxyaluminum aminoacetate, 0.1-0.2g of a pH-regulating agent and 6-10g of water. The rapamycin micelles are prepared by using the rapamycin, a surfactant and a cosurfactant in water. According to the technical scheme, the invention provides the rapamycin cataplasms which are good in target properties, good in transdermal properties, and long in lesion position residence time, and are used for treating vascular malformation.
Owner:WUHAN CONFORM PHARMA CO LTD
Stuffy nose relieving paste and preparation method thereof
InactiveCN108354971AIncrease air intakeImprove ventilationHydroxy compound active ingredientsPharmaceutical non-active ingredientsNasal cavityDihydroxyaluminum aminoacetate
The invention discloses a stuffy nose relieving paste and a preparation method thereof, and relates to the technical field of medical products. The invention mainly solves the technical problems of poor treatment effect, high cost and the like of a stuffy nose relieving paste. The invention adopts the technical scheme that the stuffy nose relieving paste comprises a carrier layer, a gel layer andan isolating membrane layer; the gel layer is prepared from the following raw materials in parts by weight: 133 parts of carbomer, 327 parts of sodium carboxymethylcellulose, 4,000 parts of glycerol,12 parts of dihydroxyaluminum aminoacetate, 835 parts of sodium polyacrylate, 1,000 to 3,000 parts of water, 50 parts of eucalyptus oil, 50 parts of wintergreen oil, 25 parts of mint, 30 parts of borneol, 60 parts of diphenhydramine, 30 to 60 parts of alcohol, and 10 to 30 parts of modifier. The raw materials prepared into the gel layer are sequentially subjected to coating, punching, checking andpackaging to obtain the stuffy nose relieving paste. According to the invention, nasal cavity passages can be expanded; the stuffy nose relieving paste is used for stuffy nose caused by cold, rhinitis and the like; snore is alleviated or relieved; an air suction volume of a sportsman is increased.
Owner:山西康灵医疗器械有限公司
Method for extracting effective component of cotton rose hibiscus leaves based on transdermal absorption technology
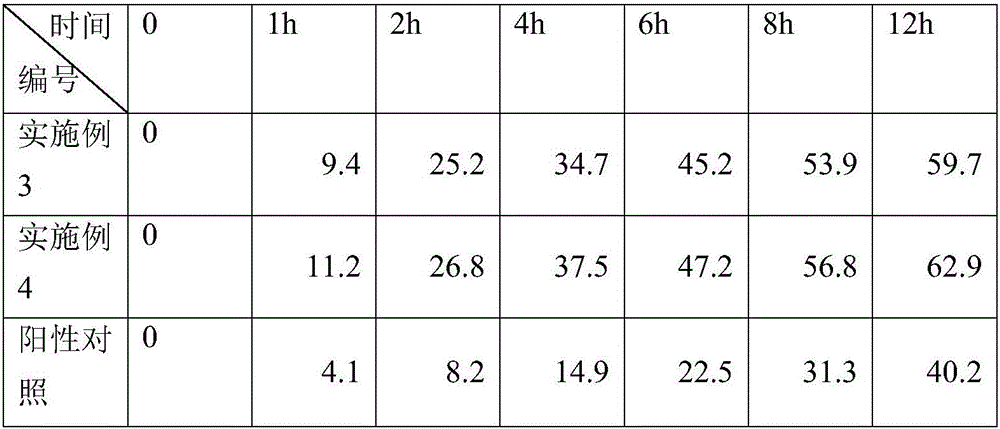
InactiveCN110638848AImprove transdermal absorption rateHigh yieldAntipyreticAnalgesicsBiotechnologyDihydroxyaluminum aminoacetate
The invention aims to provide a method for extracting an effective component of cotton rose hibiscus leaves based on a transdermal absorption technology. The method is characterized by comprising thefollowing steps: (1) gel paste is prepared, specifically, a cotton rose hibiscus leaf extract is weighed, crushed into fine powder, added into a Carbomer solution containing EDTA and evenly mixed, sodium polyacrylate and a dihydroxyaluminum aminoacetate and glycerinum mixed solution are added, even stirring is conducted, an appropriate quantity of citric acid solution is added, even stirring is conducted, and cotton rose hibiscus leaf extract gel is obtained; and (2) a transdermal absorption experiment of the cotton rose hibiscus leaf extract is conducted, specifically, pig skin is pre-treatedand then serves as a transdermal barrier, ethanol-normal saline serves as transdermal absorption fluid, the gel is smeared on treated pig skin corneum, the transdermal absorption fluid is fully injected, the liquid level is exactly in contact with a skin corium layer, bubbles are eliminated, the transdermal absorption experiment is conducted, and transdermal liquid is obtained.
Owner:湖州耕香生物科技有限公司 +1
Medical cold application plaster and preparation method thereof
PendingCN110141560AIncrease moisture contentStrong penetrating powerPeptide/protein ingredientsPharmaceutical non-active ingredientsDihydroxyaluminum aminoacetateMedicine
The invention discloses medical cold application plaster. The medical cold application plaster consists of a back lining layer, a gel layer and an antiseizing layer and is characterized in that the gel layer is prepared from the following components in percentage by mass of 4%-5.2% of sodium polyacrylate, 0.08%-0.12% of dihydroxyaluminum aminoacetate, 0.11%-0.12% of ethylenediaminetetraacetic aciddisodium salt, 0.15%-0.22% of tartaric acid, 21%-25% of glycerine, 0.01%-0.1% of a preservative, 0.1%-0.5% of a functional additive, 0.5%-2% of ethanol and the balance water. The invention further discloses a preparation method of the medical cold application plaster. The medical cold application plaster has high molecular gelatin being high in water content, effective components can rapidly penetrate into the fat layer, impregnate into subcutaneous tissue and achieve favorable effect through transdermic absorption.
Owner:广州市白云区大荣精细化工有限公司
Piroxicam cataplasm
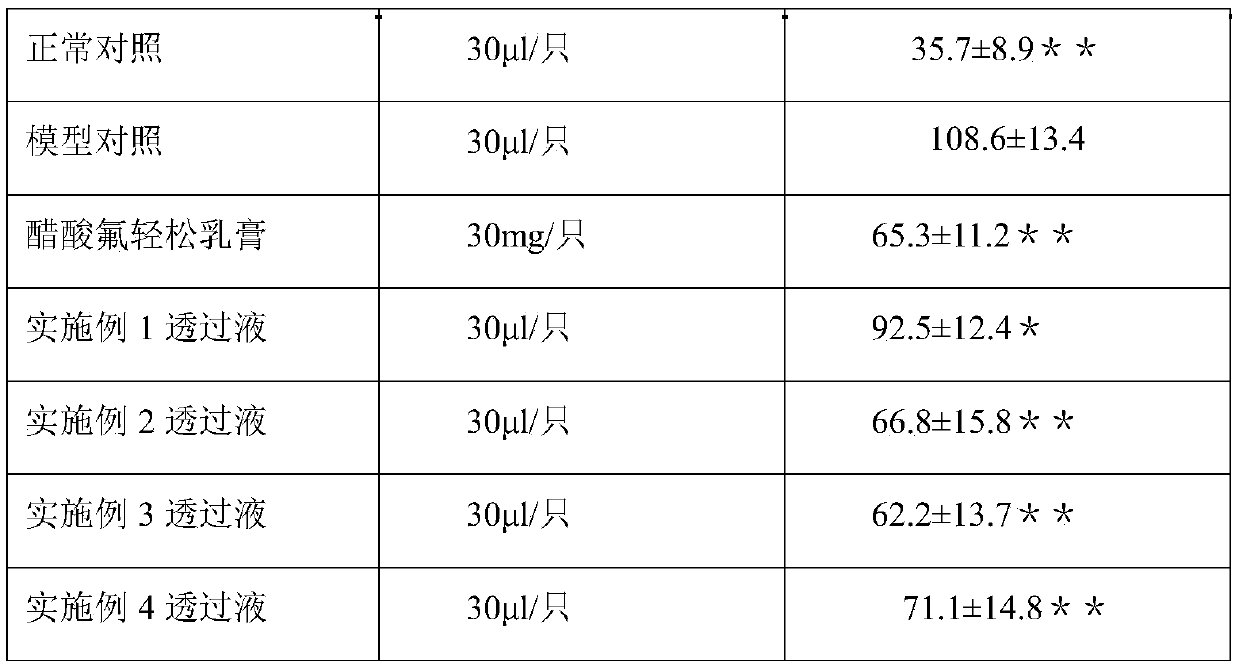
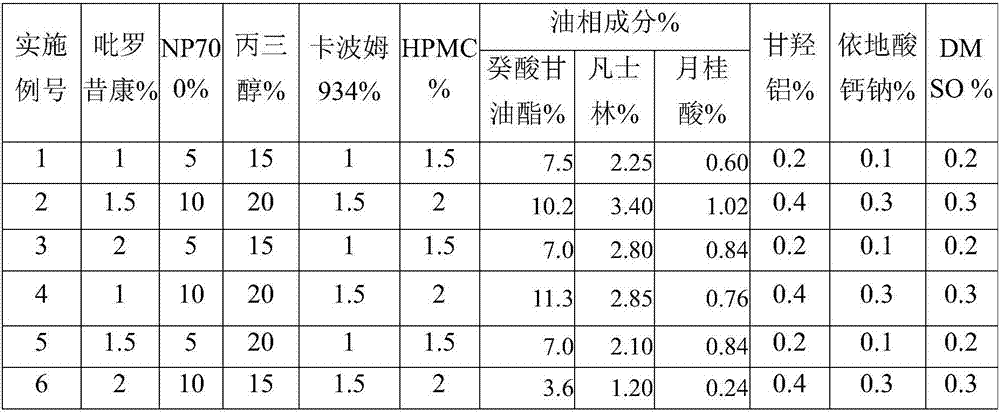
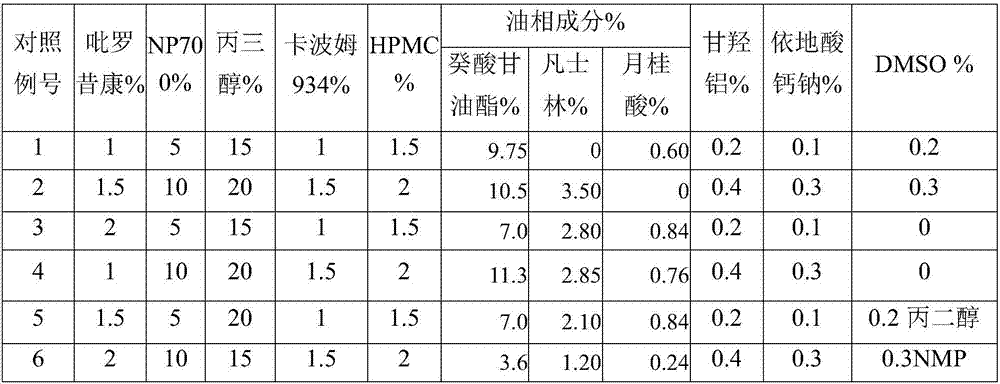
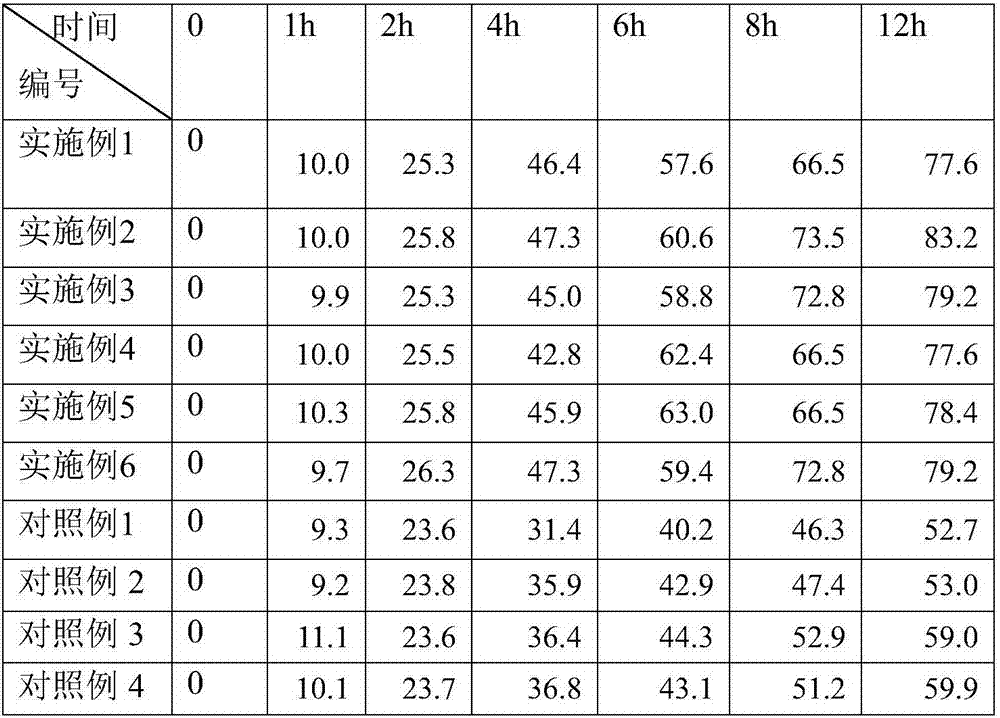
InactiveCN107028921AFast transdermal absorptionLow drug releaseOrganic active ingredientsAntipyreticDihydroxyaluminum aminoacetateGlycerol
The invention discloses piroxicam cataplasm. The piroxicam cataplasm comprises back lining layers, medicine storages and protective layers, and is characterized in that the medicine storages comprise, by weight, 1%-2% of piroxicam, 10%-15% of oil-phase components, 5%-10% of partially neutralized sodium polyacrylate, 15%-20% of glycerol, 1%-1.5% of carbomer 934, 1.5%-2% of hydroxyl propyl methyl celluloses (HPMC), pH (potential of hydrogen) regulators, 0.2%-0.4% of dihydroxyaluminum aminoacetate, 0.1%-0.3% of calcium disodium edetate, 0.2%-0.3% of dimethyl sulfoxide (DMSO), 1%-3% of fillers and the balance water, and the piroxicam is used as an active component; the oil-phase components comprise caprin, Vaseline and lauric acid, a weight ratio of the caprin to the Vaseline to the lauric acid is 1-1.2:0.3-0.4:0.08-0.12, and the piroxicam is dispersed in oil phases; the partially neutralized sodium polyacrylate is used as a water-phase component.
Owner:北京茗泽中和药物研究有限公司
Ultrahigh-drug-loading-capacity hydrogel patch and preparation method thereof
InactiveCN107343882AStrong penetrating powerProlong the action timeMacromolecular non-active ingredientsSheet deliveryDihydroxyaluminum aminoacetateGlycerol
The invention discloses an ultrahigh-drug-loading-capacity hydrogel patch and a preparation method thereof, and relates to the field of hydrogel patches. The ultrahigh-drug-loading-capacity hydrogel patch is prepared from the following raw materials in percentage by mass: 35 to 45 percent of drug-containing hydrophilic gel, 25 to 35 percent of drug-containing starch water retention agent gel, 15 to 25 percent of pre-dissolved drug plant pulp, 0.03 to 0.06 percent of surfactant, 0.2 to 0.25 percent of tartaric acid, 0.1 to 0.2 percent of dihydroxyaluminum aminoacetate cross-linking agent and 7 to 12 percent of mixture, wherein the mixture is prepared by mixing glycerol and sodium polyacrylate according to a proportion of (2 to 4) to 1. The invention also relates to the preparation method of the hydrogel patch. The drug loading capacity of the prepared hydrogel patch reaches 35 percent or above of the total mass of the patch; the ultrahigh-drug-loading-capacity hydrogel patch is multiple in the species of loaded drugs, high in drug inclusiveness and high in drug loading capacity; a horny layer of a skin can be prompted to increase multiplicatively in water quantity by the high water retention performance of the hydrogel patch, swells to be in a porous state, and is more easily penetrated through by a drug; the ultrahigh-drug-loading-capacity hydrogel patch is good in drug release performance and long in acting time.
Owner:山西海清源生物科技有限公司
Antibacterial dressing used in hepatological surgery department
ActiveCN109125781ABreathableImprove mechanical propertiesAbsorbent padsBandagesDihydroxyaluminum aminoacetatePolyvinyl alcohol
The invention discloses an antibacterial dressing used in a hepatological surgery department and relates to the technical field of medical dressings. The antibacterial dressing comprises a fiber layerI, a gel layer and a fiber layer II which are sequentially arranged from inside to outside, wherein the gel layer is made of the following raw materials in parts by weight: 45-55 parts of acrylic acid, 40-50 parts of N-hydroxymethyl acrylamide, 10-20 parts of polyvinyl alcohol, 1-2 parts of dihydroxyaluminum aminoacetate, 3-8 parts of talcum powder, 1-2 parts of a polypropylene fiber, 4-8 parts of sodium carboxymethylcellulose, 5-12 parts of polyvinylpyrrolidone, 0.6-1.2 parts of N,N-methylene bisacrylamide, 0.5-1 part of sodium persulfate and 200-300 parts of water. The antibacterial dressing is excellent in antibacterial property and mechanical property, and is beneficial to rapid recovery of wounds.
Owner:NANYANG CITY CENT HOSPITAL
Smoking-quitting hydrogel patch and preparation method thereof
ActiveCN106491569APromote softeningImprove skin penetrationOrganic active ingredientsNervous disorderDihydroxyaluminum aminoacetateAdditive ingredient
The invention discloses a smoking-quitting hydrogel patch and a preparation method thereof. The smoking-quitting hydrogel patch is prepared by preparing nicotine, polyacrylic acid and sodium polyacrylate copolymer, dihydroxyaluminum aminoacetate, tartaric acid, glycerin, high-molecular polymer having linear and / or netty crosslinking structure, ethylene diamine tetraacetic acid disodium, azone, eucalyptus oil, castor oil and purified water into hydrogel; subjecting the hydrogel to coating, film covering and curing to obtain the smoking-quitting hydrogel patch. The objective of storing and releasing nicotine is achieved by utilizing special structure and ingredients inside materials so as to realize smoking quitting effect. The smoking-quitting hydrogel patch has the advantages of quickness and continuity in release, moisture preservation, air breathability, high skin permeability, little irritation, stability, attractive appearance and comfort.
Owner:NANJING UNIV OF TECH +1
Mud flat silt based slow-release antibacterial agent for cutting propagation and preparation method thereof
InactiveCN108617653AGood waterGood water locking effectBiocideGrowth substratesDihydroxyaluminum aminoacetateAntibacterial agent
The invention relates to a mud flat silt based slow-release antibacterial agent for cutting propagation and a preparation method thereof. The slow-release antibacterial agent is prepared from the following components in parts by weight: 40-60 parts of a fine silt powder, 5-10 parts of sodium polyacrylate, 1-10 parts of crosslinking polyacrylic acid resin, 2-10 parts of nonionic polyacrylamide, 0.5-8 parts of a crosslinking agent, 2-5 parts of a bactericide and 1-3 parts of a tissue protector, wherein the fine silt powder is prepared by treating mud flat silt; the crosslinking agent is dihydroxyaluminum aminoacetate; the bactericide is at least one of copper abietate, potassium permanganate and lanthanum sulfate; the tissue protector is a mixture of oxidized and reduced glutathione. The mudflat silt based slow-release antibacterial agent for cutting propagation and the preparation method thereof disclosed by the invention have the beneficial effects that the slow-release antibacterialagent expands into gel when meeting water and has excellent water gathering and retaining effects; the antibacterial agent is gradually dissolved into water, slowly released or diffused into a cuttingmedium and used for long-term disinfection and sterilization of the cutting medium.
Owner:柯江波
Evodia rutaecarpa and ginger emplastrum and preparation method thereof
ActiveCN113413451AEasy to shapeBroaden applicationAntipyreticDigestive systemBiotechnologyDihydroxyaluminum aminoacetate
The invention discloses an evodia rutaecarpa and ginger emplastrum and a preparation method thereof. The evodia rutaecarpa and ginger emplastrum is prepared from the following raw material medicines in parts by weight: 2.6 to 3.4 parts of evodia rutaecarpa and ginger extract, 0.06 to 1 part of dihydroxyaluminum aminoacetate, 1.8 to 2.6 parts of glycerol, 0.1 to 0.5 part of propylene glycol, 1 to 1.5 parts of sodium polyacrylate, 0.03 to 0.07 part of ethylparaben, 0.04 to 0.08 part of tartaric acid, 1 to 1.4 parts of distilled water and 1.2 to 1.8 parts of kaolin. In the main medicines, the evodia rutaecarpa is compatible with the fresh ginger. The evodia rutaecarpa and the ginger are used together, and the ginger not only can restrain the drawness of the evodia rutaecarpa, but also can enhance the yang warming, cold dispelling, stomach harmonizing and adverse qi lowering effects of the evodia rutaecarpa. In the preparation process, stirring is easy, all phases are mutually fused, and the prepared paste is uniform in color, free of obvious particles, easy to coat, free of strip breaking and overflowing phenomena and excellent in product quality.
Owner:GUIZHOU UNIV
Vein applicator and preparation method thereof
InactiveCN106963850AFixed indwelling needle works wellReduce surface temperatureHydrocarbon active ingredientsHydroxy compound active ingredientsDihydroxyaluminum aminoacetateThrombophlebitis
The invention discloses a vein applicator. The vein applicator comprises a backlining layer, a gel layer and an anti-sticking layer which are sequentially arranged from bottom to top, wherein the gel layer is prepared from, by weight, 30 parts of high polymer material, 1 part of dihydroxyaluminum aminoacetate, 100 parts of Chinese violet with the relative density of 1.08%, 1 part of lycopene, 4 parts of menthol and 20 parts of medical ethanol. The high polymer material is prepared from, by mass, 0.3% of polyacrylate, 4% of polyacrylate, 33% of glycerin, 0.13% of sorbitan-80 and 0.18% of tartaric acid. The invention also discloses a preparation method of the vein applicator. The vein applicator has the advantages of fixing an indwelling needle, having the good effect of preventing and treating thrombophlebitis and the like.
Owner:东阳市人民医院
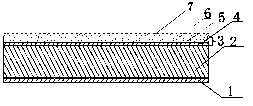
Indometacin hydrogel patch
InactiveCN107198681AFast transdermal absorptionSustained rapid releaseOrganic active ingredientsAntipyreticDihydroxyaluminum aminoacetateIndometacin
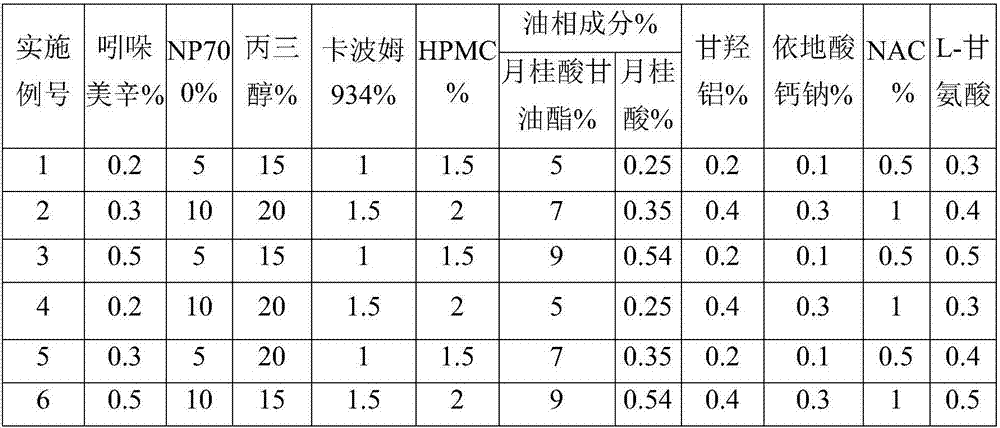
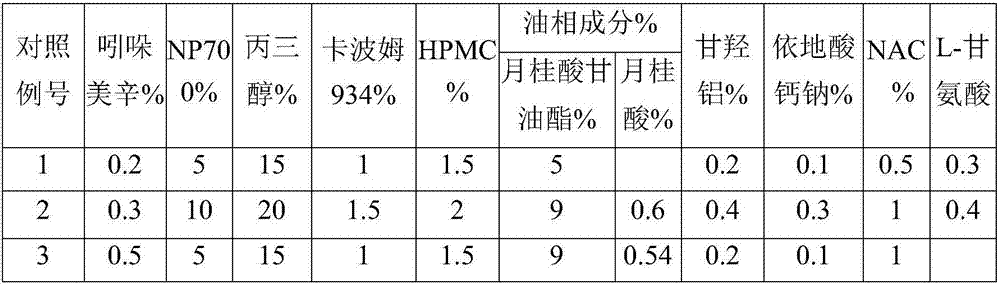
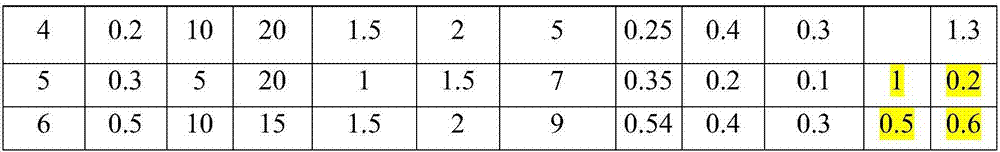
The invention discloses an indometacin hydrogel patch which comprises a back liner layer, a medicine storage layer and a protection layer. The indometacin hydrogel patch is characterized in that the medicine storage layer consists of the following components in percentage by weight: 0.3-0.5% of indometacin as an active component, 5-10% of an oil-phase component, 5-10% of partially neutralized sodium polyacrylate as a water-phase component, 15-20% of glycerol, 1-1.5% of carbomer 934, 1.5-2% of hydroxypropyl methyl cellulose (HPMC), a pH adjusting agent, 0.2-0.4% of dihydroxyaluminum aminoacetate, 0.1-0.3% of sodium calcium edentate, 0.3-0.5% of L-glycine, 0.5-1% of N-acetylcystein (NAC), 1-3% of a filling agent and the balance of water, wherein the oil-phase component comprises glycerol laurate and lauric acid at a ratio of 1:(0.05 to 0.06), and indometacin is dispersed in the oil phase.
Owner:北京茗泽中和药物研究有限公司
Convenient moxibustion patch
InactiveCN107007645AReduce volumeLightweightDevices for heating/cooling reflex pointsPharmaceutical non-active ingredientsDihydroxyaluminum aminoacetateMugwort Essential Oil
The invention discloses a convenient moxibustion patch. The convenient moxibustion patch comprises the following components: big bags and small bags, wherein each big bag contains the following ingredients: 1560g of main glue, 40.5g of dihydroxyaluminum aminoacetate, 34g of disodium and 10g of titanium dioxide; and each small bag contains the following ingredients: 70g of tartaric acid, 180g of ethyl alcohol, 60g of Chinese mugwort essential oil, 120g of supercritical ginger oil and 210g of a brilliant blue pigment. The convenient moxibustion patch is prepared by carrying out the following preparation steps: (1) firstly stirring glycerol with the big bags for five minutes, then adding ethyl alcohol-Chinese mugwort essential oil solution and stirring for five minutes; (2) then adding the small bags, the supercritical ginger oil and the pigment into water, and uniformly stirring; and (3) pouring a water phase in the step (2) into a stirring pot, and adding the oil phase in the step (1) and stirring for ten minutes. The convenient moxibustion patch disclosed by the invention is small in size, light and handy, easy to carry and use and simple in operation, an autologous heating technology is combined, and the effect of the convenient moxibustion patch is obviously better than that of the traditional moxibustion.
Owner:湖北正源生物科技有限公司
Traditional Chinese medicinal gel plaster for treating traumatic injury, blood stasis and swelling and preparation method of traditional Chinese medicinal gel plaster
The invention relates to a traditional Chinese medicinal gel plaster for treating traumatic injury, blood stasis and swelling and a preparation method of the traditional Chinese medicinal gel plaster; the problem on medication of traumatic injury, blood stasis and swelling can be effectively solved; according to the technical scheme, the traditional Chinese medicinal gel plaster is prepared from, by weight, 5-15g of radix angelicae sinensis, 2-10g of radix angelicae dahuricae, 5-15g of fructus gardeniae, 5-10g of processed rhizoma arisaematis, 5-15g of radix saposhnikoviae, 5-15g of flos carthami and 25-78g of a matrix, wherein the matrix is prepared from sodium polyacrylate, polyvinyl pyrrolidone, dihydroxyaluminum aminoacetate, tartaric acid, ethylenediamine tetraacetic acid, kaolin, glycerol and 1,2-propanediol at the ratio of 2.38:2.25:1.0:0.15:0.025:1.88:16:8. The traditional Chinese medicinal gel plaster, which is prepared on the basis of modern pharmaceutical technology, has functions of promoting blood circulation, removing stasis, diminishing inflammation and alleviating pain; the traditional Chinese medicinal gel plaster is applicable to such symptoms as traumatic injury, blood stasis and swelling, and the like; and the traditional Chinese medicinal gel plaster is an innovation of medicines for treating the traumatic injury, blood stasis and swelling.
Owner:HENAN UNIV OF CHINESE MEDICINE
A kind of cataplasm of total coumarin cataplasm for improving the bioavailability of ancestralin
ActiveCN107714835BExcellent adhesionImprove ductilityAntipyreticAnalgesicsDihydroxyaluminum aminoacetateEdetic Acid
The invention provides a girald daphne bark total-coumarins cataplasm for improving the daphnetin bioavailability. The girald daphne bark total-coumarins cataplasm is prepared from girald daphne barktotal-coumarins extractives, sodium polyacrylate NP700, dihydroxyaluminum aminoacetate, disodium edetate dehydrate, glycerin and deionized water, wherein the mass ratio is 0.85-1.05:5.5-6.5:0.14-0.18:0.12-0.20:30-35:40-50; according to the girald daphne bark total-coumarins extractives, the content of total-coumarins is larger than 50% of total solids by weight, and the content of daphnetin is larger than 20% of total solids by weight. The daphnetin in the girald daphne bark total-coumarins cataplasm has the obvious slow release characteristic, and the defects that commercially available daphnetin in girald daphne bark tablets is rapid in absorption and elimination, short in half-life period, low in bioavailability and large in blood concentration volatility are overcome; in the transdermal drug delivery route, the daphnetin can well achieve transdermic absorption, on the continuous applying condition, the stable and efficient blood concentration can be maintained for a long time, andmeanwhile the bioavailability is improved.
Owner:SHANDONG UNIV OF TRADITIONAL CHINESE MEDICINE
A kind of hydrogel patch for smoking cessation and preparation method thereof
ActiveCN106491569BPromote softeningImprove skin penetrationOrganic active ingredientsNervous disorderDihydroxyaluminum aminoacetateAdditive ingredient
Owner:NANJING TECH UNIV +1
A heat-sensitive warm water gel and double-sided transparent film color-changing antipyretic patch
ActiveCN108410105BSensitive to detect temperatureAchieve color transitionDiagnostic recording/measuringSensorsDihydroxyaluminum aminoacetateWarm water
The invention discloses a heat-sensitive temperature-sensing hydrogel and a double-sided transparent film change-color fever cooling patch. The heat-sensitive temperature-sensing hydrogel comprises the following raw materials in parts by weight: 5 to 50 parts of glycerin, 0.5 to 30 parts of sodium polyacrylate, 0.05 to 10 parts of dihydroxyaluminum aminoacetate, 0.01 to 10 parts of titanium dioxide, 0.01 to 20 parts of SupGuard GM-BP, 0.01 to 30 parts of thermochromic microcapsule resin, 0.01 to 15 parts of mint and 20 to 100 parts of water. The heat-sensitive temperature-sensing hydrogel is sensitive in color change within the temperature range of the human body, the double-sided transparent film change-color fever cooling patch prepared by the heat-sensitive temperature-sensing hydrogelis sensitive in color change within the temperature range of the human body, and has good air breathability, and fever cooling conditions can be visually observed.
Owner:武汉南雪药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com