Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
50 results about "Dienedione" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
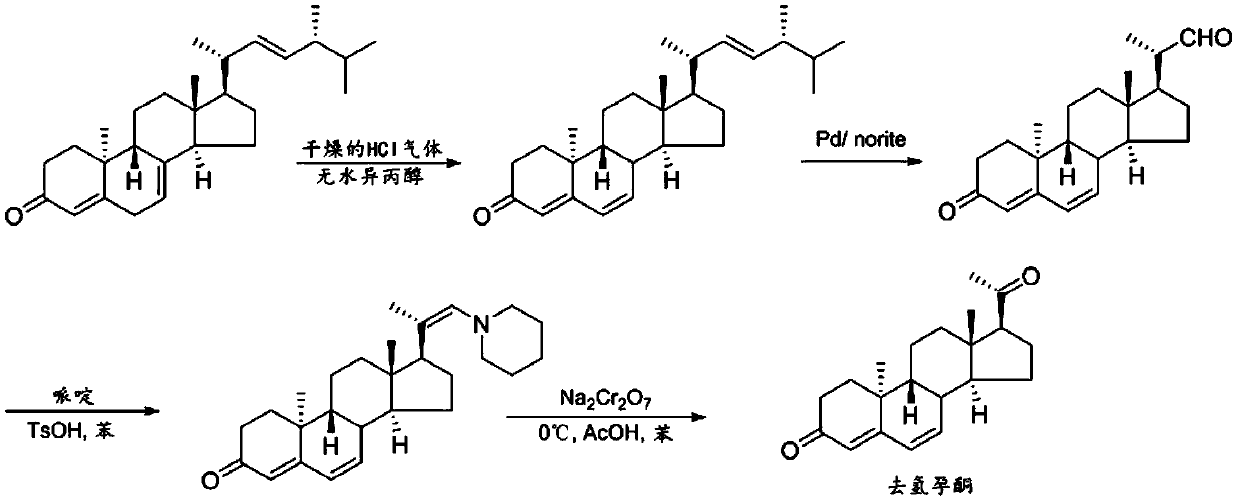
Dienedione, also known as estra-4,9-diene-3,17-dione, is a synthetic, orally active anabolic-androgenic steroid (AAS) of the 19-nortestosterone group that was never introduced for medical use. It is thought to be a prohormone of dienolone. The drug became a controlled substance in the US on January 4, 2010, and is classified as a Schedule III anabolic steroid under the United States Controlled Substances Act. Previous to this, it was sold as a bodybuilding supplement within the United States, and often mistakenly marketed as a prohormone for trenbolone, a veterinary steroid. Prior to its scheduling, it was part of a number of supplements that were seized during FDA enforcement of Bodybuilding.com for selling unapproved new drugs. The actual active metabolite, dienolone, is almost identical to trenbolone structurally, but lacks the C11 double bond.
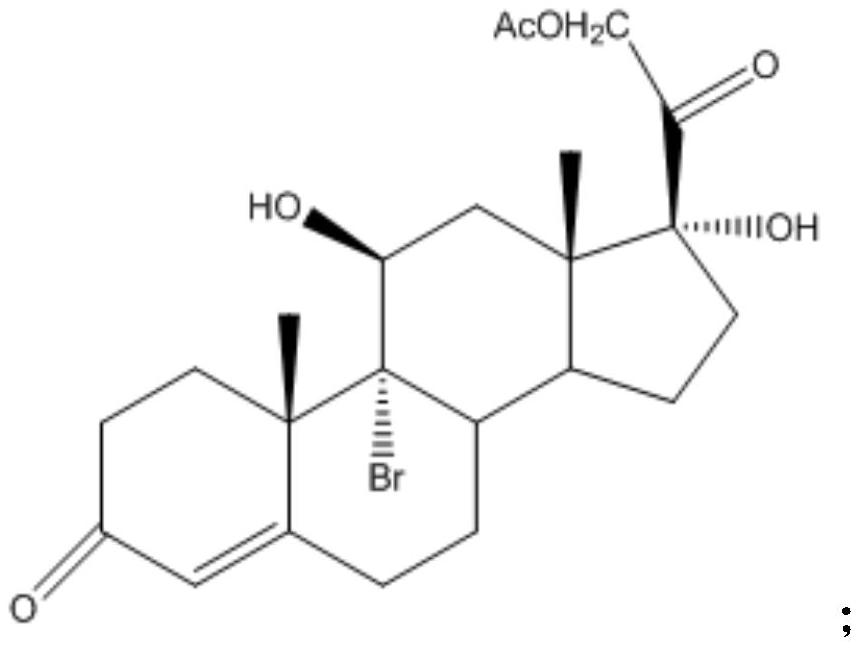
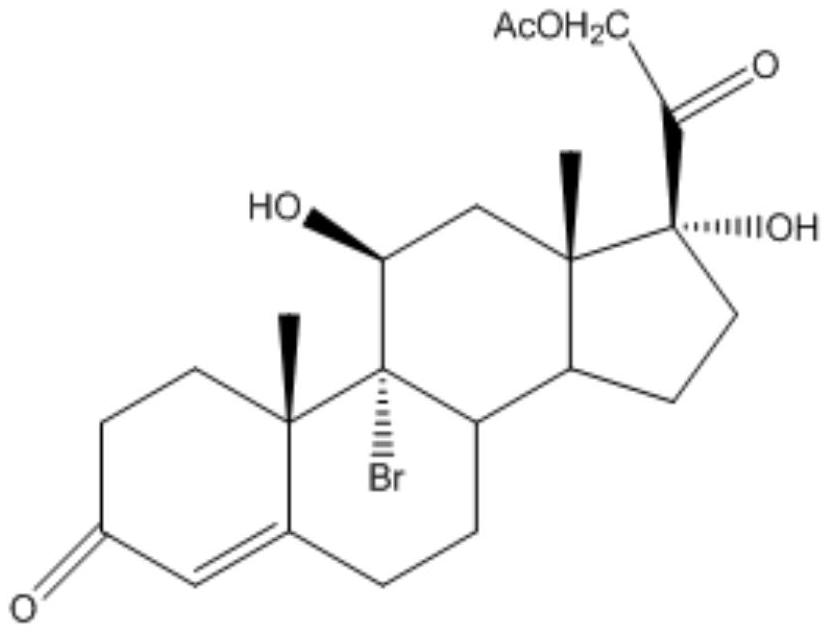
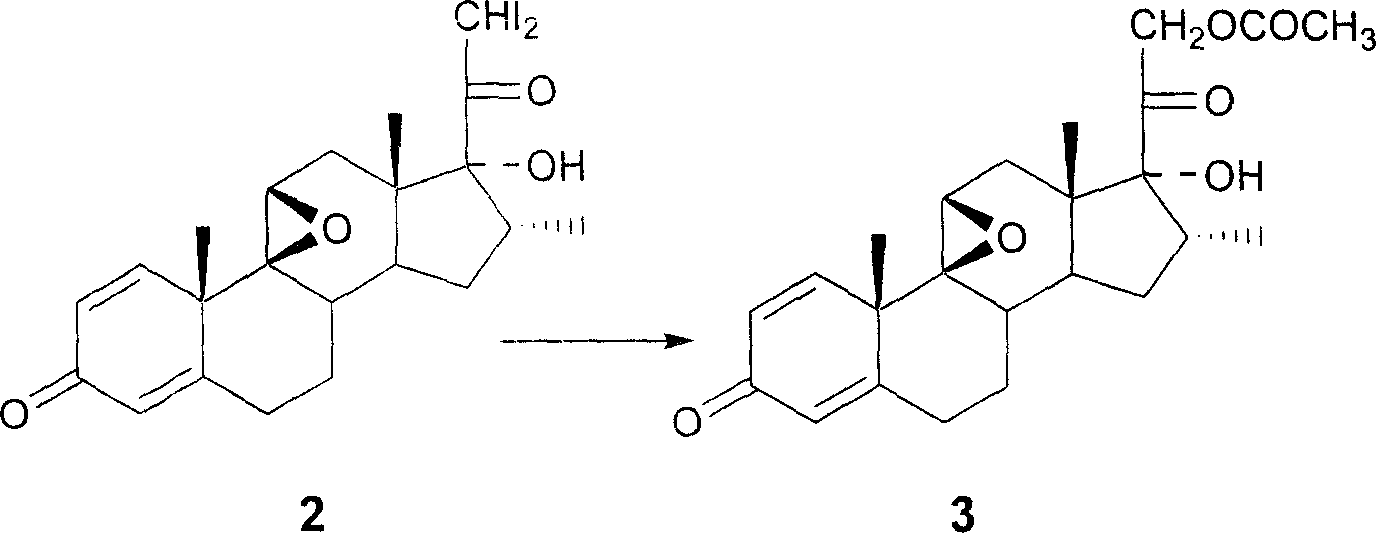
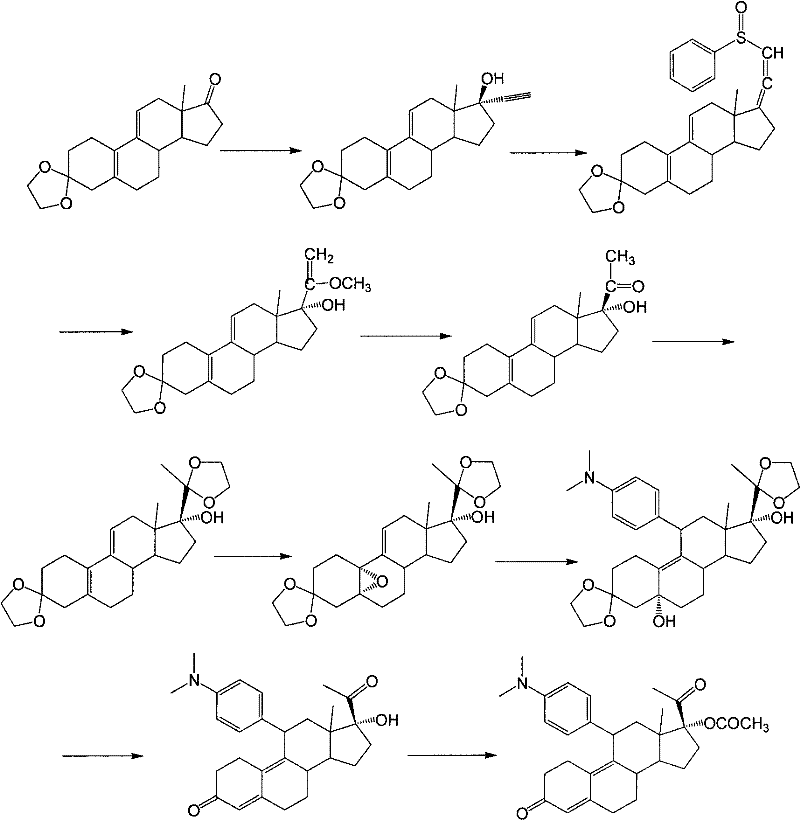
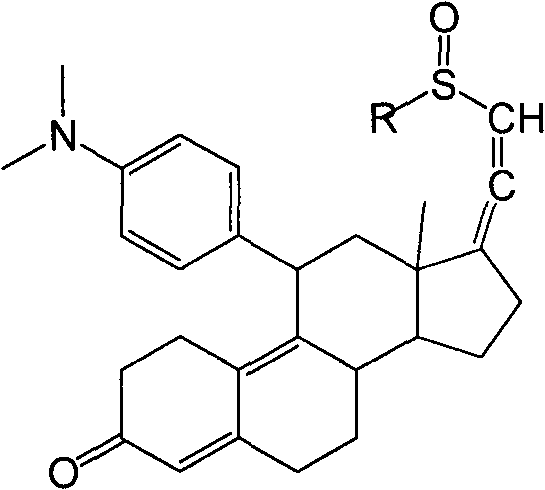
Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process
The present invention relates to a new industrial process for the synthesis of solvate- free 17a-acetoxy-11ss-[4-(N,N-dimethyl-amino)-phenyl]-19-norpregna-4,9-diene-3,20-dione [CDB -2914] of formula (I) which is a strong antiprogestogene and antiglucocorticoid agent. The invention also relates to compounds of formula (VII) and (VIII) used as intermediates in the process. The process according to the invention is the following: i) 3-(ethylene-dioxy)-estra-5(10),9(11)-diene-17-one of formula (X) is reacted with potassium acetilyde formed in situ in dry tetrahydrofuran by known method, ii) the obtained 3-(ethylene-dioxy)-17a-ethynyl-17ss-hydroxy-estra-5(10),9(11)-diene of formula (IX) is reacted with phenylsulfenyl chloride in dichloromethane in the presence of triethylamine and acetic acid, iii) the obtained isomeric mixture of 3-(ethylene-dioxy)-21-(phenyl-sulfinyl)-19-norpregna-5(10),9(11),17(20),20-tetraene of formula (VIII) is reacted first with sodium methoxide in methanol, then with trimethyl phosphite, iv) the obtained 3-(ethylene-dioxy)-17a-hydroxy-20-methoxy-19-norpregna-5(10),9(11),20-triene of formula (VII) is reacted with hydrogen chloride in methanol, then v) the obtained 3-(ethylene-dioxy)-17a-hydroxy-19-norpregna-5(10),9(11l); -diene-20- one of formula (VI) is reacted with ethylene glycol hi dichloromethane in the presence of trimethyl orthoformate and p-toluenesulfonic acid by known method, vi) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-19-norpregna- 5(10),9(11)-diene of formula (V) is reacted with hydrogen peroxide in a mixture of pyridine and dichloromethane in the presence of hexachloroacetone by known method, vii) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-5,10-epoxy-19-norpregn-9(11)-ene of formula (IV), containing approximately a 1:1 mixture of 5a,10a- and 5ss,10ss-epoxides, is isolated from the solution and reacted with a Grignard reagent obtained from 4-bromo-N,N-dimethyl-aniline in tetrahydrofuran.
Owner:RICHTER GEDEON NYRT
Method for obtaining high-purity 17α-acetoxy-11β-(4-n,n-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione
The invention discloses a method of acquiring high-purity 17 alpha-acetoxy-11 beta-(4-N, N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione, comprising the following steps: a) putting the acquired crude 17 alpha-acetoxy-11 beta-(4-N, N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione in a proper solvent system to generate a pure 17 alpha-acetoxy-11 beta-(4-N, N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione solid; b) separating the 17 alpha-acetoxy-11 beta-(4-N, N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione solid; and c) carrying out recrystallization on the acquired solid. The compound used as a new oral emergency contraception can be taken in 120 h after unprotected sexual intercourse of women without a reduction of emergency contraception effect with the delay of the time of using drugs, and has good safety and survivability simultaneously.
Owner:SICHUAN UNIV
Novel steroid compound, and preparation method and application thereof
The invention relates to a steroid compound, and a preparation method and application of the compound. The traditional method for preparing 17alpha-acetoxyl-11beta-(4-N,N-dimethylaminophenyl)19- norpregna -4,9-diene-3,20-diketone has the problems of low yield, incapability of industrial production and the like. The invention provides a novel steroid compound, and the preparation method and the application thereof. The steroid compound can be used for preparing 17alpha-acetoxyl-11beta-[4-(N,N-dimethylamino)-phenyl]-19- norpregna -4,9-diene-3,20-diketone. The preparation method is high in yield, mild in reaction condition, simple and easy to obtain raw materials and easy for industrial production.
Owner:CHINA RESOURCES ZIZHU PHARMA
Preparation method of dydrogesterone intermediate
ActiveCN111171101AConcentrated luminous bandsHigh yieldChemical recyclingKetal steroidsPhotocatalytic reactionUltraviolet
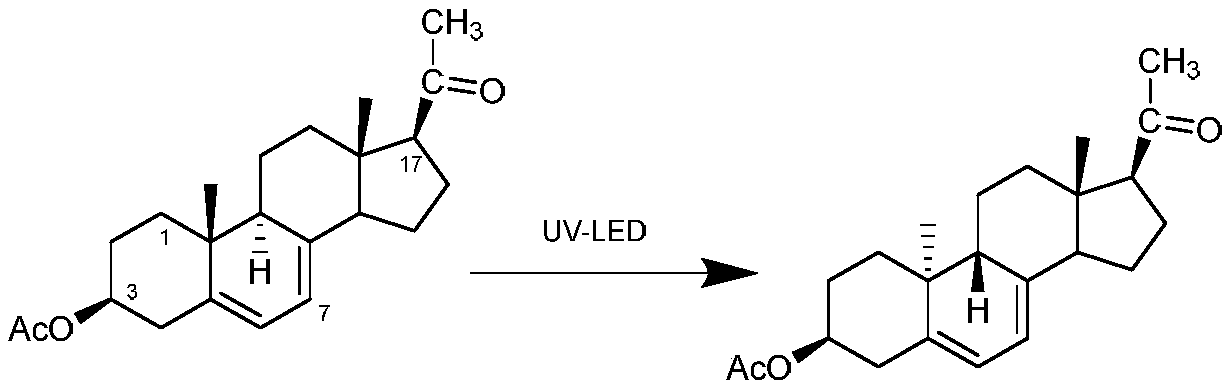
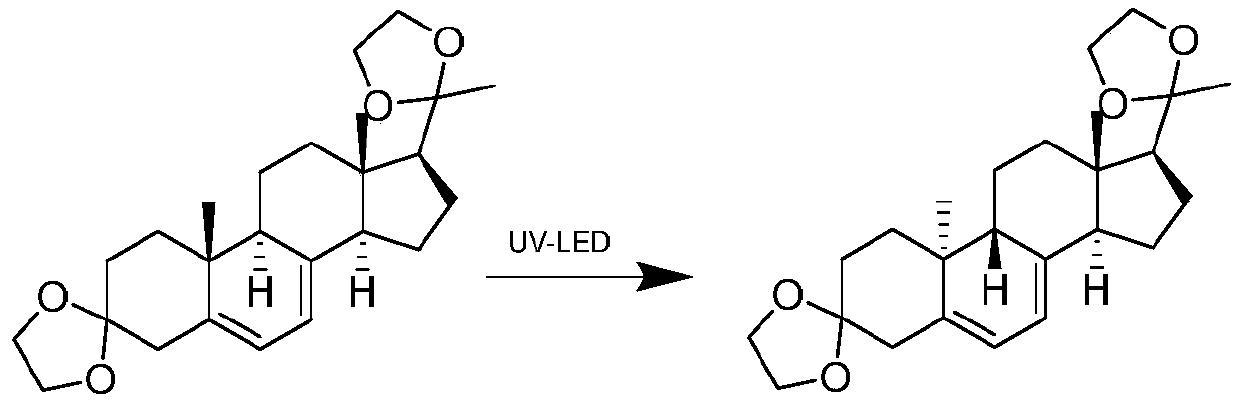
The invention discloses a preparation method of a dydrogesterone intermediate. The preparation method is characterized by comprising the following steps: dissolving 5,7-diene steroid compound in an organic solvent to obtain a solution as a raw material; and carrying out a photocatalytic reaction and separating to obtain the dydrogesterone intermediate, wherein the 5,7-diene steroid compound is 7-dehydropregnenolone acetate, pregna-5,7-diene-3,20-diketodivinyl ketal, 7-dehydropregnenolone, ergosterol or pregna-5,7-diene-3,20-diketo-3-vinyl ketal, and a lamp used in the photocatalytic reaction comprises an LED ultraviolet lamp with the wavelength range of 295-335 nm. The preparation method of the dydrogesterone intermediate is high in yield, low in cost, safer in preparation and friendlier to environment.
Owner:上海璟兆实业有限公司
Preparation method of steroid intermediate
The invention discloses a preparation method of a steroid intermediate. The preparation method comprises the following steps: adding a first solvent, a first acid solution, water and 17-alpha-hydroxypregnenolone steroid-4,9-diene-3,20-diketone acetate into a first reaction tank, and uniformly stirring; adding a bromination agent in four times at 2-4 DEG C, maintaining the temperature for reactingfor 2h, and carrying out thin-layer chromatography analysis until the reaction of the raw materials is finished; and regulating the pH value to be more than 7, regulating the pH value back to 6, carrying out reduced pressure concentration until the first solvent is completely concentrated, adding water, cooling to 5 DEG C, and carrying out centrifugation, cleaning, spin-drying and drying, so as toobtain the steroid intermediate, namely cortisone. According to the preparation method, an oxidant and a catalyst which contain heavy metal ions are not used, the used solvent can be recycled and reused, wastewater does not contain heavy metal ions in the production process, the method belongs to an environment-friendly process and has very good process prospects, and the yield and purity of theproduct are high.
Owner:JIANGSU YUANDA XIANLE PHARMA
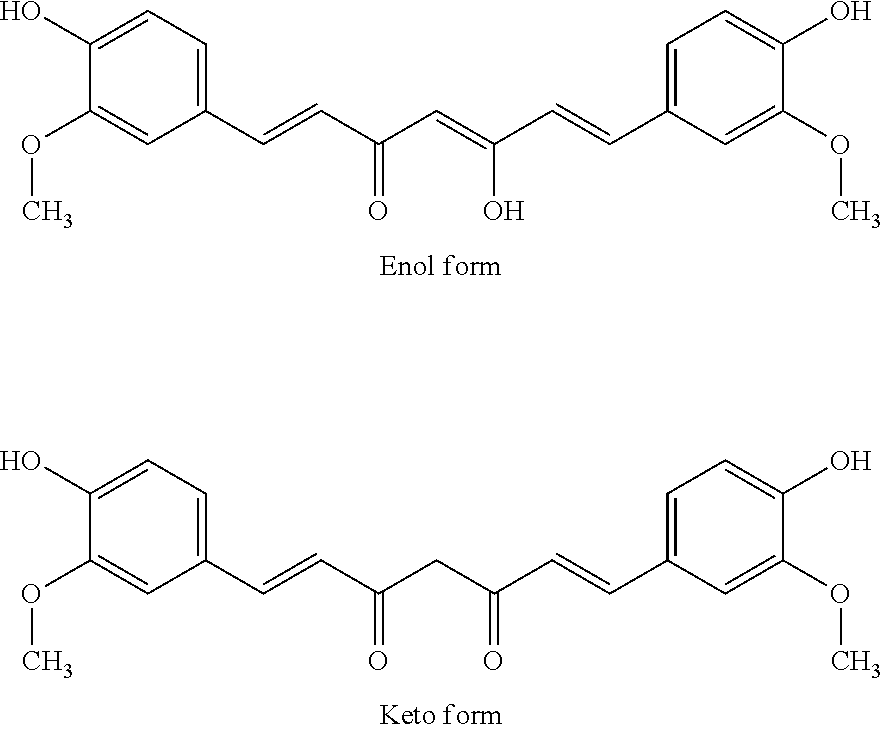
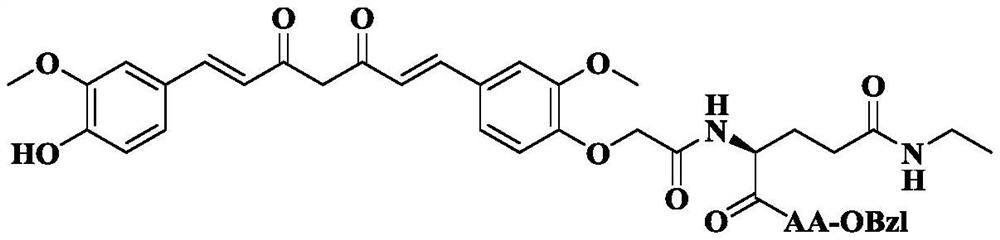
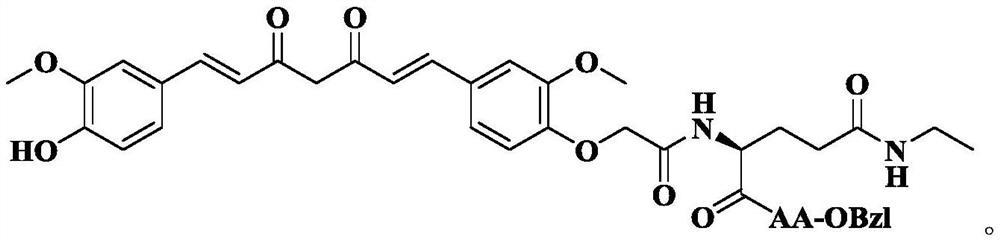
Phenylglycyl amino acid benzyl ester modified curcumin, synthesis, activity and application thereof
The invention discloses 1-(4)-Hydroxy-3-Methoxyphenyl)-7- Oxyacetylcarbamoyl-AA-OBzl-Methoxyphenyl)-1,6-Heptadiene-3,5-diketone of the following formula: (wherein AA is selected from L-Lys residue, L-Asn residue, L-Pro residue, L-Gln residue, L-Ser residue and L-Thr residue), the preparation methods are disclosed, and the antitumor growth activities are disclosed, the anti-inflammatory activitiesare disclosed, thus the use in the preparation of anti-tumor drugs and anti-inflammatory drugs is disclosed. The formulas are shown in the description.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Preparation method for prednisone acetate and intermediate of same
The invention relates to the field of preparation of steroid drugs and an intermediate of the same, and in particular relates to a preparation method for prednisone acetate. The method comprises the steps of taking 11 alpha, 17 alpha-dyhydroxy Pregnene-1,4-diene-3,20-dione as an initial material, oxidizing to obtain 17 alpha-hydroxy Pregnene-1,4-diene-3,11,20-trione, and carrying out iodization reacting to obtain the prednisone acetate. The invention provides a novel oxidation technology more suitable for production of the intermediate of the prednisone acetate, the synthesis path has the characteristics of low cost and simple operation, environment pollution pressure can be greatly reduce, and the yield and quality of the prednisone acetate can reach a satisfactory level.
Owner:JIANGSU YUANDA XIANLE PHARMA
Method for manufacturing cyproterone acetate intermediate 17a-hydroxy progesterone-1, 4, 6-triene-3, 20-diketone
InactiveCN103290087ADDQ is highly toxicEasy to handleMicroorganism based processesFermentationBiotechnologyChemical synthesis
The invention relates to a method for manufacturing a cyproterone acetate intermediate 17a-hydroxy progesterone-1, 4, 6-triene-3, 20-diketone. The method includes performing microbial fermentation and dehydrogenation on 17a-hydroxy progesterone-4, 6-diene-3, 20-diketone to obtain the 17a-hydroxy progesterone-1, 4, 6-triene-3, 20-diketone. Microbial fermentation and dehydrogenation culture media comprise 1.2-1.6(wt)% of glucose, 1.8-2.6(wt)% of corn steep liquor, 0.4-0.6(wt)% of peptone, 0.4-0.7(wt)% of potassium dihydrogen phosphate, 0.01(wt)% of defoamers and the balance water, and the pH (potential of hydrogen) of a mixture of the glucose, the corn steep liquor, the peptone and the potassium dihydrogen phosphate is regulated by a proper amount of sodium hydroxide and then ranges from 6.0 to 6.5. The method has the advantages that the microbial fermentation and dehydrogenation reaction selectivity is high, a product is free of side effects, the purity of the product is 97%, the content of the maximum impurity component is 1.5%, the yield of the product is greatly increased and reaches 80%, and environmental pollution is obviously reduced as compared with the traditional chemical synthesis process.
Owner:YUEYANG HUANYU PHARMA
Preparation method of high-purity hydrocortisone
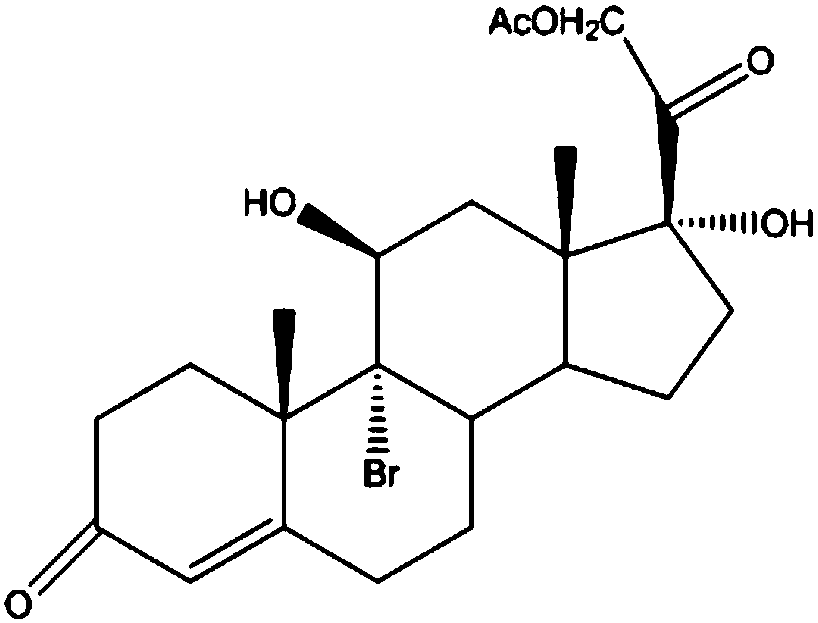
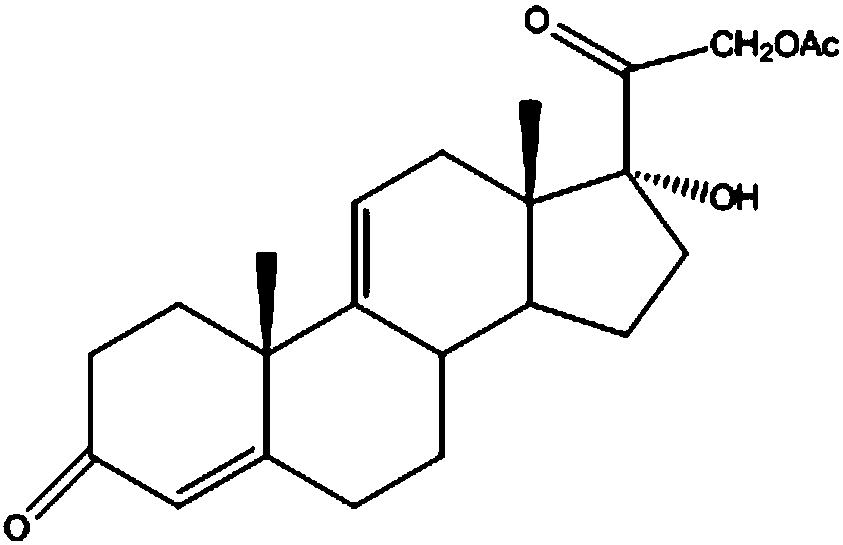
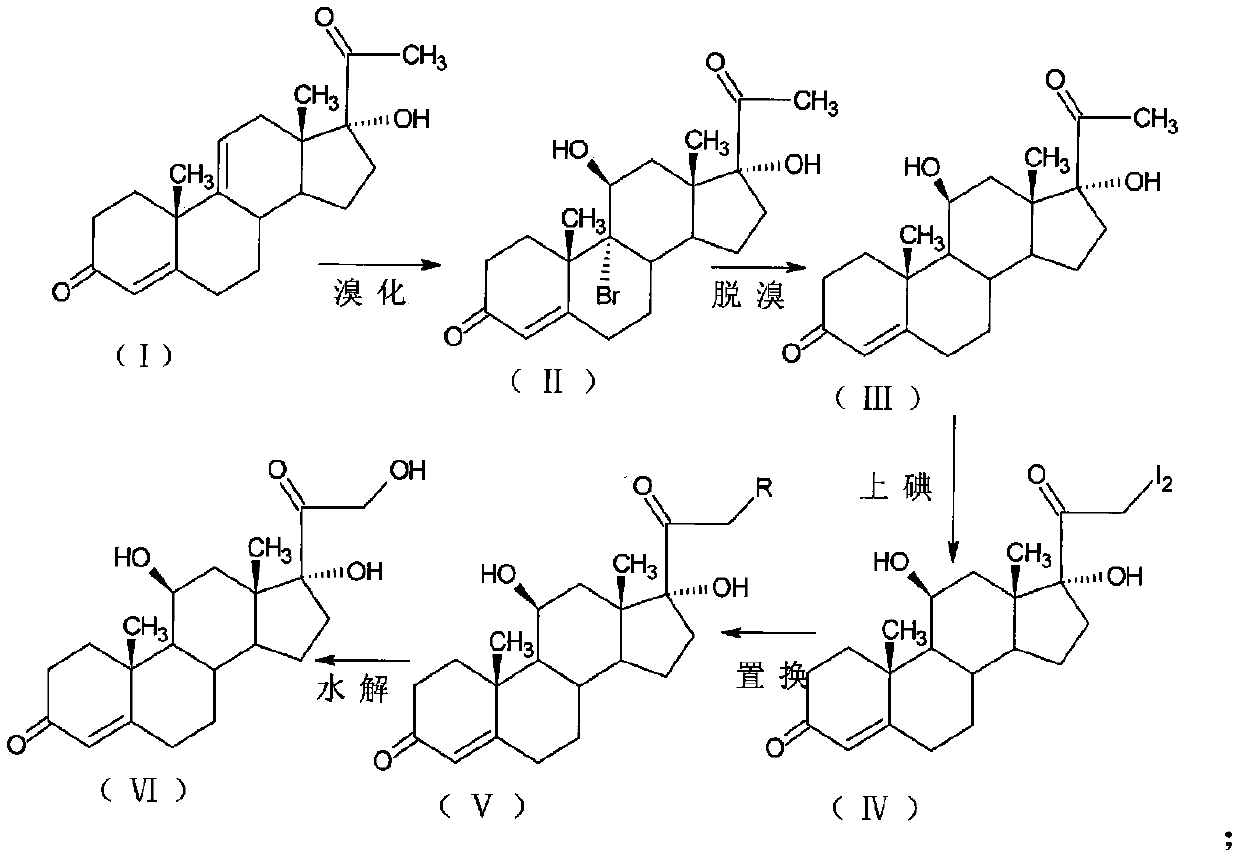
The invention discloses a preparation method of high-purity hydrocortisone, and belongs to the technical field of preparation and processing of medicines. According to the method, 17alpha-hydroxypregna-4, 9 (11)-diene-3, 20-diketone-21-acetate is used as a starting material, and the high-purity hydrocortisone is prepared through three steps of bromo-hydroxyl, debromination and hydrolysis. According to the preparation method of the high-purity hydrocortisone disclosed by the invention, the reaction process can be effectively shortened by improving the defects of a traditional process, the generation of impurities during the reaction process is controlled, the use of iodine with characteristics of high toxicity and environmental unfriendliness is avoided, the environmental pollution is reduced, and the method has characteristics of high overall conversion rate, simple operation and wide market prospect, and is suitable for industrial production.
Owner:ZHEJIANG SHENZHOU PHARMA
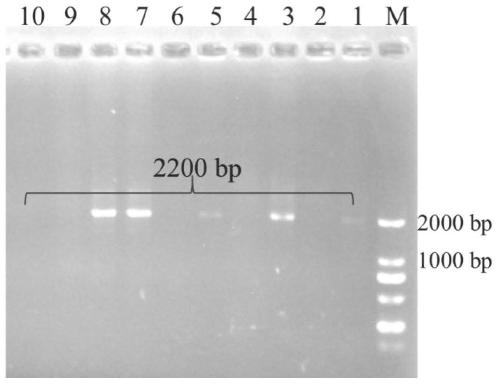
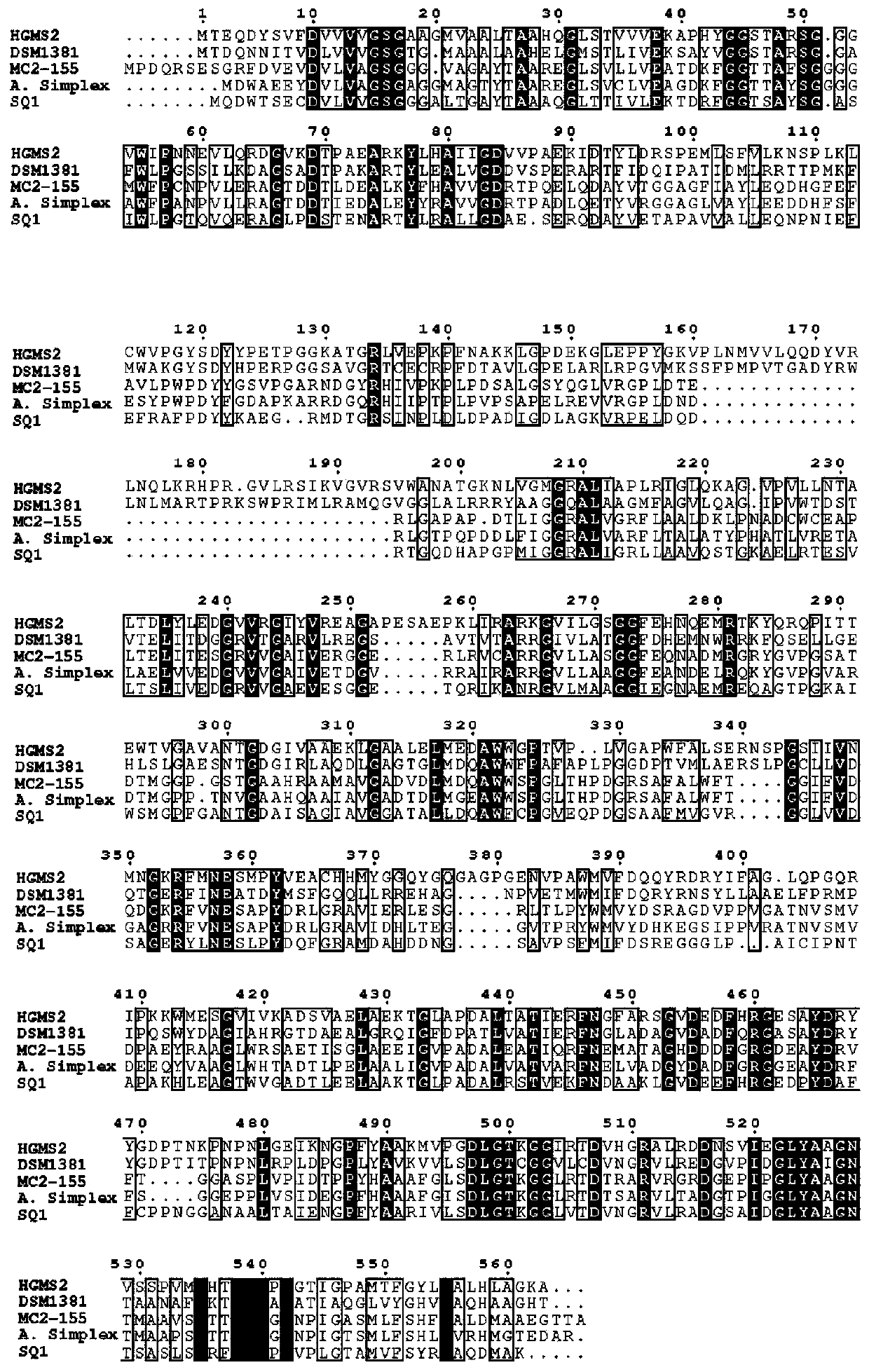
3-sterone-1, 2-dehydrogenase as well as gene sequence and application thereof
ActiveCN111500600AImprove solubilityHas high enzymatic activityOxidoreductasesFermentationEscherichia coliAndrostane
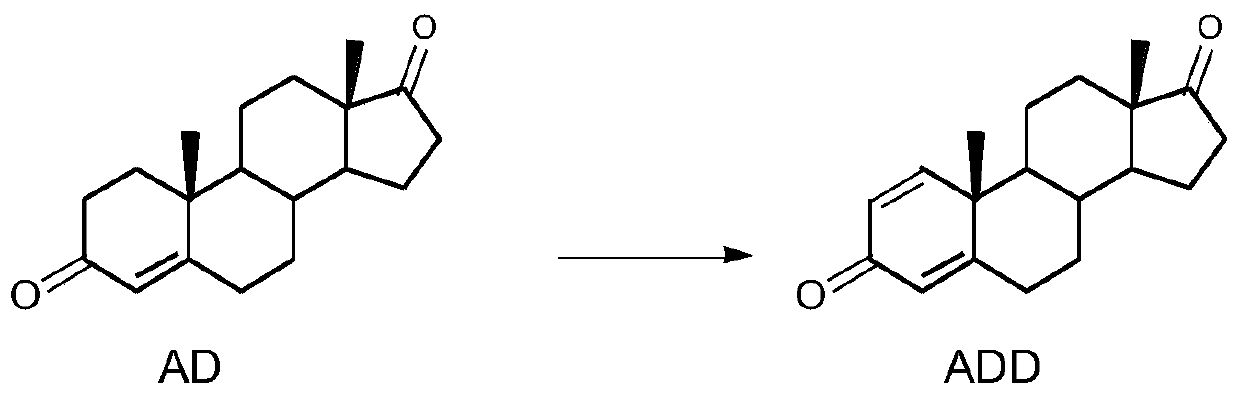
The invention provides 3-sterone-1, 2-dehydrogenase as well as a gene sequence and application thereof. The invention discloses a gene sequence for expressing 3-sterone-1, 2-dehydrogenase, the gene sequence is shown as SEQ ID. 4, and the gene sequence is used for expression in escherichia coli. The 3-ketosterone-1, 2-dehydrogenase disclosed by the invention can be used for efficiently catalyzing androstane-4-ene-3, 17-dione (4-AD) reaction to generate androstane-1, 4-diene-3, 17-dione (ADD), and has high enzymatic activity. In addition, the soluble 3-sterone-1, 2-dehydrogenase is obtained, andthe solubility of the enzyme is improved.
Owner:武汉艾默佳华生物科技有限公司
Preparation method for [17alpha, 16alpha-d] methyl oxazoline
The invention discloses a preparation method for a denazacort key intermediate [17alpha, 16alpha-d] methyl oxazoline steroids. The steps include: dissolving [16, 17alpha-epoxy-pregnane-20-methyl formate hydrazine acetyl-1,4-diene-3,11-diketone] in trichloromethane, adding [16, 17alpha-epoxy-pregnane-20-methyl formate hydrazine acetyl-1,4-diene-3,11-diketone] and additives into a pressure reaction kettle, leading ammonia to the reaction kettle to a certain pressure under the condition of stirring, and reacting at certain temperature; and dissolving the obtained compound crude products in glacial acetic acid, adding a certain amount of anhydride under the condition of stirring, and controlling reaction temperature. After the reaction, reaction liquid is poured into 500mL of cold 10% sodium hydroxide solution and stirred for 1 hours, and the denazacort key intermediate [17alpha, 16alpha-d] methyl oxazoline steroids is obtained after suction filtration. The method achieves safe and clean production of denazacort, is favorable for reducing environment pollution, shortens a production period, reduces production cost for enterprises, improves production safety, and is remarkable in social benefit, environment benefit and economical benefit.
Owner:JIANGXI JUNYE BIOLOGICAL PHARM CO LTD
3-sterone-1,2-dehydrogenase and application thereof
The invention provides 3-sterone-1,2-dehydrogenase and an application thereof. An amino acid sequence of the 3-sterone-1,2-dehydrogenase is as shown in SEQ ID.2. The KsdD211 of the invention can catalyze a reaction of androsta-4-ene-3,17-dione (4-AD) to produce androst-1,4-diene-3,17-dione (ADD, 1,4-androstenedione), and the TLC analysis verifies the high enzymatic activity of the KsdD211. The HPLC analysis of the KsdD211catalytic reaction shows that the KsdD211 completely converts 4-AD into ADD within 18 h.
Owner:HUBEI UNIV OF TECH
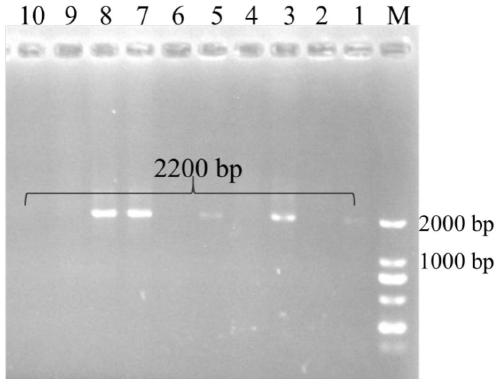
Method for producing androstadienedione by degrading phytosterol through microorganisms
InactiveCN111808830ARich genetic resourcesHigh purityBacteriaMicroorganism based processesBiotechnologyPlant sterol
The invention relates to the technical field of biology, and provides a method for producing androstadienedione by degrading phytosterol through microorganisms. According to the invention, a new 3-sterone-1-dehydrogenase gene is cloned from Mycobacterium neoaurum DSM 1381 for the first time; through genetic engineering means, recombinant bacteria capable of efficiently expressing 3-ketosterol-1-dehydrogenase are obtained, and when the concentration of substrate phytosterol in a conversion culture medium is as high as 5g / L, the purity of the product androstadienedione in a conversion solution can be 95% or above, so that the residue of the byproduct androstenedione is effectively reduced, and the purity of the product androstadienedione is improved.
Owner:SHANGHAI ADVANCED RES INST CHINESE ACADEMY OF SCI
Preparation method of high-quality prednisone acetate and intermediate thereof
InactiveCN110862431AHigh feeding concentrationGood conversion effectMicroorganism based processesSteroidsHydrocortisoneDehydrogenation
The invention discloses a preparation method of high-quality prednisone acetate and an intermediate thereof. The method includes the steps of: taking epihydrocortisone as the raw material, firstly conducting microbial dehydrogenation to obtain 11alpha, 17alpha, 21-trihydroxy-pregn-1, 4-diene-3, 20-dione intermediate, then carrying out esterification reaction to obtain 11alpha, 17alpha, 21-trihydroxy-pregn-1, 4-diene-3, 20-dione-21-acetate, and finally carrying out oxidation reaction to obtain prednisone acetate. The method provided by the invention solves the technical difficulty of non-idealintroduction of C1, 2 double bond in the traditional fermentation production process of prednisone acetate. The cortisone acetate prepared by the method provided by the invention has high quality andextremely low impurity content, greatly improves the multidirectional application of prednisone acetate, and meanwhile, the process route has the characteristics of low cost and simple operation.
Owner:HUAZHONG PHARMA
Biological dehydrogenation method of androstenedione C1 and 2 loci
ActiveCN110656147AReduce degradation rateStrong response specificityBacteriaMicroorganism based processesBiotechnologyDehydrogenation
The invention relates to production methods of steroid medicine intermediates, and specifically relates to a biological dehydrogenation method of androstenedione C1 and 2 loci. The biological dehydrogenation method of the androstenedione C1 and 2 loci comprises the following steps: taking 4-androstene-3,17-dione as a substrate and simple nocardia bacterium fluid as an enzyme source, adding soybeanoil at a concentration of 100-200 ml / L and tween-80 at a concentration of 0.1-2 g / L into a transformation system, and carrying out transformation reaction at 29-31 DEG C until completion of the reaction so as to obtain a reaction product; and then, separating and purifying the reaction product so as to obtain the 1,4-androdiene-3,17-dione. On the basis of adopting a common direct conversion method, the soybean oil and the tween-80 are added according to the biological dehydrogenation method of the androstenedione C1 and 2 loci, so that conversion rate can be greatly improved that the conversion rate can be up to 96% or above; and moreover, the product is low in degradation rate, high in reaction specificity, relatively fast in conversion time and high in product quality. In addition, sterile environment is not required that only routine outdoor operation is enough; and thus, the biological dehydrogenation method of the androstenedione C1 and 2 loci is easy to operate, and suitable forindustrial large-scale production.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
Method for synthesizing desonide impurity
The invention discloses a method for synthesizing desonide impurity 11 [beta], 16 [alpha], 17 [alpha]-trihydroxypregna-1, 4-diene-3, 20-dione-21 aldehyde-16, 17-acetal acetone. The method comprises the following steps: taking desonide as an initial raw material, catalyzing by using anhydrous cupric sulfate, introducing air to oxidize, carrying out hydrolysis reaction under the catalysis of acid, and drying by using sodium sulfate to obtain the desonide impurity 11 [beta], 16 [alpha], 17 [alpha]-trihydroxypregna-1, 4-diene-3, 20-dione-21 aldehyde-16, 17-acetal acetone. The synthesis route is short, the reaction condition is mild, the impurity can be obtained without column chromatography, and the purity of the obtained impurity is 97% or above.
Owner:CHONGQING HUABANGSHENGKAI PHARM
1,7-diaryl-1,6-heptadiene-3,5-dione derivatives, methods for the production and use thereof
ActiveUS11191855B2Inactivate microorganisms more efficientlyEfficient inactivationAntibacterial agentsBiocideDiketoneAryl
Owner:UNIVSKLINIKUM REGENSBURG +1
A kind of preparation method of high-purity fluocinolone
The invention provides a preparation method of high-purity fluocinolone acetonide. The preparation method comprises the following steps: (1) performing hydrolysis reaction on 11 beta-hydroxyl-16 alpha,17-[(1-methyl ethylidene)-bis (oxygen)]-21-(acetoxy)-6 alpha,9-difluorobenzene-1,4-diene-3,20-diketone; (2) refining a 11 beta-hydroxyl-16 alpha,17-[(1-methyl ethylidene)-bis (oxygen)]-6 alpha,9-difluorobenzene-1,4-diene-3,20-diketone crude product to obtain a 11 beta-hydroxyl-16 alpha,17-[(1-methyl ethylidene)-bis (oxygen)]-6 alpha,9-difluorobenzene-1,4-diene-3,20-diketone I crystal; (3) refining the 11 beta-hydroxyl-16 alpha,17-[(1-methyl ethylidene)-bis (oxygen)]-6 alpha,9-difluorobenzene-1,4-diene-3,20-diketone I crystal to obtain a fluocinolone acetonide finished product.
Owner:ZHEJIANG REACHALL PHARMA
A method for treating 16α-methylandrost-4,9(11)-diene-3,17-dione mother liquor
ActiveCN110745848BAmino compound purification/separationOrganic compound preparationDistillationOrganosolv
The embodiment of the present invention provides a method for processing 16α-methylandrosta-4,9(11)-diene-3,17-dione mother liquor, the method comprising: adding a sufficient amount of carbonic acid to the mother liquor The salt is subjected to a precipitation reaction, and the precipitate is taken out after the reaction is complete to obtain crude lithium carbonate; the solution after the precipitation is taken out is distilled to a thick state, and then the thick liquid is filtered to obtain a filter residue and a filtrate; the filter residue is put into a strong alkali solution After mixing, leave it still to obtain a layered solution; take the upper layer of the layered solution to distill to obtain diisopropylamine; take the lower layer of the layered solution to pass through chlorine gas and add an organic solvent for extraction reaction to obtain bromine; The aqueous solution obtained after the extraction reaction is distilled together with the filtrate to obtain solid waste containing organic phosphorus. The embodiment of the present invention can effectively recover diisopropylamine, lithium, bromine and water in the 16α-methylandroster-4,9(11)-diene-3,17-dione mother liquor, and can efficiently remove the mother liquor organophosphorus in.
Owner:SHANDONG SITO BIO TECHNOLOGY CO LTD +1
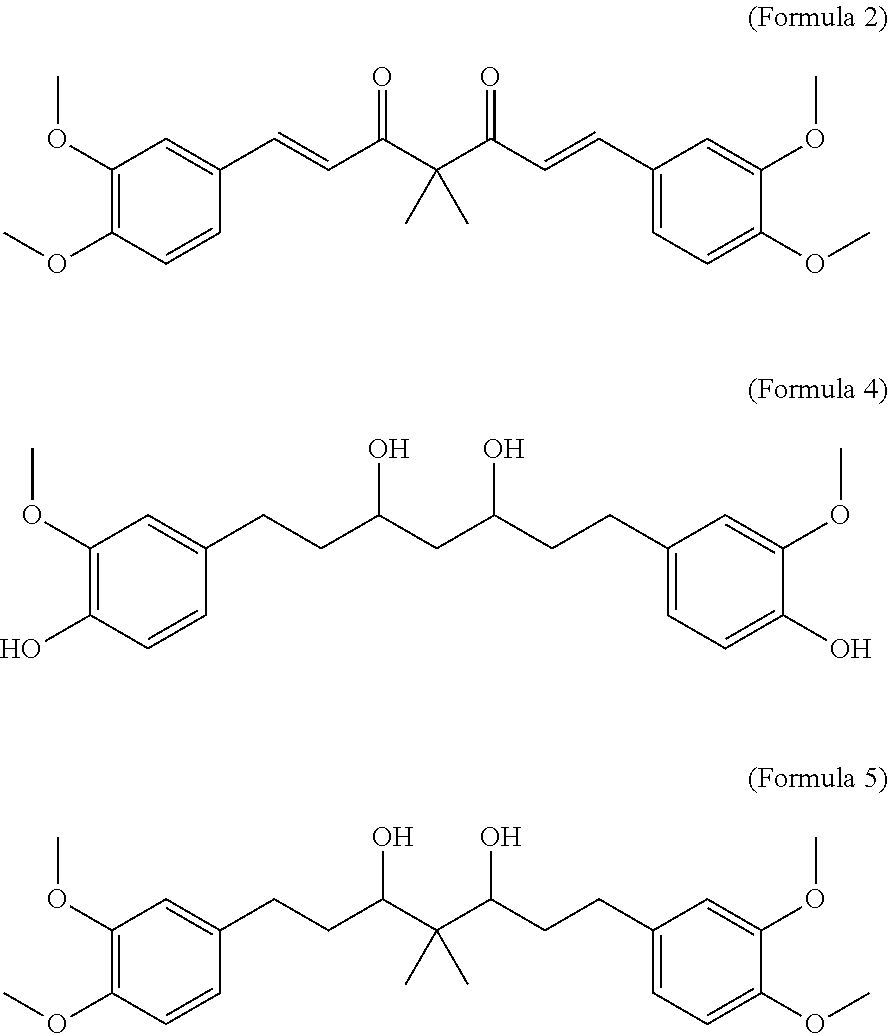
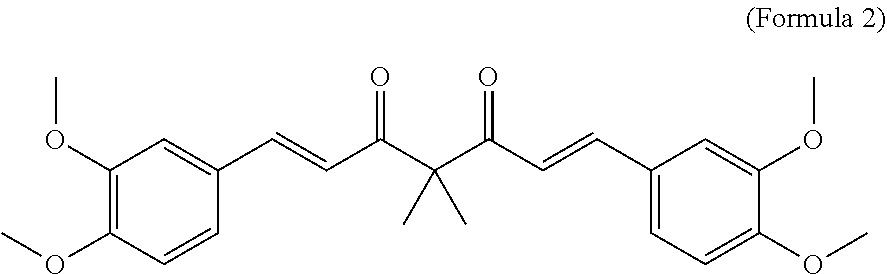
A personal care composition
Disclosed is a personal care composition and a method of providing antiperspirant and anti-inflammation using certain curcuminoid derivatives. The composition comprises: (i) a compound of the Formula 1 Ar—CHnCHn—X.C(R)2—X.CHnCHn—Ar (Formula 1) wherein Ar is a substituted or unsubstituted phenyl group; R is H or CH3; X is CH(OH) group or C═O group; n has the value 1 or 2; and, (ii) a topically acceptable base comprising at least 0.1% of a fragrance wherein, when n=1, the compound of (Formula 1) is 1E,6E)-1,7-bis(3,4-dimethoxyphenyl)-4,4-dimethylhepta-1,6-diene-3,5-dione (Formula 2), and when n=2, the compound of (Formula 1) is 1,7-bis(4-hydroxy-3-methoxyphenyl) heptane-3,5-diol (Formula 4) or is 1,7-bis (3,4-dimethoxyphenyl)-4,4-dimethylheptane-3,5-diol (Formula 5).
Owner:CONOPCO INC D B A UNILEVER
Method of acquiring high-purity 17 alpha-acetoxy-11 beta-(4-N, N-dimethylaminophenyl)-19-norpregna-4, 9-diene-3, 20-dione
Owner:SICHUAN UNIV
Preparation of 11β-hydroxy-1,4-diene-3,20-dione steroids by joint fermentation of Absidia and Arthrobacter
ActiveCN105779555BHigh yieldReduce stepsMicroorganism based processesFermentationArthrobacterDienedione
Owner:TIANJIN JINYAO GRP
A new sterone c27-monooxygenase derived from mycobacterium aureus and its application
ActiveCN106282080BIncrease productionImprove productivityBacteriaMicroorganism based processesMicroorganismSide chain
The invention discloses a new sterone C27-monooxygenase derived from Mycobacterium aureus and its application, and belongs to the technical fields of genetic engineering and enzyme engineering. The present invention uses methods of gene knockout and enhanced expression to screen out three isoenzymes of SMO, the key enzyme in the degradation process of sterol side chains, in Mycobacterium aureus. They were respectively expressed in Mycobacterium aureus, which has high production of androstadienedione (ADD), and the ADD production was significantly improved, among which SMO2 had the most obvious effect. By overexpressing SMO2, the final yield of ADD was increased from 5.2g / L to 7.3g / L. The present invention provides useful guidance for the industrialization of microbial fermentation to improve ADD production.
Owner:JIANGNAN UNIV
A kind of preparation method of cortisone acetate
ActiveCN108373492BLow priceMild reaction conditionsKetal steroidsPtru catalystThin layer chromatographic
Owner:JIANGSU YUANDA XIANLE PHARMA
Momertasone furoate intermediate 21-hydroxyl preparing process
ActiveCN100389121CChange the situation of complete dependence on importsRealize localizationSteroids preparationEpoxyKetone
The present invention is compound 9beta, 11beta-epoxy-17alpha, 21-dihydroxy-16alpha-methyl-1, 4-pregnanediene-3,20-dione, as the momertasone furoate intermediate, and its production process. The intermediate 9beta, 11beta-epoxy-17alpha, 21-dihydroxy-16alpha-methyl-1, 4-pregnanediene-3,20-dione is prepared through hydrolyzing 9beta, 11beta-epoxy-17alpha, 21-dihydroxy-16alpha-methyl-1, 4-pregnanediene-3,20-dione, 21-ester (compound III) and the preparation process includes the following steps: dissolving compound III in methanol completely; dropping NaOH-MeOH solution after lowering the tempera ture to 0-5 deg.c to produce TLC tracking reaction and stopping dropping after finishing reaction; filtering and concentrating the filtrate to separate out white solid; filtering and drying to obtain the target product.
Owner:天津药业集团有限公司
Preparation method of dehydroxymethasone intermediate
The invention provides a preparation method of a dehydroxymethasone intermediate, which comprises the following steps: by taking 21-hydroxypregna-1, 4, 9 (11), 16-tetraene-3, 20-diketone-21-acetate as a starting material, sequentially carrying out bromine hydroxylation, epoxidation, methylation and hydrolysis reaction, so as to obtain the dehydroxymethasone intermediate 9beta, 11 beta-epoxy-21-hydroxy-16 alpha-methyl progesterone-1, 4-diene-3, 20-diketone. The preparation process route provided by the invention has fewer reaction steps, the quality and yield of the product have obvious competitiveness, dangerous chemical processes are not involved, the method is green and clean, and the atom economy is improved; the preparation process has a wide industrial application prospect.
Owner:JIANGSU YUANDA XIANLE PHARMA +1
Theanyl amino acid benzyl ester modified curcumin, its synthesis, activity and application
The invention discloses 1-(4-hydroxyl-3-methoxyphenyl)-7-(4-oxyacetyltheanyl-AA-OBzl-3-methoxyphenyl)-1,6 -Heptadiene-3,5-dione (where AA is selected from L-Lys residues, L-Asn residues, L-Pro residues, L-Gln residues, L-Ser residues and L-Thr residues residues), disclose their preparation method, disclose their anti-tumor growth activity, and disclose their anti-inflammatory activity, thus the present invention discloses their application in the preparation of anti-tumor drugs and anti-inflammatory drugs.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Preparation method of 9β, 10α-pregna-4,6-diene-3,20-dione
The invention relates to a preparation method of 9β,10α-pregnant-4,6-diene-3,20-dione. The preparation method uses 9β,10α-pregnant-5,7-diene-3,20-dione diacetal (compound A) as raw material, and deprotects it under acidic conditions to obtain 9β,10α-pregnant- 4,7-diene-3,20-dione (compound B), and then rearranged under basic conditions to give 9β,10α-pregnantrol-4,6-diene-3,20-dione (compound C ). The method of the invention is simple, environmentally friendly and has extremely high yield.
Owner:TAIZHOU HISOUND PHARMA CO LTD
1,7-diaryl-1,6-heptadiene-3,5-dione derivatives, methods for the production and use thereof
PendingUS20220054671A1Inactivate microorganisms more efficientlyEfficient inactivationAntibacterial agentsBiocideDiketoneAryl
Owner:UNIVSKLINIKUM REGENSBURG +1
Theanyl amino acid benzyl ester modified curcumin, its synthesis, activity and application
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00221.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00231.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00232.PNG)









![Preparation method for [17alpha, 16alpha-d] methyl oxazoline Preparation method for [17alpha, 16alpha-d] methyl oxazoline](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/28dd9628-42fb-4f2b-802e-1bfb286190a9/88406DEST_PATH_IMAGE008.PNG)
![Preparation method for [17alpha, 16alpha-d] methyl oxazoline Preparation method for [17alpha, 16alpha-d] methyl oxazoline](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/28dd9628-42fb-4f2b-802e-1bfb286190a9/899868DEST_PATH_IMAGE001.PNG)