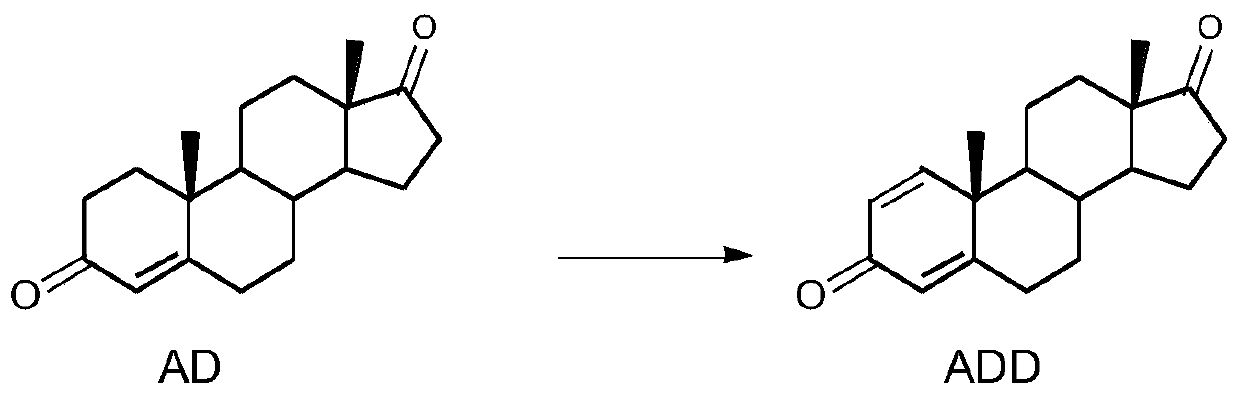
Biological dehydrogenation method of androstenedione C1 and 2 loci
A kind of androstenedione and biological technology, applied in the field of biological dehydrogenation of androstenedione C1, 2, can solve the problems of low conversion rate, complicated operation, high degradation rate, etc., and achieve fast conversion time, simple operation and high degradation low rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 Simple Nocardia seed culture
[0025] Species: Nocardia simplex
[0026] Incline medium: 0.1-1% glucose, 0.1-1% corn steep liquor, 0.1-0.5% peptone, 0.01-0.15% potassium dihydrogen phosphate, 0.01-0.05% yeast extract, 0.01-2% agar powder; pH 7.0 -7.2. Culture conditions: 25-40°C, 1-5d.
[0027] Primary seed medium: 0.1-1% glucose, 0.1-1% corn steep liquor, 0.1-0.5% peptone, 0.01-0.15% potassium dihydrogen phosphate, 0.01-0.05% yeast extract; pH 7.0-7.2. Culture conditions: 100ml culture medium in 500ml shake flask, shake culture, rotation speed 50-200rpm, 25-40℃, culture time 10-72h.
[0028] Secondary seed medium: glucose 0.1-1%, corn steep liquor 0.1-1%, peptone 0.1-0.5%, potassium dihydrogen phosphate 0.01-0.25%, 4-androstene-3,17-dione 0.05% and foam enemy 0.02%; pH 7.0-7.2. Culture conditions: 100ml culture medium in 500ml shake flask, shake culture, rotation speed 50-200rpm, 25-40℃, culture time 10-72h.
[0029] OD in cultured Nocardia simplex ...
Embodiment 2
[0030] Embodiment 2 shakes flask transformation
[0031] Seed culture was carried out according to Example 1, the primary seed solution was inserted into the secondary seed medium for 10 hours, 2g4-AD was added to the cultured 100ml secondary seeds, and transformed for 72 hours, transformation conditions: 200rpm, 30±1°C. After the conversion, the sample was sent to the liquid phase, the normalized content of ADD was 82.19%, and the normalized content of 4-AD was 17.18%.
Embodiment 3
[0032] Embodiment 3 shake flask transformation
[0033] Carry out seed cultivation according to Example 1, insert the primary seed liquid into the secondary seed medium and cultivate for 16 hours, add 2 g of 4-AD crushed to 200 meshes into the cultivated 100 ml secondary seeds, transform for 72 hours, transformation conditions: 200 rpm, 30±1°C. After the conversion, the sample was sent to the liquid phase, the normalized content of ADD was 85.16%, and the normalized content of 4-AD was 14.49%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com