Momertasone furoate intermediate 21-hydroxyl preparing process
A hydroxyl and dihydroxy technology, applied in the preparation of steroids, chemical instruments and methods, steroids, etc., can solve the problems of relying on imports, structure and physical and chemical properties that have not been reported, and achieve the effect of localization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Compound 9β, 11β-epoxy-17α, 21-dihydroxy-16α-methyl-1,4-pregnadiene-3,20-dione provided by the present invention consists of 9β, 11β-epoxy-17α, 21 -Dihydroxy-16α-methyl-1,4-pregnadiene-3,20-dione, 21-ester [compound 3] is prepared by hydrolysis, the specific process is as follows:
[0022] Add 0.5g of compound 3, 13mL of methanol and start stirring, continue to add methanol until compound 3 is completely dissolved, drop the temperature at 0-5°C and add NaOH-MeOH solution dropwise, follow the reaction with TLC, stop the dropwise addition after the reaction is complete, stop stirring, Filtration, the filtrate was concentrated to precipitate a white solid, which was filtered and dried to obtain the finished compound.
[0023] Pass the obtained finished compound through a chromatographic column, the mobile phase is chloroform:methanol=10:3, and obtain the refined compound.
Embodiment 2
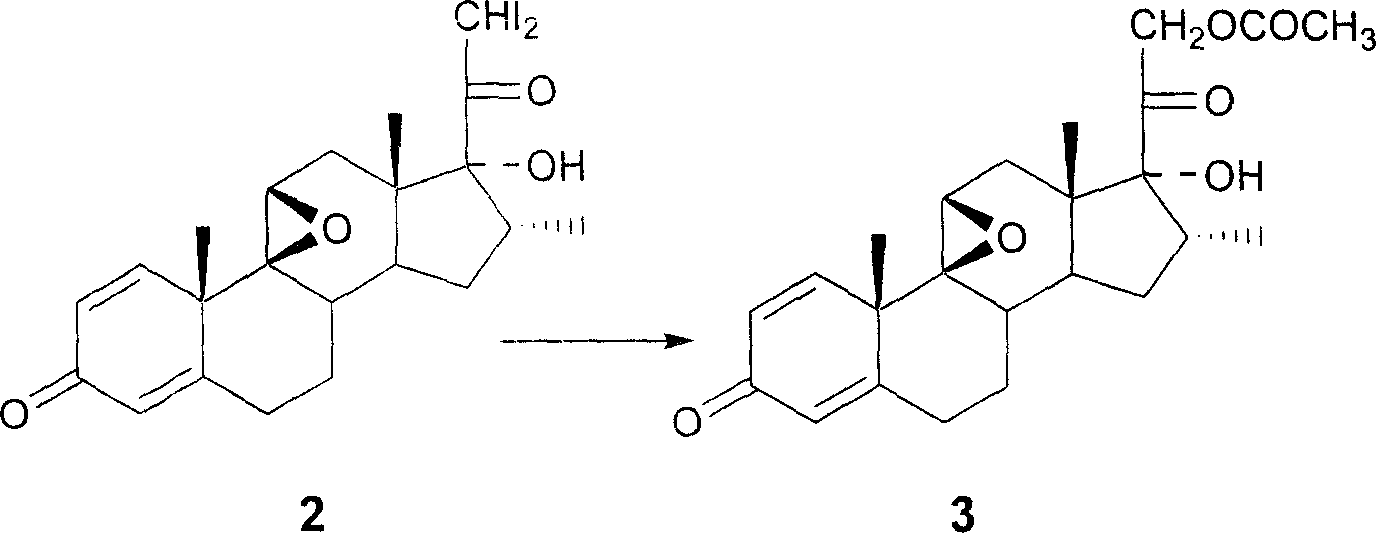
[0025] The above compound 3 is obtained by substitution of 9β, 11β-epoxy-17α-hydroxyl-16α-methyl-21-iodo-1,4-pregnadiene-3,20-dione [compound 2], the specific process as follows:
[0026] Dissolve 3.0g of iodide (compound 2) and 4.0g of potassium acetate in 15mL of DMF at room temperature, and then raise the temperature according to a gradient: 1 hour to 30°C → 1 hour to 40°C → 1 hour to 50°C, at 50 ℃ for another 1 hour to cool down for post-treatment to obtain the crude product of compound 3.
[0027] The crude product of compound 3 was passed through the chromatographic column, and the mobile phase was chloroform:methanol=5:1, and the final refined product compound 3 was obtained.
[0028] The chemical reaction formula is:
[0029]
[0030] Structural characterization of compound 3
[0031] Elemental Analysis C 24 h 30 o 6
[0032] Measured value (%): C69.42, H7.14;
[0033] Calculated (%): C69.56, H7.24.
[0034] Elemental analysis (MS (FAB)), infrared spectrosc...
Embodiment 3
[0036] The above-mentioned compound 2 was obtained by iodination of 9β, 11β-epoxy-17α-hydroxyl-16α-methyl-1,4-pregnadiene-3,20-dione (compound 1), and the specific process was as follows:
[0037]Add 6mL of methanol and 0.5g of calcium chloride, stir to dissolve, then add chloroform to dissolve 3.56g of compound 1, cool down to below 10°C after dissolution, add calcium oxide, and continue to drop to 0°C (0°C±2°C) . Add 2.55 g of iodine solution dropwise, and continue to insulate and stir for 1 hour after dropping. Then lower it below 0°C, add the pre-cooled ammonium chloride aqueous solution, stand still for 30 minutes after adding, filter the chloroform layer, extract the water layer with 12mL chloroform four times, combine the chloroform solution, concentrate to nearly dry, add 1.5mL methanol , continued to concentrate, and filtered to obtain light yellow solid compound 2.
[0038] The chemical reaction formula is:
[0039]
[0040] Structural characterization of compo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com