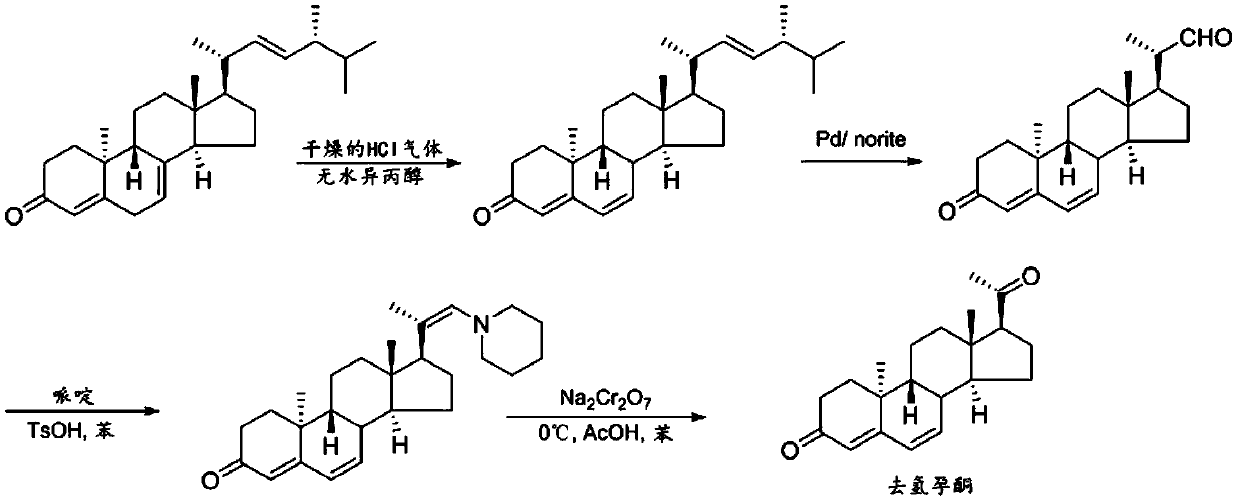
Preparation method of 9β, 10α-pregna-4,6-diene-3,20-dione
A technology of diketone and diene, which is applied in the field of preparation of steroidal compounds, can solve problems such as low yield, low total yield, and difficulty in obtaining
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of compound B
[0032] Under the protection of nitrogen, put 22g of 9β,10α-pregna-5,7-diene-3,20-dione diethylene diacetal (compound A) into 500mL of ethanol, add dropwise 40mL of 8.5% dilute sulfuric acid. After the dropwise addition, the mixture was refluxed for 1 h under the protection of nitrogen. After the reaction was completed, it was added to the ice-water mixture, filtered, and dried to obtain 16.2 g of compound B, melting point: 145-147°C, yield 94.40%, and content about 97.2%.
[0033] Take 4g of compound B and recrystallize twice with acetone-water to obtain about 2.0g of light yellow crystals, melting point: 147.5~148℃, specific rotation: [α] D 25 =+106~+114°, content: 99.21%.
[0034] IR: 870, 1232, 1419, 1464, 1618, 1663, 1701, 2965, 3464cm -1 .
[0035] 1 H NMR (δ, ppm, CDCl 3 ): 0.55(s,3H); 1.01(s,3H); 1.39-1.43(m,1H); 1.55-1.80(m,6H); 1.96-1.99(m,1H); 2.08(s,3H); 2.16-2.18 (m, 3H); 2.32-2.46 (m, 3H); 2.63-2.67 (m,...
Embodiment 2
[0037] Embodiment 2: the preparation of compound B
[0038] Under the protection of nitrogen, put 3g of compound A into 50ml of 50% acetic acid, stir and react at 60-65°C for 30min, then pour it into a large amount of ice water, filter to obtain the crude product, and recrystallize from acetone-water to obtain 1.8g of compound, B Melting point: 147-148°C. About 0.36 g of compound B was recovered from the mother liquor, melting point: 146-147.5°C. The total yield is about 92.31%.
Embodiment 3
[0039] Embodiment 3: the preparation of compound B
[0040] Put 5.0g of Compound A into 75mL of dichloromethane, add 25ml of 5% hydrochloric acid dropwise at 0-5°C, keep warm and stir for 1h, then pour it into a large amount of ice water, stir for 10min, then let stand to separate layers, and the water layer Extract again with 75 ml of dichloromethane. The dichloromethane layers were combined and washed with saturated NaHCO 3 After the aqueous solution was washed twice, it was concentrated under reduced pressure and dichloromethane was evaporated to dryness to obtain 3.7 g of compound B, melting point: 146.2-147.8°C, yield about 94.87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com