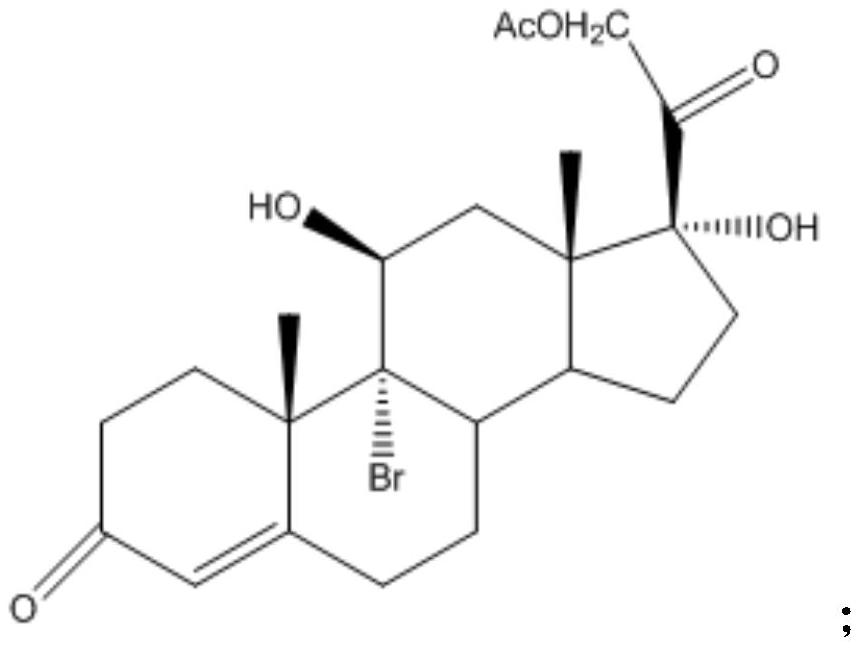
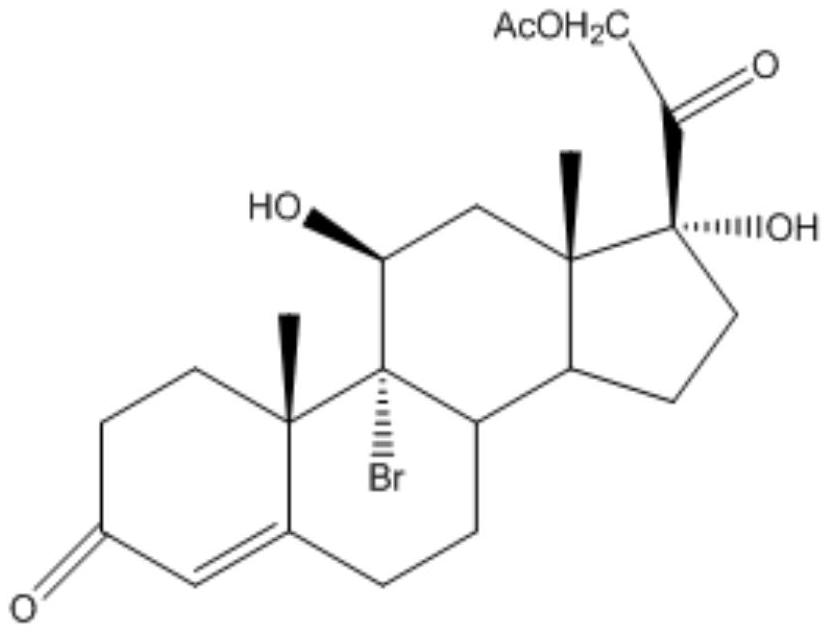
A kind of preparation method of cortisone acetate
A technology of cortisone acetate and acid solution, applied in chemical instruments and methods, steroids, organic chemistry, etc., can solve the problems of containing large heavy metal ions, etc., and achieve the effects of low price, good process prospects, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Steroid intermediate A
[0041] Add 20 parts by volume of acetone to the dry first reaction tank, feed nitrogen, stir and add 1 part by mass of 17α-hydroxypregna-4,9-diene-3,20-dione acetate, stir After 15 minutes, cool down to 0-5°C, add 0.06 parts by volume of perchloric acid and 2 parts by volume of water, add 0.5 parts by mass of dibromohydantoin four times at 2-4°C, with an interval of 30 minutes each time, After the addition is completed, keep the temperature for 2 hours, and take a sample for detection and analysis by thin-layer chromatography until the raw materials are completely reacted;
[0042] After completion of the reaction, add dropwise a concentration of 10% sodium sulfite aqueous solution to the reaction solution to adjust the pH>7. For the distillate, add 2 parts by volume of water, and then steam until there is no acetone smell, that is, the concentration is complete;
[0043] Add 25 parts by volume of water, stir and cool down to 0-5°C, discha...
Embodiment 2
[0053] (1) Steroid intermediate A
[0054]Add 15 parts by volume of acetone to the dry first reaction tank, feed nitrogen, stir and add 0.8 parts by mass of 17α-hydroxypregna-4,9-diene-3,20-dione acetate, stir After 15 minutes, cool down to 0-5°C, add 0.04 parts by volume of perchloric acid and 1 part by volume of water, add 0.4 parts by mass of dibromohydantoin four times at 2-4°C, with an interval of 30 minutes each time, After the addition is completed, keep the temperature for 1 hour, and take a sample and analyze it by thin-layer chromatography until the raw material is completely reacted;
[0055] After completion of the reaction, add dropwise concentration of 10% sodium sulfite aqueous solution to the reaction solution to adjust pH>7. For the distillate, add 2 parts by volume of water, and then steam until there is no acetone smell, that is, the concentration is complete;
[0056] Add 20 parts by volume of water, stir and cool down to 0-5°C, discharge and centrifuge, ...
Embodiment 3
[0065] (1) Steroid intermediate A
[0066] Add 25 parts by volume of acetone to the dry first reaction tank, feed nitrogen, stir and add 1.2 parts by mass of 17α-hydroxypregna-4,9-diene-3,20-dione acetate, stir After 15 minutes, cool down to 0-5°C, add 0.08 parts by volume of perchloric acid and 3 parts by volume of water, add 0.6 parts by mass of dibromohydantoin four times at 2-4°C, with an interval of 30 minutes each time, After the addition is completed, keep the temperature for 3 hours, and take a sample and analyze it by thin-layer chromatography until the raw material is completely reacted;
[0067] After completion of the reaction, add dropwise concentration of 10% sodium sulfite aqueous solution to the reaction solution to adjust the pH>7. For the distillate, add 2 parts by volume of water, and then steam until there is no acetone smell, that is, the concentration is complete;
[0068] Add 30 parts by volume of water, stir and cool down to 0-5°C, discharge and centr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com