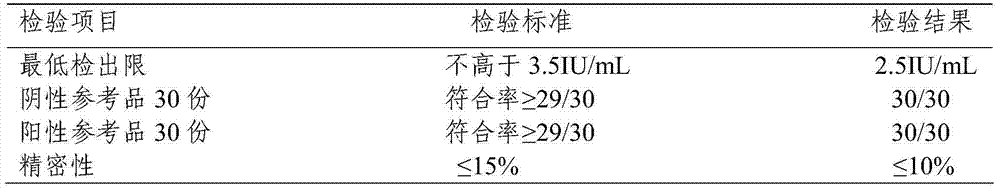
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Allergen extract" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A complex mixture consisting of allergenic proteins derived from natural sources. May be used for diagnosis or therapy. Extracts used for diagnosis have the same active ingredients as those used for therapy but may differ by concentration, diluent or other additives.
Toothpaste for Delivery of Allergens to Oral Mucosa
ActiveUS20160228539A1Reduce concentrationImprove concentrationCompounds screening/testingCosmetic preparationsAllergen extractToothpaste
Pro-toothpaste compositions formulated to receive a volume of allergen solution to provide toothpastes that exhibit efficacy for oral mucosal immunotherapy (OMIT) and acceptable consumer and product stability properties, along with kits of pro-toothpaste, allergen / extracts of allergens, and optionally compounding means and / or specialized toothbrushes are provided. Toothpastes suitable and effective for OMIT and methods for managing allergic symptoms and for reducing risk of allergy in people without symptoms employing the pro-toothpastes, toothpastes and kits of the invention are also disclosed.
Owner:ALLOVATE
Process for producing an allergen extract
The present invention relates to a process for producing an allergen extract from a biological allergen source material comprising the steps ofa) contacting the source material with a liquid extraction agent to produce a allergen extract mixture containing allergens dissolved in a liquid phase and a solid phase consisting of source material residues,b) subjecting the allergen extract mixture to a first separation step to remove the solid phase to produce a crude allergen extract,c) subjecting the crude allergen extract to a low molecular fraction removal step to remove molecules having a molecular size of less than 10 kDa, andd) carrying out step c) until the allergen extract has conductivity of below 2000 μS / cm at 25° C. to obtain a purified allergen extract.
Owner:ALK ABELLO SA
Anaphylactic disease allergen colloidal gold diagnostic test strip and preparation method thereof
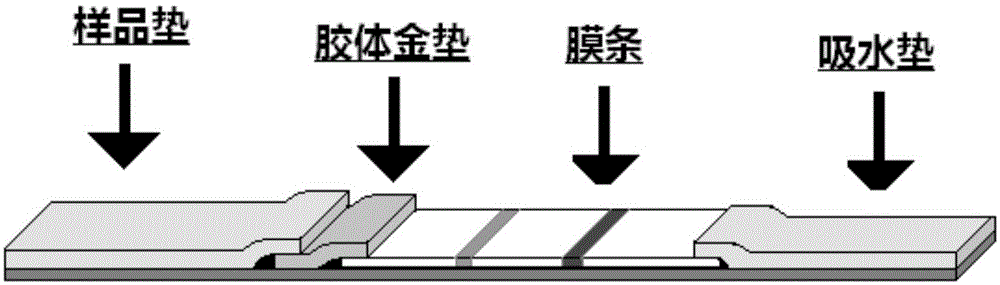
The invention discloses an anaphylactic disease allergen colloidal gold diagnostic test strip. The test strip comprises a sample hole, a colloidal gold pad, a film strip, an absorbent pad and a PVC board, wherein the film strip is coated with inhalation group allergen I, inhalation group allergen II, a food group allergen and a mouse anti-goat IgG antibody, so as to form three detecting strips and one quality control strip. Each allergen energy included in the test strip can represent allergen molecules of 80 percent of patients, and on the detecting film strip, a symbolic allergen coated molecule replaces an allergen extract, so that the purpose of quickly diagnosing the anaphylactic disease technically is achieved. The time for detecting by using the test strip lasts for 5 to 30 minutes, the specificity is high, the sensitivity is high, the testing is simple and quick, on-site detecting is achieved, special training for operators is not required, operation can be finished according to specification, and quick diagnosis to anaphylactic diseases can be achieved.
Owner:北京新华联协和药业有限责任公司
Pharmaceutical product comprising mite allergen extract(s) and a method for the manufacture thereof
The invention relates to a pharmaceutical product comprising an allergen extract or an allergoid thereof for the treatment and / or prevention of allergy and allergic asthma caused by house dust mites, which extract comprises at least one extract of mite bodies selected from the following groups a)-b): a) An extract of Der p mite bodies, and b) An extract of Der f mite bodies, and at least one extract of mite cultures selected from the following groups c)-g): c) An extract of Der p faecal particles, d) An extract of Der f faecal particles, e) An extract of Der f whole mite culture, f) An extract of an Der p whole mite culture, and g) a combination of extracts c) to f).
Owner:ALK ABELLO SA
Food allergen dynamic digestion model and in-vitro simulation evaluation method
InactiveCN110531059AReduce experimental errorReduce space station ratioBiological testingHuman bodyColonic adenocarcinoma
The invention discloses a food allergen dynamic digestion model and an in-vitro simulation evaluation method. The method mainly comprises the steps of establishing an allergen dynamic digestion modeland an in-vitro simulation evaluation method, simulating a biochemical reaction condition of the stomach and duodenum of a human body through a digestive system simulation device, providing a method for constructing an allergen in-vitro simulated dynamic digestion experiment in the simulated evaluation method, constructing a Caco-2 (human cloned colonic adenocarcinoma cells) small intestine epithelial cell model to simulate small intestine absorption and transport effects, and constructing a KU812 (human peripheral blood basophilic leukemia cells) cell model to evaluate the ability of mast cell degranulation induced by food allergen digestion and transport products. The method of the invention is suitable for evaluating the digestion and sensitization of a purified food allergen or an allergen extracting solution containing a complex food matrix.
Owner:OCEAN UNIV OF CHINA
Water-containing liquid allergic reaction bacterin preparation for mouth mucosa drug administration
InactiveCN101791399APharmaceutical delivery mechanismAllergen ingredientsAllergic transfusion reactionMouth mucosa
The invention relates to a water-containing liquid bacterin preparation and the purpose thereof for treating and preventing allergic reaction. The preparation contains an allergen extract which exists in water-containing liquid or suspension containing a protein stabilizer and derives from one or more kinds of artemisia plant pollen.
Owner:ALK ABELLO SA
Minor allergen control to increase safety of immunotherapy
InactiveUS20110200641A1Improve securityEasy to controlAllergen ingredientsImmunoglobulins against plantsAllergen extractMedicine
Owner:ALK ABELLO SA
Process for producing an allergen extract
The present invention relates to a process for producing an allergen extract from a biological allergen source material comprising the steps ofa) contacting the source material with a liquid extraction agent to produce a allergen extract mixture containing allergens dissolved in a liquid phase and a solid phase consisting of source material residues,b) subjecting the allergen extract mixture to a first separation step to remove the solid phase to produce a crude allergen extract,c) subjecting the crude allergen extract to a low molecular fraction removal step to remove molecules having a molecular size of less than 10 kDa, andd) carrying out step c) until the allergen extract has conductivity of below 2000 μS / cm at 25° C. to obtain a purified allergen extract.
Owner:ALK ABELLO SA
Pharmaceutical product comprising mite allergen extract(s) and a method for the manufacture thereof
The invention relates to a pharmaceutical product comprising an allergen extract or an allergoid thereof for the treatment and / or prevention of allergy and allergic asthma caused by house dust mites, which extract comprises at least one extract of mite bodies selected from the following groups a)-b): a) An extract of Der p mite bodies, and b) An extract of Der f mite bodies, and at least one extract of mite cultures selected from the following groups c)-g): c) An extract of Der p faecal particles, d) An extract of Der f faecal particles, e) An extract of Der f whole mite culture, f) An extract of an Der p whole mite culture, and g) a combination of extracts c) to f).
Owner:ALK ABELLO SA
London plane tree pollen allergen extract, extracting solution and preparation method thereof
ActiveCN110064052AEfficient diagnosis of allergic diseasesImprove effectivenessCompounds screening/testingPowder deliveryDiseaseFiltration
The invention relates to a London plane tree pollen allergen extract and an extracting solution and a preparation method thereof. The amino acid sequences of London plane tree pollen contained in theLondon plane tree pollen allergen extract are shown in SEQ ID NO.4 and SEQ ID NO.5, and the extracting solution can be prepared by London plane tree pollen collection, drying, degreasing, extracting,ultra-filtration concentrating, freeze-drying, re-dissolving and other process methods. The London plane tree pollen allergen extracting solution has the characteristic of high specificity, and the London plane tree pollen component is fully extracted, the total biological titer is stable, the validity period is long, and the aseptic effect is good. Its stock solution can be effectively used for skin prick test diagnosis of allergic reaction diseases, can be used for in-vitro basophil activation test diagnosis, intradermal test diagnosis and specific immunotherapy after proper dilution (10-2 to 10-20), and can effectively diagnose and carry out specific immunotherapy on allergic reaction diseases induced by London plane tree pollen.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
Periplaneta americana allergen preparation production method
ActiveCN103920147AGood repeatabilityReduce mistakesAllergen ingredientsRespiratory disorderAllergen extractFreeze-drying
The present invention provides a periplaneta americana allergen preparation production method, which comprises: (1) inactivating periplaneta americana in a refrigerator, removing intestinal tracts, washing with 75% ethanol, carrying out air-drying, and placing in the refrigerator so as to be spare; (2) taking an appropriate amount of the frozen periplaneta americana, and grinding into powder in liquid nitrogen; (3) degreasing with acetone, placing into the refrigerator to freeze, and carrying out freeze-drying in a vacuum freeze dryer to obtain powder; (4) weighing the periplaneta americana powder, adding an extraction solution, carrying out centrifugation, and sucking the supernatant, wherein the supernatant is a periplaneta americana allergen extract; (5) adopting specific antibodies to identify the content and the proportion of the main allergen in the periplaneta americana allergen crude extract; and (6) according to the identification result in the step (5), adding recombinant protein expressed by yeast or insects. With the periplaneta americana allergen crude extract extracted by using the method, the main allergen protein of the periplaneta americana can be obtained to the maximal degree, the extract has good repeatability, and the error is less than 30%.
Owner:何韶衡
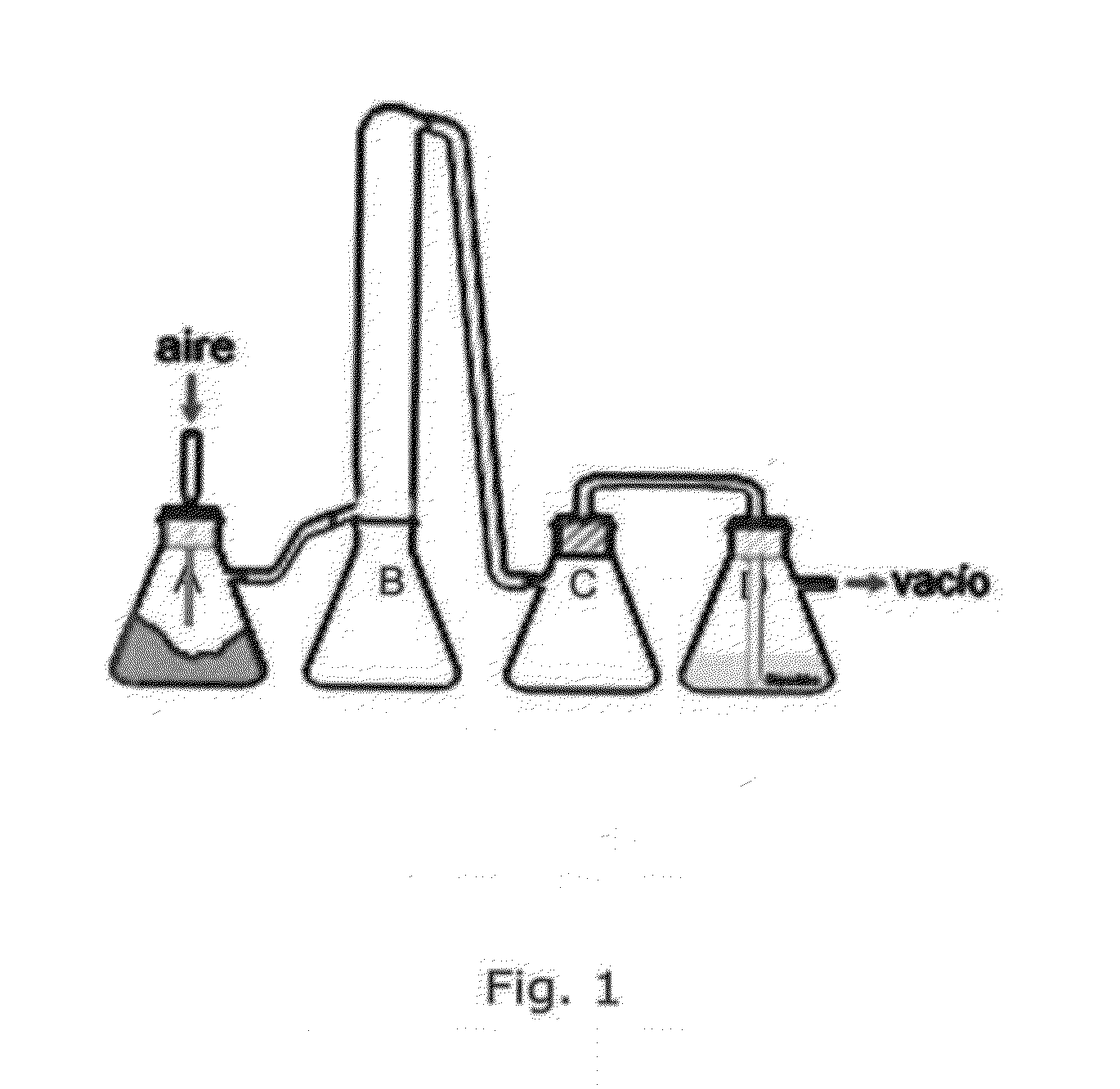
Filter screen antianaphylaxis detection method and apparatus
ActiveCN106771116AAntiallergic ObservationEasy to chooseBiological testingAllergen extractAir cleaning
The invention discloses a filter screen antianaphylaxis detection method, which is used for detecting antianaphylaxis of a filter screen in an air purification apparatus. The method comprises the following steps: S100: cutting the filter screen into a sample sheet A with a diameter of 50 mm; S200: respectively sterilizing the sample sheet A, a sample sheet B and a sample sheet C; S300: placing the sample sheet A into a 250ml sterile triangular flask A; S400: respectively dropwise adding and dyeing a dilute solution of an allergen solution onto the sample sheet A and the sample sheet B; S500: standing the sterile triangular flask A, a sterile triangular flask B and a sterile triangular flask C in a space with an ambient temperature of 25 DEG C for one hour; and S600: respectively adding 10ml of allergen extract liquid into the sterile triangular flask A, the sterile triangular flask B and the sterile triangular flask C. The invention also provides a filter screen antianaphylaxis detection apparatus. A nonwoven fabric is used as a comparison reference, so that the antianaphylaxis of the sample filter screen and a comparison result with the nonwoven fabric can be detected.
Owner:CHINA HOUSEHOLD ELECTRIC APPLIANCE RES INST
Process for producing an allergen extract
ActiveUS9884111B2Reduce allergiesPromote aggregationAllergen ingredientsDepsipeptidesAllergen extractMedicine
Owner:LETI PHARMA SL
Methods of purifying an allergen extract
PendingUS20220110983A1High in proteinIncrease contentProtozoa material medical ingredientsPlant ingredientsAllergen extractPharmaceutical drug
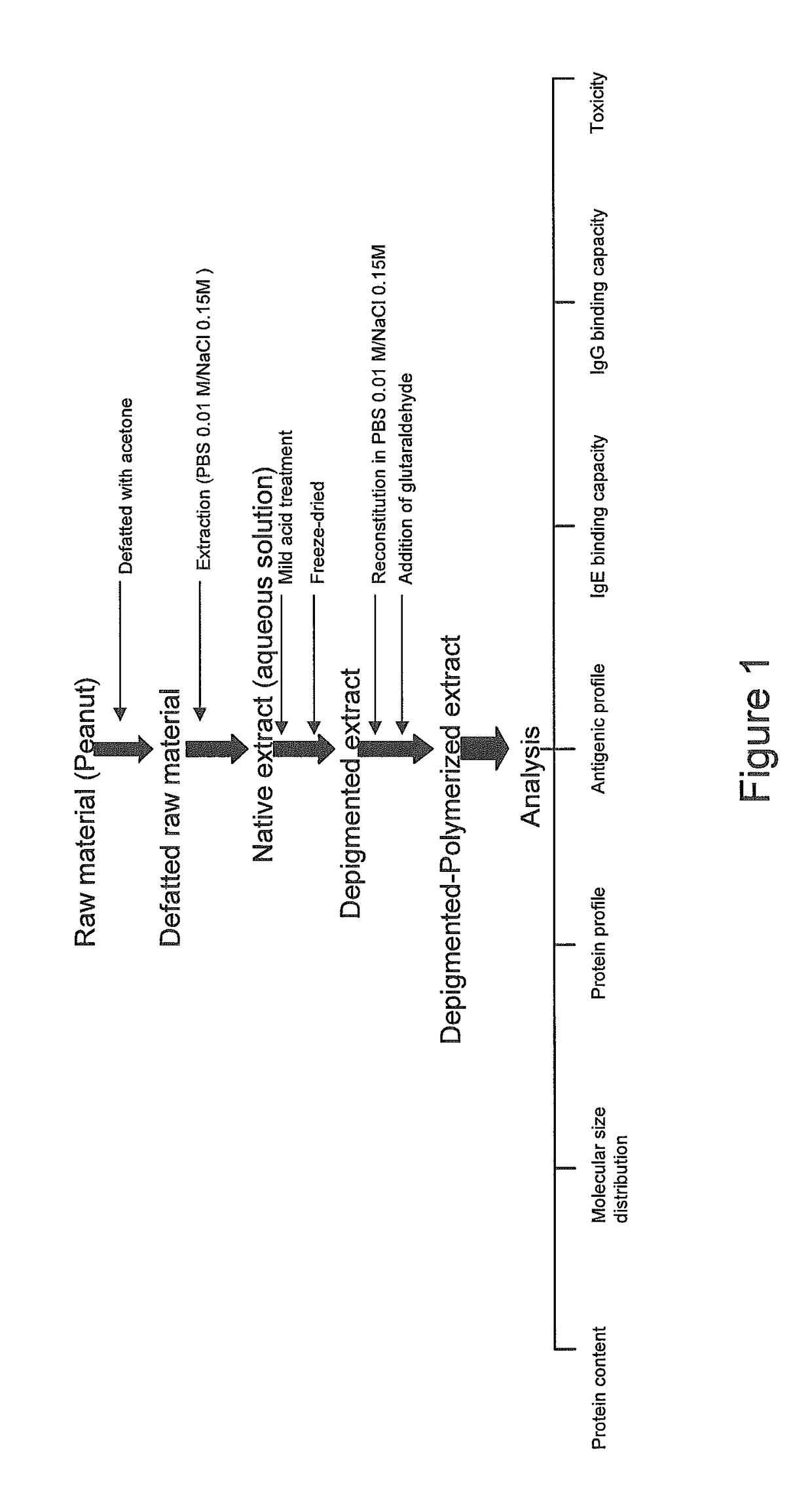
The invention relates to processes for producing semi-purified and purified allergen extracts and pharmaceutical compositions and vaccines for use in the diagnosis and treatment of allergy. In one aspect of the invention, a process for producing a depigmented allergen extract is provided, the process comprising: a) basifying a native allergen extract; and b) removing molecules having a molecular size of less than 3.5 kDa; and c) adjusting the pH to neutrality; thereby to produce a depigmented allergen extract.
Owner:LETI PHARM SL
Ginkgo leaf powder for injection and its preparation
The invention relates to a ginkgo leaf extract powder injection, which contains 1 weight part of ginkgo leaf extract, 1-4 weight parts of hydroxypropyl beta cyclodextrin, and 0.02- 0.08 parts by weight. Its preparation method is: take ginkgo biloba extract, add hydroxypropyl beta cyclodextrin and mix in proportion, add acid-base regulator, filter, and the filtrate is ultrafiltered through a 50000-150000 Dalton ultrafiltration membrane, ultrafiltration The liquid is directly aseptically freeze-dried or aseptically spray-dried to form a powder for injection. On the basis of fully retaining the active ingredients, the preparation effectively removes impurities such as macromolecules, pyrogens, particles (including resin exfoliation), microorganisms, etc. in the original extract, and the safety and stability of the preparation are significantly improved.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Allergen colloidal gold diagnostic test strip for allergic diseases and preparation method thereof
The invention discloses an anaphylactic disease allergen colloidal gold diagnostic test strip. The test strip comprises a sample hole, a colloidal gold pad, a film strip, an absorbent pad and a PVC board, wherein the film strip is coated with inhalation group allergen I, inhalation group allergen II, a food group allergen and a mouse anti-goat IgG antibody, so as to form three detecting strips and one quality control strip. Each allergen energy included in the test strip can represent allergen molecules of 80 percent of patients, and on the detecting film strip, a symbolic allergen coated molecule replaces an allergen extract, so that the purpose of quickly diagnosing the anaphylactic disease technically is achieved. The time for detecting by using the test strip lasts for 5 to 30 minutes, the specificity is high, the sensitivity is high, the testing is simple and quick, on-site detecting is achieved, special training for operators is not required, operation can be finished according to specification, and quick diagnosis to anaphylactic diseases can be achieved.
Owner:北京新华联协和药业有限责任公司
Fraxinus pennsylvanica pollen allergen extract and immersion liquid of fraxinus pennsylvanica pollen allergen extract and preparation method thereof
ActiveCN110078803AEfficient diagnosis of allergic diseasesImprove effectivenessCompounds screening/testingPowder deliveryDiseaseBasophilic Granulocyte
The invention provides a fraxinus pennsylvanica pollen allergen extract and immersion liquid of the fraxinus pennsylvanica pollen allergen extract and a preparation method thereof. The fraxinus pennsylvanica pollen allergen extract contains an amino acid sequence, which is as shown in SEQ ID NO.4, of fraxinus pennsylvanica pollen allergen protein. The immersion liquid is prepared through technology methods of fraxinus pennsylvanica pollen collecting, drying, degreasing, extracting, ultrafiltration concentrating, freeze-drying, redissolving and the like, and main components of the immersion liquid are a fraxinus pennsylvanica pollen allergen, glycerin, sodium chloride, phenol and the like. The fraxinus pennsylvanica pollen allergen immersion liquid has the character of high specificity, a fraxinus pennsylvanica allergic protein component is fully extracted, a total biological value is stable, a validity period is long, and an aseptic effect is good; and stoste of the immersion liquid ofthe fraxinus pennsylvanica pollen allergen extract can be effectively used for skin prick test diagnosis of allergic reaction diseases, and can be used for in-vitro basophilic granulocyte activationtest diagnosis and specific immunotherapy after being properly diluted so that the allergic reaction diseases induced by fraxinus pennsylvanica pollen can be effectively diagnosed, and can be subjected to specific immunotherapy.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
A kind of ashwagandha pollen allergen extract, its leaching solution and preparation method thereof
ActiveCN110078803BEfficient diagnosis of allergic diseasesImprove effectivenessCompounds screening/testingPowder deliveryBiotechnologyDisease
The present invention provides the present invention relates to an infusion solution of allergen of C. chinensis pollen, an infusion solution thereof and a preparation method thereof. The allergen extract of C. chinensis pollen contains the amino acid sequence of C. chinensis pollen allergen protein as shown in SEQ ID NO.4. The infusion solution is prepared by the process of collecting, drying, defatting, extracting, ultrafiltration concentration, freeze-drying, redissolving and the like of C. chinensis pollen, and its main components are C. chinensis pollen allergen, glycerin, sodium chloride, phenol, etc. . The allergen infusion solution of C. chinensis pollen has the characteristics of high specificity, sufficient extraction of allergenic protein components of C. chinensis, stable total biological titer, long validity period and good aseptic effect; the original solution can be effectively used for the treatment of allergic diseases. The skin prick test can be used in the diagnosis of in vitro basophil activation test and specific immunotherapy after appropriate dilution.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
Immunogenic proteins and fragments thereof from allergenic mites
ActiveUS10556936B2Microbiological testing/measurementAllergen ingredientsAllergen extractBiochemistry
The invention relates to novel immunogenic polypeptides identified in house dust mites and storage mites, which have the potential to be used in allergy immunotherapy, for diagnostic purposes, eventually via production of antibodies binding the polypeptide or for characterising allergen extracts of house dust mites and storage mites.
Owner:ALK ABELLO SA +1
Immunogenic proteins and fragments thereof from allergenic mites
ActiveUS20190202875A1Microbiological testing/measurementAllergen ingredientsAllergen extractImmunogenicity
The invention relates to novel immunogenic polypeptides identified in house dust mites and storage mites, which have the potential to be used in allergy immunotherapy, for diagnostic purposes, eventually via production of antibodies binding the polypeptide or for characterising allergen extracts of house dust mites and storage mites.
Owner:ALK ABELLO SA +1
Allergen-specific IgE antibody detection kit and preparation method thereof
PendingCN113552338ASmall amount of sampleShorten the timeMaterial analysisReagent stripAllergen extract
The invention belongs to the field of clinical laboratory diagnostics, and relates to an allergen-specific IgE antibody detection kit and a preparation method thereof. The reagent strip comprises a membrane strip, a sample pad, a colloidal gold pad, a water absorption pad and a PVC plate; the membrane strip is provided with at least one detection line, and the detection line is coated with an allergen extracting solution; the coating concentration of the allergen extracting solution is 0.5 to 5 mg / mL. The colloidal gold reagent strip and the kit disclosed by the invention can be used for detecting fingertip blood and whole blood, so that the bedside detection of allergens in a real sense is realized; the patient can be diagnosed in time, and even if the patient himself / herself can buy a reagent for self-inspection, so that the possibility is provided for early discovery and early treatment of more allergic disease patients.
Owner:GLALLERGEN CO LTD
Veterinary product
The present invention relates to a purified veterinary allergen extract enriched. In particular, the invention relates to a purified veterinary allergen extract enriched with Der f 15 and Der f 18. The invention further relates to use of the allergen extract as a veterinary product, and its use in treating allergy, in particular house dust mite allergy in mammals, more particularly, dogs.
Owner:莱蒂个人实验室
Toothpaste for Delivery of Allergens to Oral Mucosa
Pro-toothpaste compositions formulated to receive a volume of allergen solution to provide toothpastes that exhibit efficacy for oral mucosal immunotherapy (OMIT) and acceptable consumer and product stability properties, along with kits of pro-toothpaste, allergen / extracts of allergens, and optionally compounding means and / or specialized toothbrushes are provided. Toothpastes suitable and effective for OMIT and methods for managing allergic symptoms and for reducing risk of allergy in people without symptoms employing the pro-toothpastes, toothpastes and kits of the invention are also disclosed.
Owner:ALLOVATE
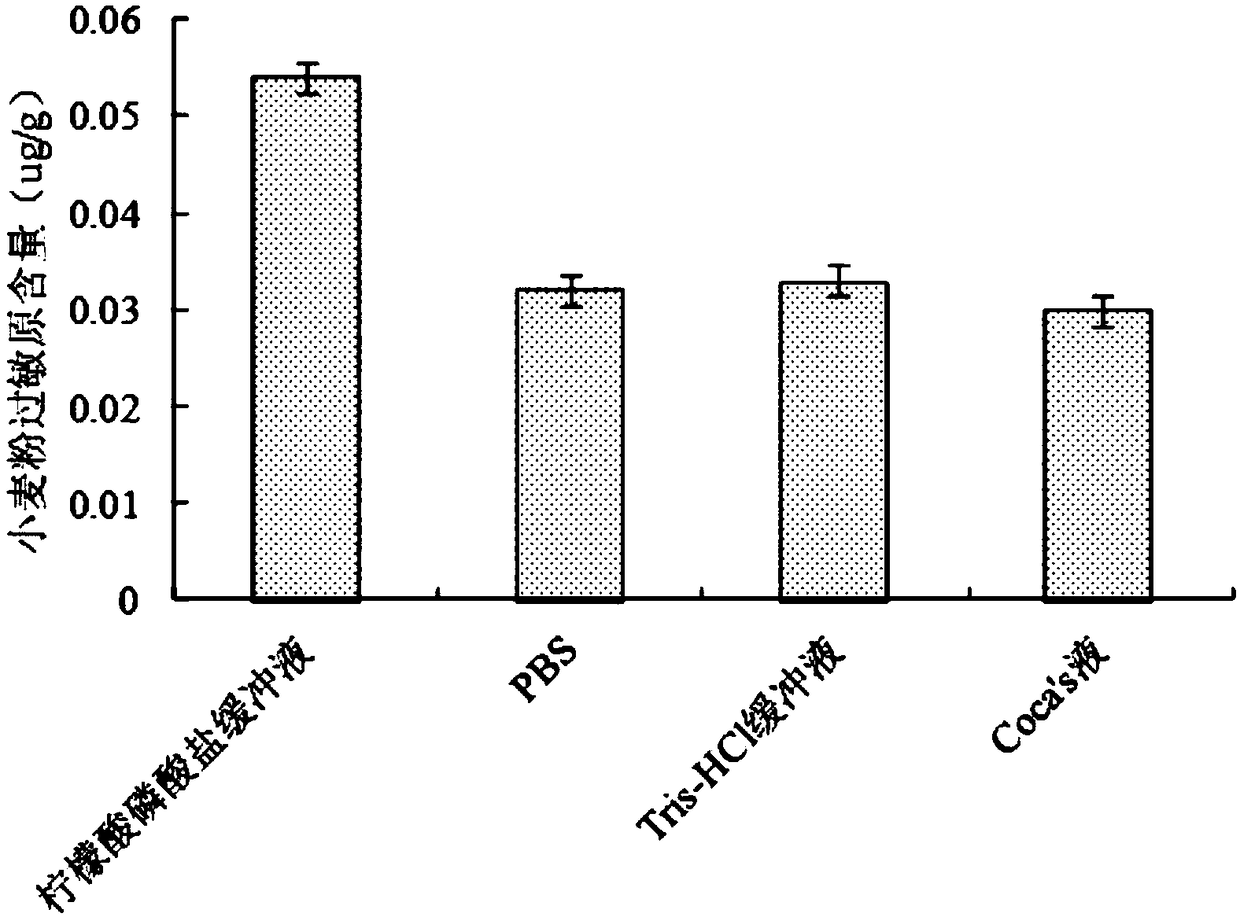
High-efficiency wheat flour allergen extracting agent and using method thereof
ActiveCN108530513AIncreased levels of allergensIncrease color depthPeptide preparation methodsColor/spectral properties measurementsCITRATE ESTERPhosphate
The invention discloses a high-efficiency wheat flour allergen extracting agent and a using method thereof, relating to the field of protein extraction, and aiming to provide a high-efficiency wheat allergen extracting agent, which has a high extraction rate and ensures high allergic immune activity of extracted wheat allergen, and the using method thereof. The high-efficiency wheat flour allergenextracting agent comprises a citrate phosphate buffer solution, in which the concentration of citric acid is 3 to 5 mmoL / L, and the concentration of phosphate is 70.3 to 147.3 mmoL / L, and the pH of the citrate phosphate buffer solution is 7.0 to 9.0. The using method of the high-efficiency wheat flour allergen extracting agent comprises the steps of weighing, extracting and detecting. The contentof allergen extracted by the high-efficiency wheat flour allergen extracting agent disclosed by the invention is 59.4%-106.3%, 54.5%-100% and 70%-120% higher than that of a phosphate buffer solution,tri(hydroxymethyl) amino methane hydrochloric acid and a Coca's solution, respectively; and the allergen obtained with the extracting agent has a rich variety of allergen subunits and high allergic immune activity.
Owner:HEBEI AGRICULTURAL UNIV. +1
A filter anti-allergic detection method and device
ActiveCN106771116BAntiallergic ObservationEasy to chooseBiological testingAllergen extractEngineering
The invention discloses a method for detecting the anti-allergic properties of a filter, which is used to detect the anti-allergenic properties of the filter in an air purification device. It includes the following steps: S100: Cut the filter into a sample piece A with a diameter of 50 mm; S200: Sterilize sample A, sample B and sample C respectively; S300: Place sample A in a 250 ml sterile Erlenmeyer flask A. ; S400: Dye the dilution of the allergen solution on sample A and sample B; S500: Place sterile flask A, sterile flask B and sterile flask C in a space with an ambient temperature of 25 degrees Celsius. Let stand for 1 hour; S600: Add 10 ml of allergen extraction solution to sterile Erlenmeyer flask A, sterile Erlenmeyer flask B and sterile Erlenmeyer flask C respectively. The invention also provides a filter anti-allergy detection device. The present invention uses non-woven fabric as a comparison standard and can detect the anti-allergenicity of the sample filter compared with the non-woven fabric.
Owner:CHINA HOUSEHOLD ELECTRIC APPLIANCE RES INST
Virus removal and inactivation treatment process for preparation of dust mite allergen
The present invention relates to the virus elimination and deactivation process in preparing dermatophagoide allergen immersion, and viruses are eliminated and deactivated by means of several combined methods. Specifically, the combined process includes: 1.dry heating process including dry roasting of the instrument at 300 deg.c, heating culture medium and extracting buffer liquid at 80 deg.c for 72 hr, verifying the dry heating case for deactivating virus at least every half year and performing bacteria-free operation; 2. filtering process to filter allergen extracting liquid with filtering membrane to eliminate viruses of molecular weight greater than 100 KDa; 3. ultrasonic process to deactivate viruses via single cyclic path exposed to microwave energy; 4. chromatographic process to eliminate viruses of molecular weight greater than 100 KDa with Sephadex S-100 molecular sieve; and 5. verifying all the virus eliminating and deactivating processes.
Owner:SHENZHEN UNIV
Standardized Subcutaneous Allergen Immunotherapy
InactiveUS20170252297A1Low costReduce concentrationAllergen ingredientsPharmaceutical delivery mechanismPersonalizationCost effectiveness
The invention provides a process for the standardization of subcutaneous allergen immunotherapy formulas resulting in increasing availability and decreasing costs, allowing for expanded and improved relief for a greater number of allergy sufferers.This invention provides a lower cost treatment to increase availability of the benefits of subcutaneous immunotherapy via standardization of most common seasonal or year round allergens for treatment. Standardizing a formula containing multiple allergen types allows a large population of potential users to benefit from a wide spectrum of included allergen sensitivities. Mass production of non-customized formulas allows for cost reduction, which breaks down a major barrier to current demand. The availability of non-individualized reduced allergen extract concentration strength formula allows for cost-effective availability in less-specialized delivery channels, which breaks down the other major barrier to current demand.
Owner:YU STEVEN RONDER
A kind of preparation method of Periplaneta americana allergen preparation
ActiveCN103920147BGood repeatabilityReduce mistakesAllergen ingredientsRespiratory disorderFreeze-dryingAllergen extract
Owner:何韶衡
Process for producing an allergen extract
ActiveUS20150306211A1Promote aggregationReduce allergiesAllergen ingredientsPlant peptidesAllergen extract
Owner:LETI PHARMA SL
Methods of purifying allergen extract
PendingCN113825518AProtozoa material medical ingredientsPlant ingredientsAllergen extractPharmaceutical drug
The invention relates to processes for producing semi-purified and purified allergen extracts and pharmaceutical compositions and vaccines for use in the diagnosis and treatment of allergy. In one aspect of the invention, a process for producing a depigmented allergen extract is provided, the process comprising: a) basifying a native allergen extract; and b) removing molecules having a molecular size of less than 3.5 kDa; and c) adjusting the pH to neutrality; thereby to produce a depigmented allergen extract.
Owner:莱蒂生物制药公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com