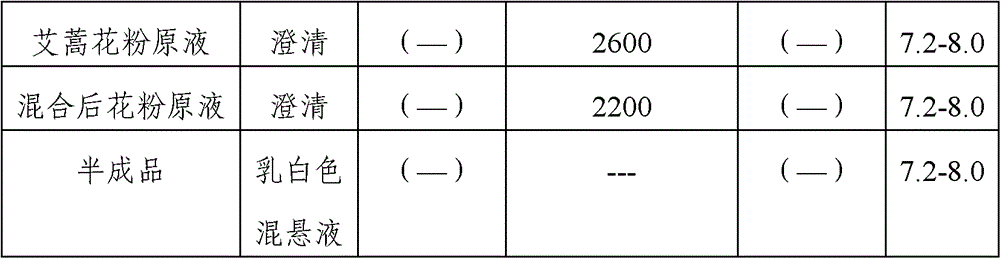Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
42 results about "Pollen allergen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
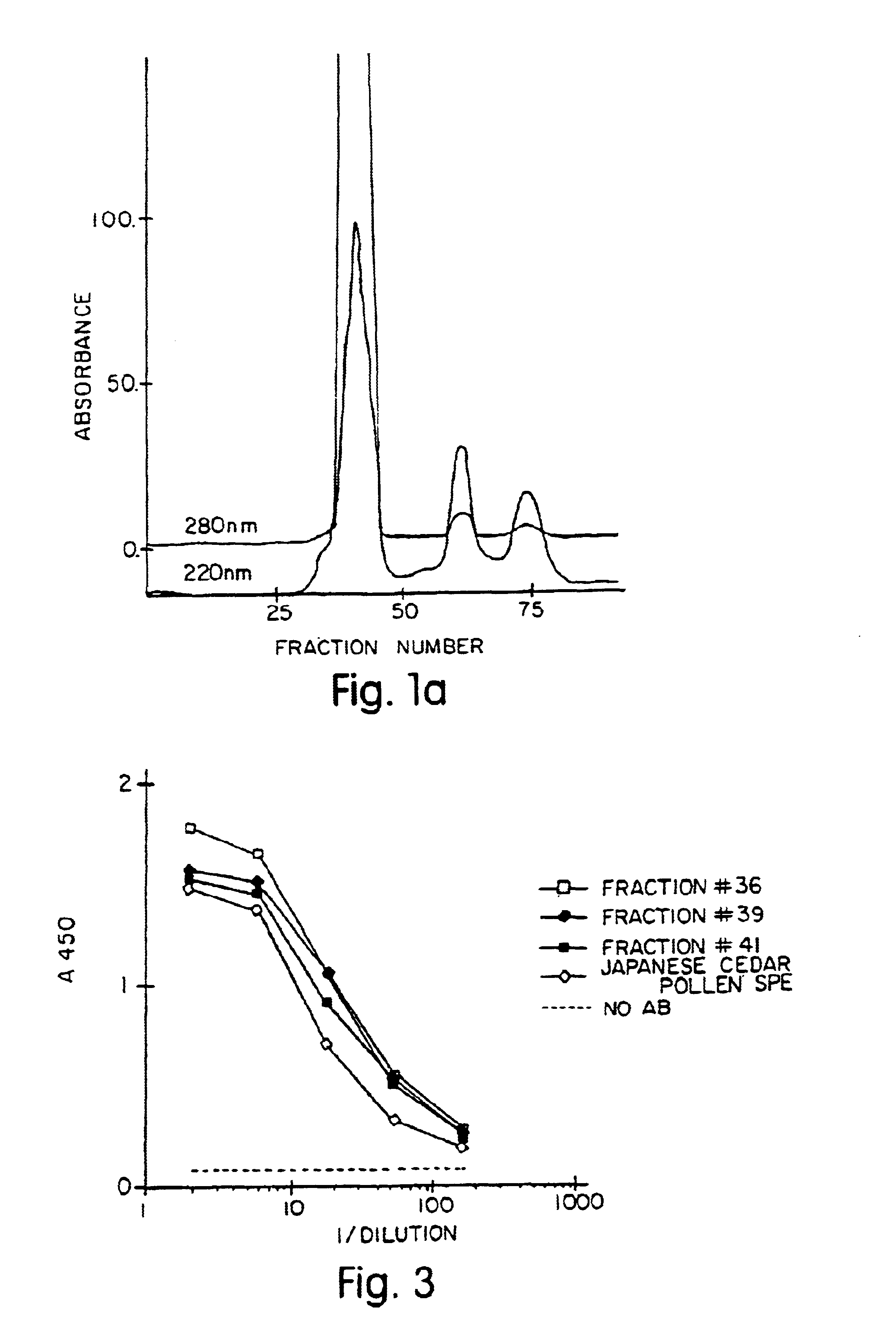
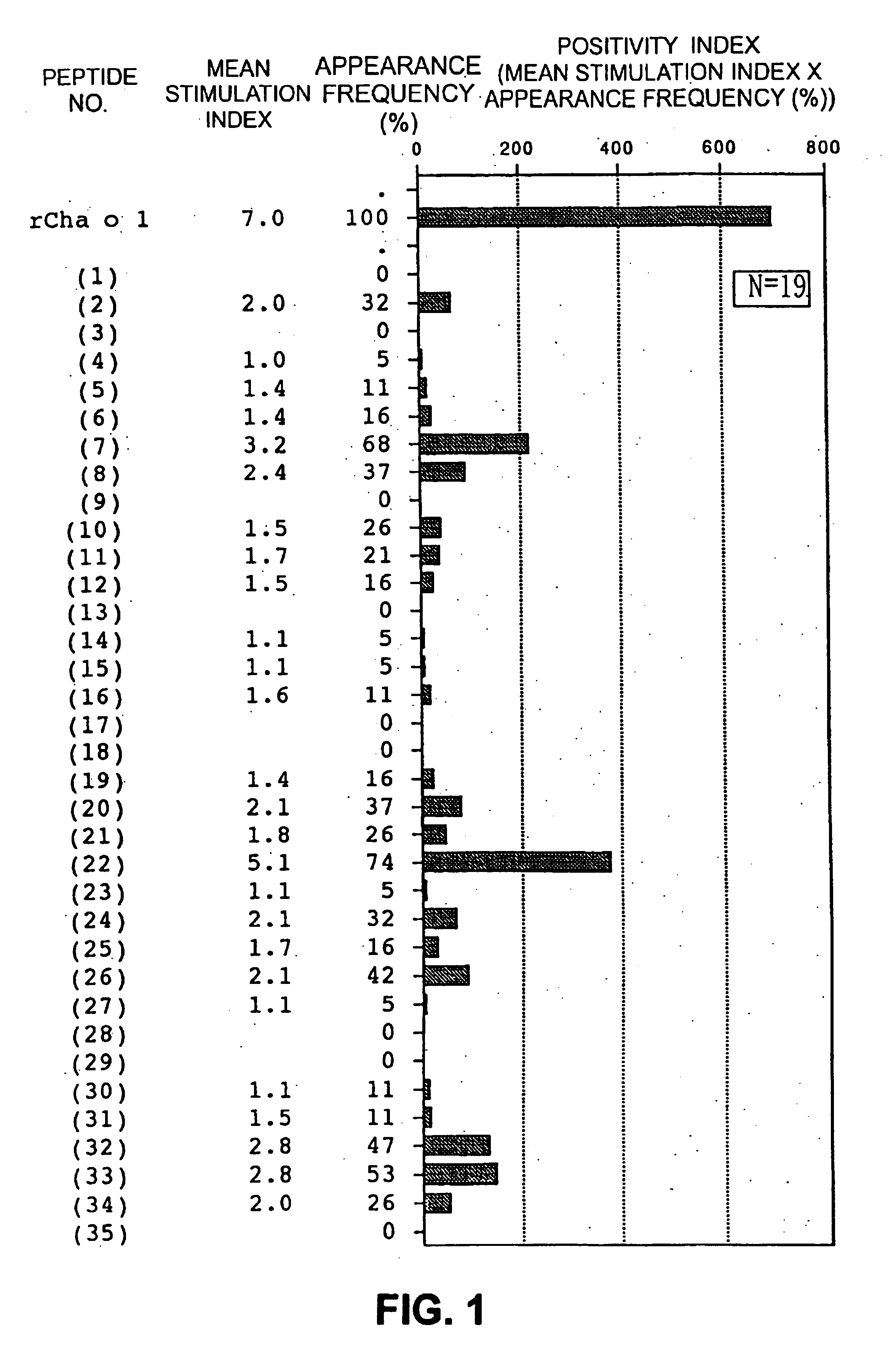
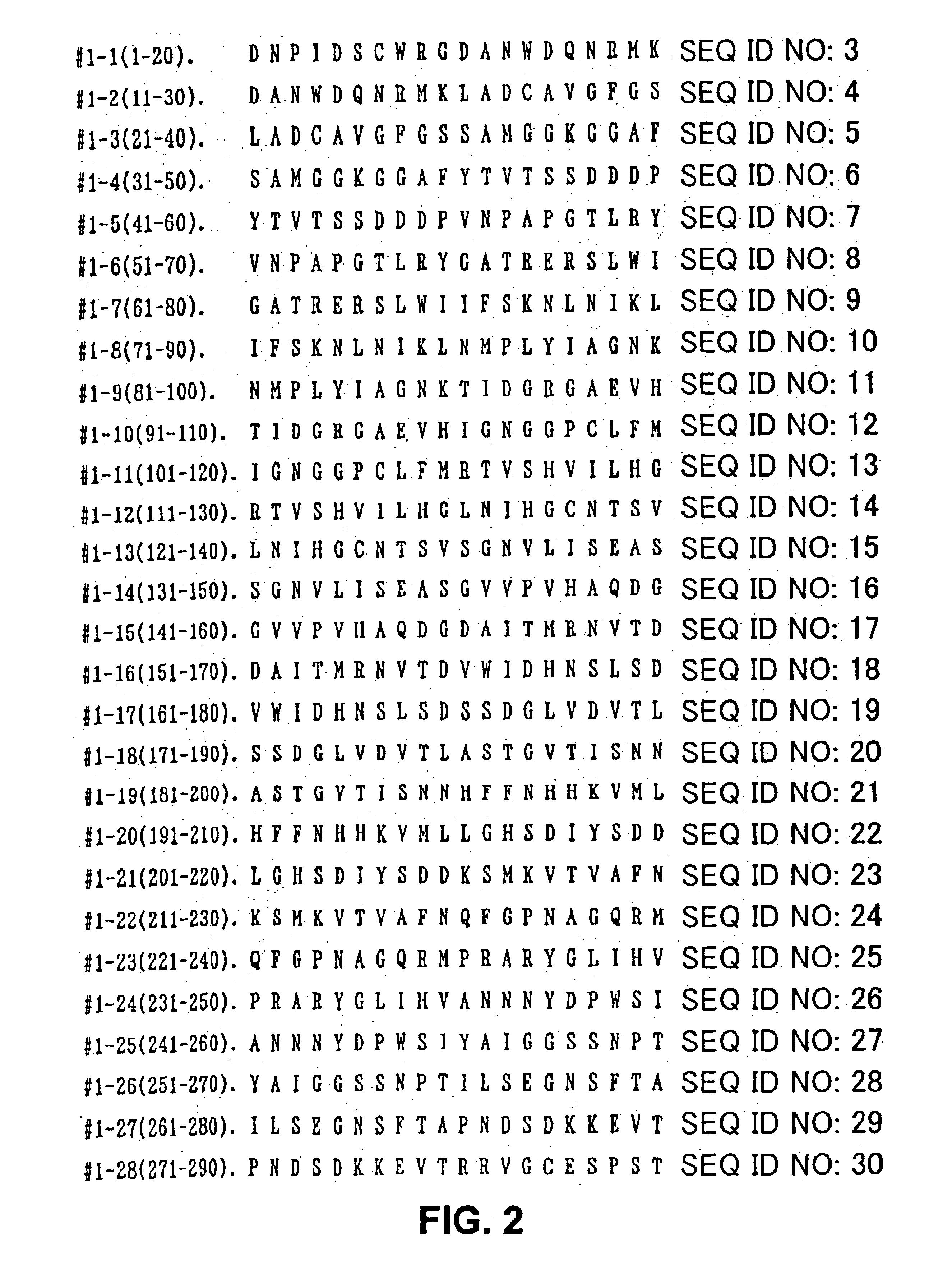
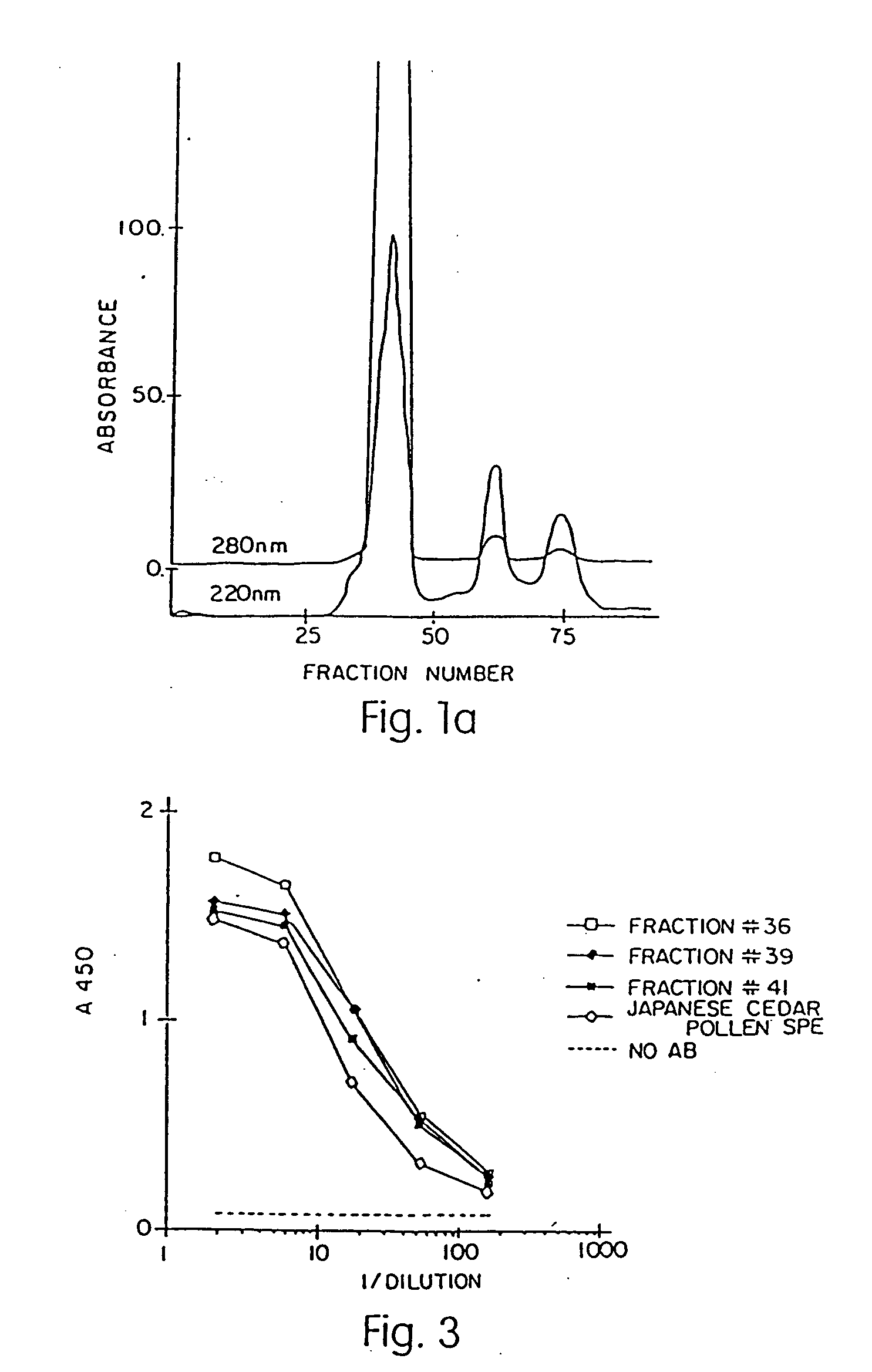
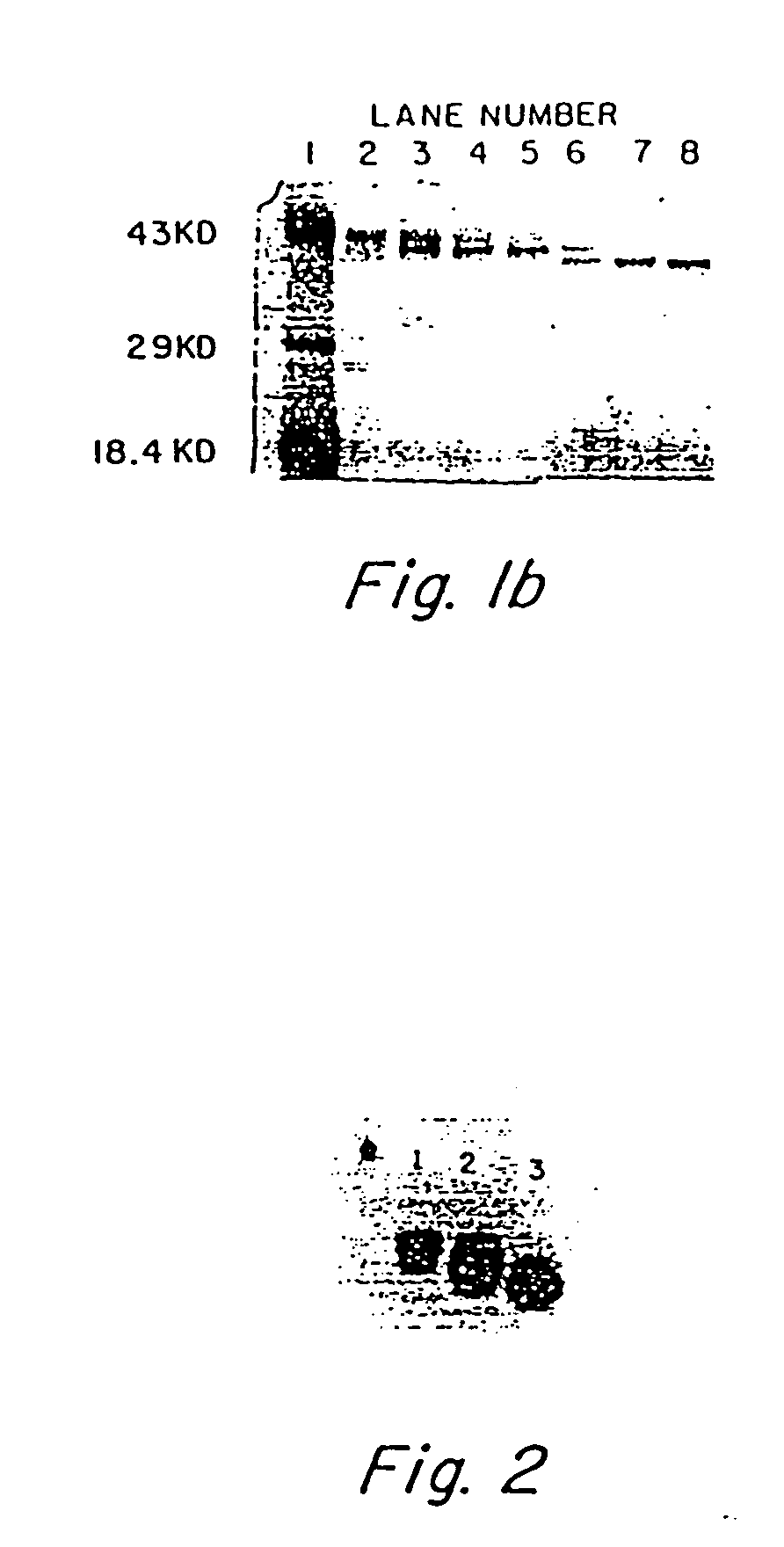
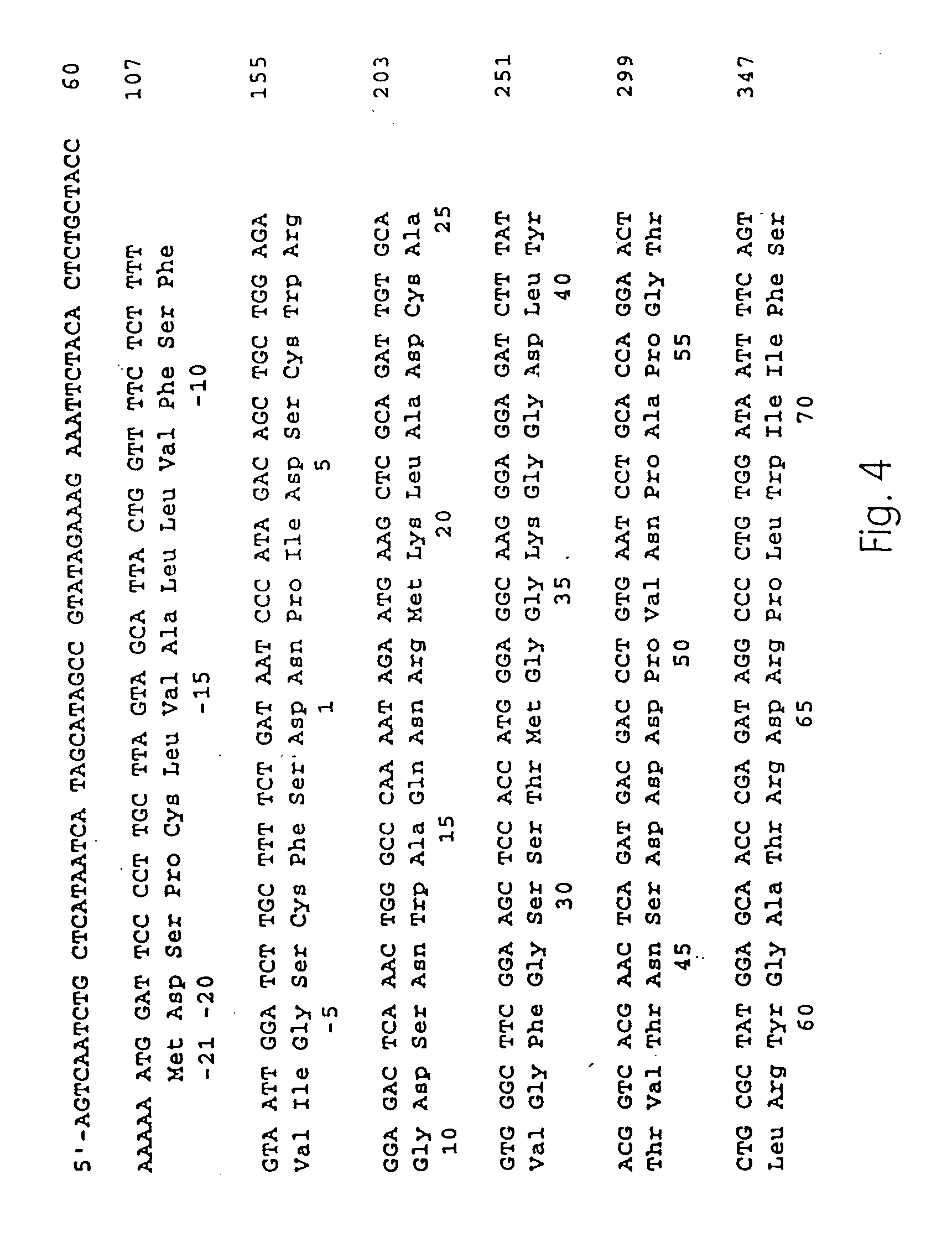
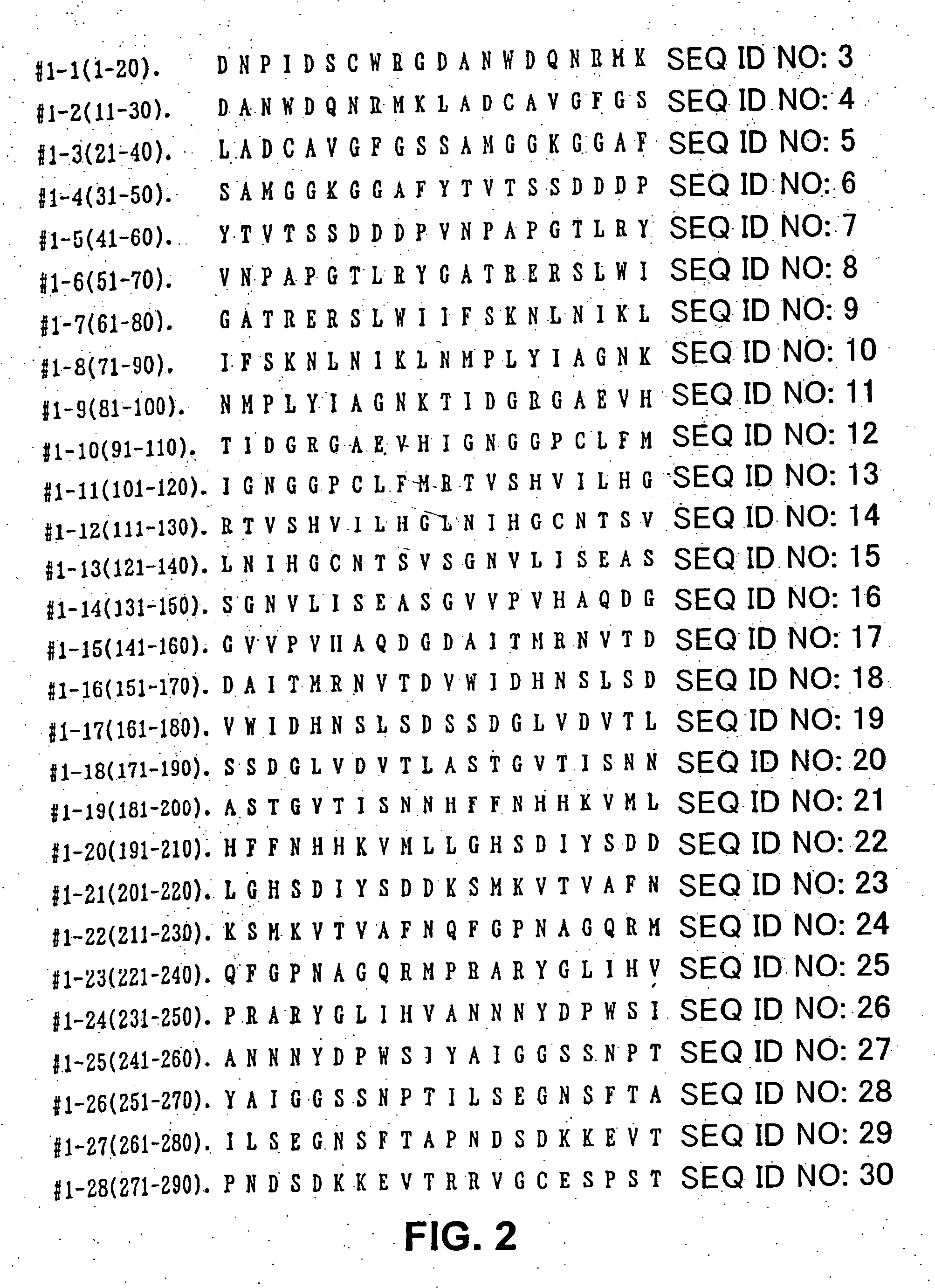
Allergenic proteins and peptides from Japanese cedar pollen
InactiveUS6982326B1Diagnosing, treating, and preventing Japanese cedar pollinosisSugar derivativesPeptide/protein ingredientsSide effectNucleic acid sequencing
The present invention provides nucleic acid sequences coding for the Cryptomeria japonica major pollen allergen Cry j I, Cry j II, Jun s I and Jun v I and fragments or peptides thereof. The present invention also provides purified Cry j I, Cry j II, Jun s I and Jun v I and at least one fragment thereof produced in a host cell transformed with a nucleic acid sequence coding for Cry j I, Cry j II, Jun s I and Jun v I or at least one fragment thereof, and fragments of Cry j I, Cry j II, Jun s I or Jun v I or at least one fragment thereof, and fragments of Cry j I, Cry j II, Jun s I or Jun v I prepared synthetically. Cry j I, Cry j II, Jun s I and Jun v I and fragments thereof are useful for diagnosing, treating, and preventing Japanese cedar pollinosis. The present invention also provides isolated peptides of Cry j I and Cry j II. Peptides within the scope of the invention comprise at least one T cell epitope, or preferably at least two T cell epitopes of Cry j I or Cry j II. The invention also pertains to modified peptides having similar or enhanced therapeutic properties as the corresponding naturally-occurring allergen or portion thereof but having reduced side effects. Methods of treatment or of diagnosis of sensitivity to Japanese cedar pollens in an individual and therapeutic compositions, and multipeptide formulations comprising one or more peptides of the invention are also provided.
Owner:MERCK PATENT GMBH
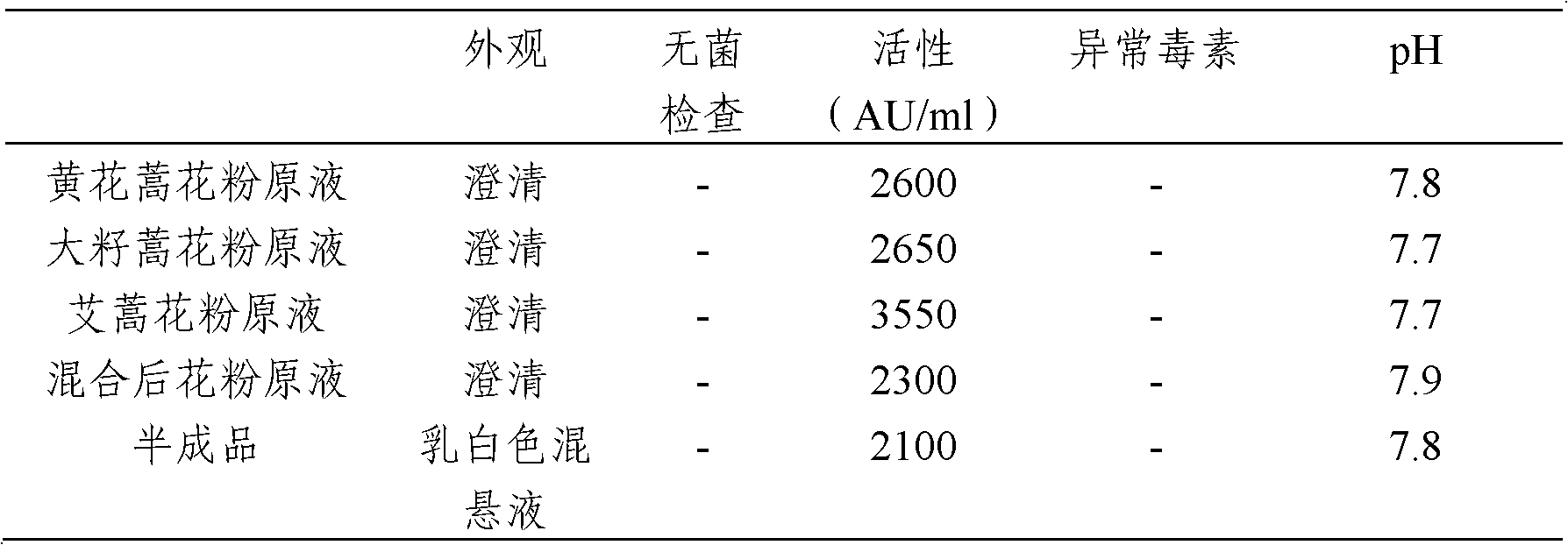
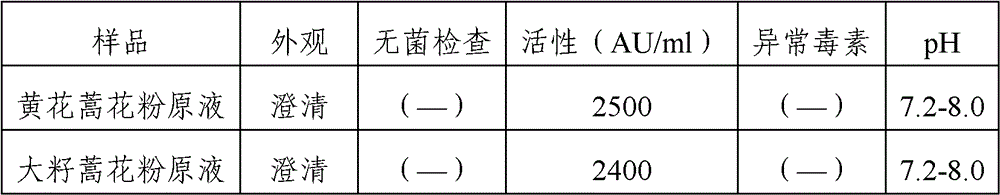
Stable medicinal composition containing artemisia pollen allergen and preparation method thereof
The invention provides a stable liquid medicinal composition. The liquid medicinal composition contains therapeutically effective amount or diagnostically effective amount of artemisia pollen allergen, buffering effective amount of buffer solution with pH of 5.0 to 7.0, and pharmaceutically acceptable carrier. The form of the liquid medicinal composition is preferable oral liquid, injection, sublingual buccal tablets, aerosol, nasal agent or skin pricking agent. The invention also provides a method for preparing the liquid medicinal composition. The liquid medicinal composition provided by the invention can be used for treating or diagnosing various allergic diseases caused by artemisia pollen, and has broad application prospect and clinical value in the field of allergic disease diagnosis and treatment.
Owner:ZHEJIANG WOLWO BIOTECH
Chemiluminiscence diagnostic kit for sensitization allergens and preparation method thereof
InactiveCN103033611AHigh sensitivityGood repeatabilityChemiluminescene/bioluminescenceSorbentWestern blot
The invention provides a chemiluminiscence diagnostic kit for sensitization allergens and a preparation method thereof. The diagnostic kit comprises a microporous plate coated with the sensitization allergens, a horseradish peroxidase-labeled anti-human IgE (immunoglobulin e) antibody, chemiluminiscence substrate solutions A and B, and a washing solution, wherein the sensitization allergens comprise one or more of an insect allergen, a pollen allergen, a fungus allergen and a food allergen. According to the diagnostic kit, the difference of the sensitivities of different proteins in one diagnostic kit is overcome, and the detection sensitivity of each allergen is enhanced to the maximum extent by repeatedly testing and adjusting the coating amount of the allergens, the concentration of the enzyme-labeled antibody and the formulae and the concentrations of the chemiluminiscence substrate solutions. The chemiluminiscence diagnostic kit is higher in detection sensitivity, safe, reliable, simple and convenient to operate and low in cost in comparison with the ELISA (enzyme linked immunosorbent assay), western-blot and RAST (radioallergo-sorbent test) diagnostic kits for the allergens.
Owner:北京新华联协和药业有限责任公司
Artemisia pollen allergen vaccine, and preparation method and application thereof
InactiveCN102552900AImprove effectivenessGood curative effectAllergen ingredientsImmunological disordersAllergic dermatitisDisease
The invention provides an artemisia pollen allergen vaccine, and a preparation method and application thereof. The artemisia pollen allergen vaccine comprises an artemisia sieversiana pollen allergen, an artemisia annua pollen allergen and an artemisia argyi pollen allergen. Compared with a single artemisia pollen allergen vaccine, the combined vaccine has the advantages that the effectiveness of treatment is improved while the safety can be ensured; and the artemisia pollen allergen vaccine can be used for treating allergic dermatitis, chronic urticaria, allergic rhinitis and allergic asthma which are caused by allergy to multiple artemisia pollens.
Owner:北京新华联协和药业有限责任公司
T cell epitope peptide
InactiveUS7112329B1Increase productionInhibit productionImmunoglobulin superfamilySenses disorderBet v I allergenAllergy
Owner:MEIJI CO LTD
Application of acidic buffer in stable pollen allergen activity
ActiveCN101496901AStable allergen activityAerosol deliveryInorganic non-active ingredientsPollenBuffer solution
The invention discloses application of an acid buffer solution with the pH value of between 3.0 and 6.0 (the preferred pH value is between 4.0 and 5.0) in stabilizing the activity of pollen allergen. The invention also discloses a pharmaceutical composition, which contains at least one allergen from pollen, the acid buffer solution with the pH value of buffer effective amount of between 3.0 and 6.0, and a pharmaceutically acceptable carrier. The pharmaceutical composition can be stored, used and transported at normal temperature.
Owner:ZHEJIANG WOLWO BIOTECH
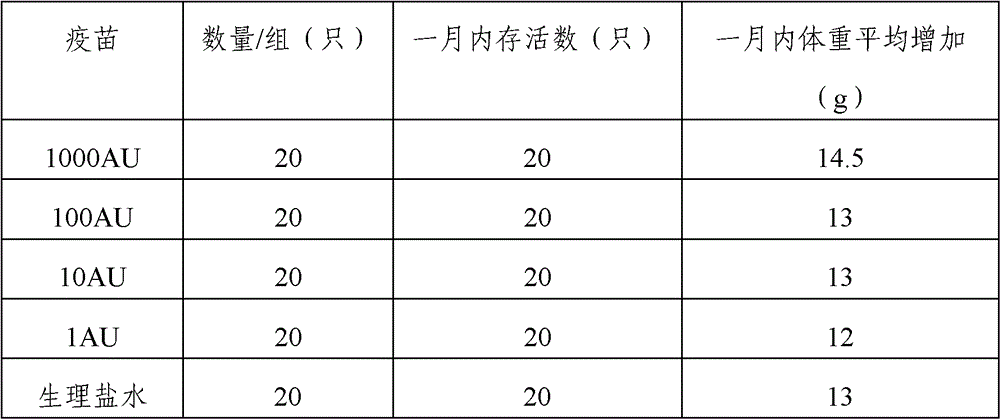
Artemisia pollen allergen vaccine and preparation method thereof
ActiveCN102512672AReduce doseLow cost of treatmentAllergen ingredientsRespiratory disorderDiseaseAdjuvant
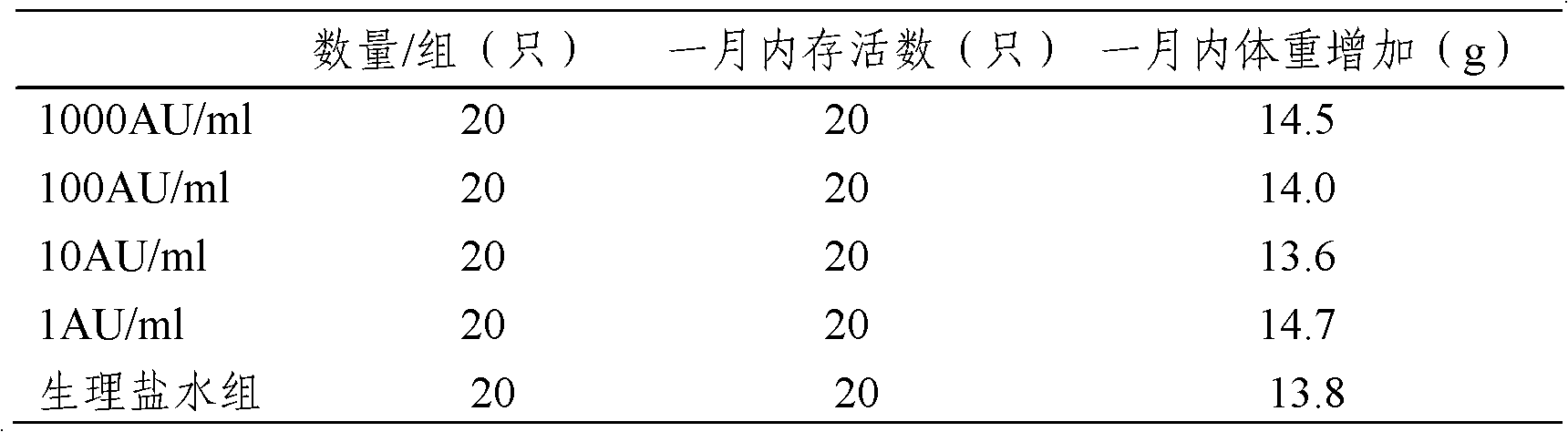
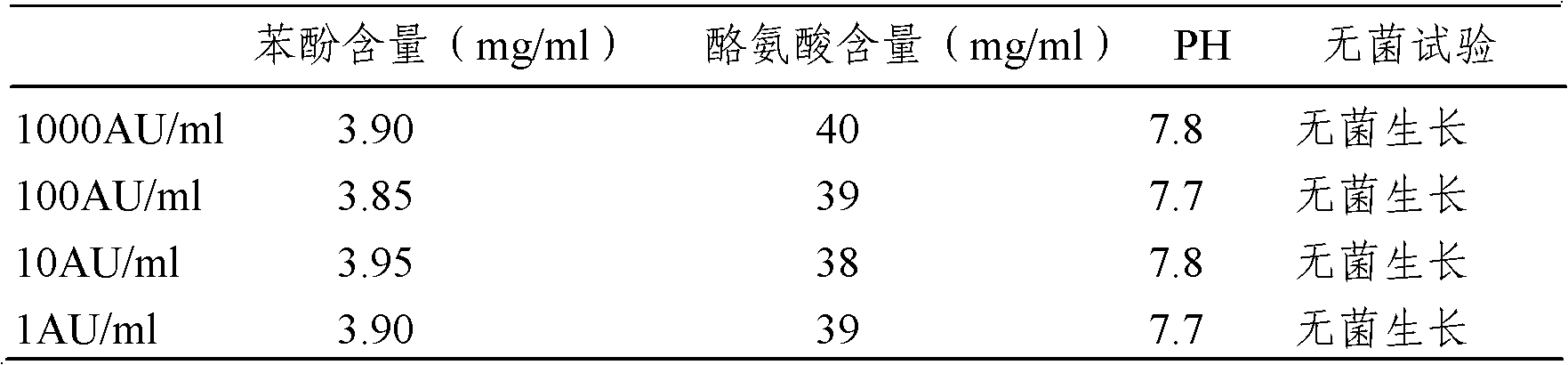
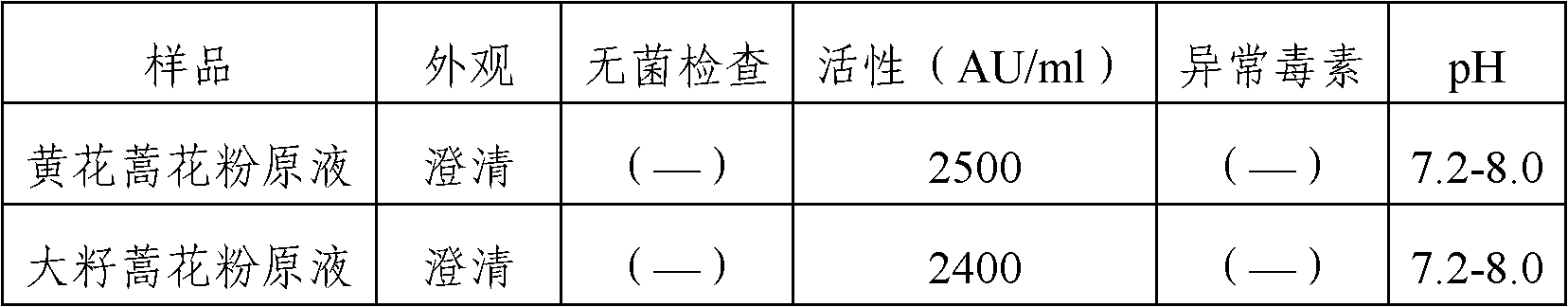
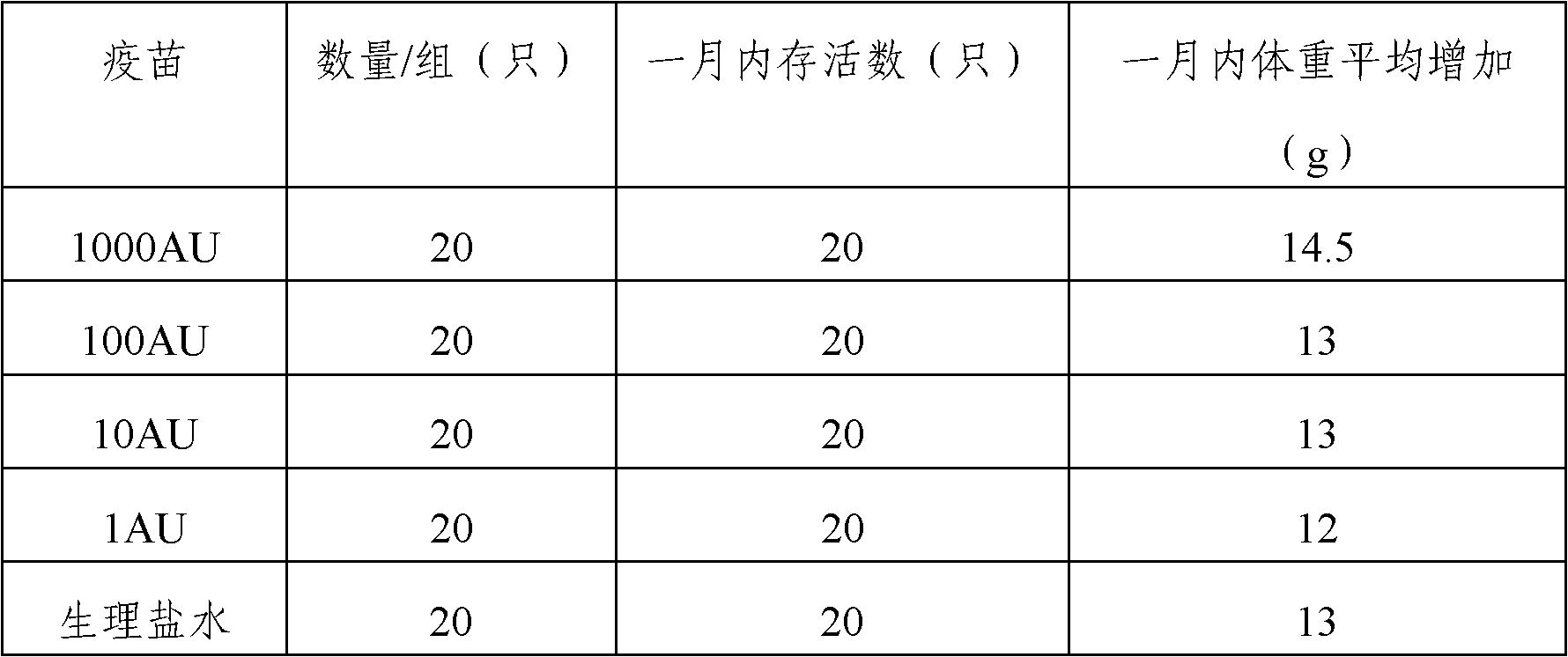
The invention provides an artemisia pollen allergen injection vaccine comprising artemisia pollen allergen and an L-tyrosine adjuvant, wherein the artemisia pollen allergen comprises sievers wormwood herb pollen allergen, sweet wormwood herb pollen allergen, and mugwort pollen allergen. The activity of the sievers wormwood herb pollen allergen is 1-1000AU / ml, the activity of the sweet wormwood herb pollen allergen is 1-1000AU / ml, and the activity of the mugwort pollen allergen is 1-1000AU / ml. The invention also provides a preparation method of the vaccine, and an application of the vaccine in preparing medicines used for treating anaphylactic diseases. According to the invention, three types of artemisia pollen allergen are adopted, such that the efficacy is higher than that of single artemisia pollen allergen. The artemisia pollen allergen is adsorbed onto the L-tyrosine adjuvant, such that an in-vivo release-retarding effect is provided, and efficacy and safety of the medicines are improved. The administration dosage of the vaccine during an entire treatment period is greatly lower (by approximately 100 times) than that of sublingual drops. With the vaccine, the treatment cost is greatly lower than that of sublingual drops immunological desensitization, such that patient burden of medical treatment is reduced.
Owner:北京新华联协和药业有限责任公司
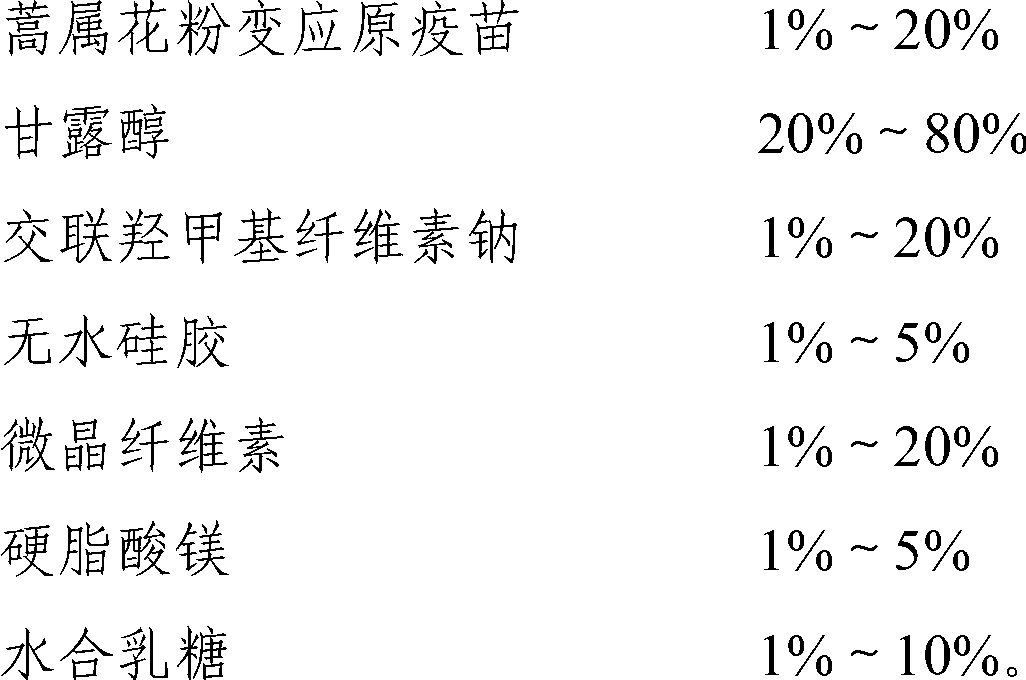
Artemisia pollen allergen vaccine lozenge and preparation method thereof
ActiveCN102988979AImprove effectivenessEasy to transportAllergen ingredientsPill deliveryPollenArtemisia annua
The invention provides Artemisia pollen allergen vaccine lozenge and a preparation method thereof, wherein the lozenge is prepared by mixing idiotoxins of five kinds of sensitized Artemisia pollens and pharmaceutically acceptable carriers; and the five kinds of sensitized Artemisia plants are Artemisia sieversiana Willd, Artemisia annua L., Artemisia argyi Levl.et Vant, A.ordosica krasch and A.sphaerocep hala krasch. The Artemisia pollen allergen vaccine disclosed by the invention includes idiotoxins of five kinds of main sensitized Artemisia pollens in China; compared with the single Artemisia pollen allergen vaccine, the Artemisia pollen allergen vaccine disclosed by the invention is more effective; furthermore, compared with the sublingual drop, the sublingual lozenge is more steady; and the administration dosage is more accurate.
Owner:北京新华联协和药业有限责任公司
Platanus pollen allergen mixture
InactiveCN104083758AImprove toleranceReduce seizuresAllergen ingredientsRespiratory disorderPlatanus orientalisPlatanus occidentalis
The invention provides a platanus pollen allergen mixture and a preparation method thereof. The mixture is prepared by mixing three types of platanus pollen allergens and a pharmaceutically acceptable carrier, wherein the three sensitizing platanus plants are platanus occidentalis, platanus acerifolia and platanus orientalis. The platanus pollen allergen mixture comprises three main sensitizing platanus pollen allergens, and is more effective than a single platanus pollen allergen preparation.
Owner:北京新华联协和药业有限责任公司
Injectable artemisia pollen allergen vaccine and preparation method thereof
ActiveCN102512673AImprove effectivenessImprove securityAllergen ingredientsRespiratory disorderAllergic dermatitisChronic urticaria
The invention relates to an injectable artemisia pollen allergen vaccine and a preparation method thereof. The injectable artemisia pollen allergen vaccine contains artemisia pollen allergens and an aluminum adjuvant, wherein the artemisia pollen allergens comprise artemisia sieversiana pollen allergens, sweet wormwood pollen allergens and argy wormwood pollen allergens. The injectable artemisia pollen allergen vaccine has obvious effects of treating hypersensitivity diseases such as allergic dermatitis, chronic urticaria, allergic rhinitis and allergic asthma.
Owner:北京新华联协和药业有限责任公司
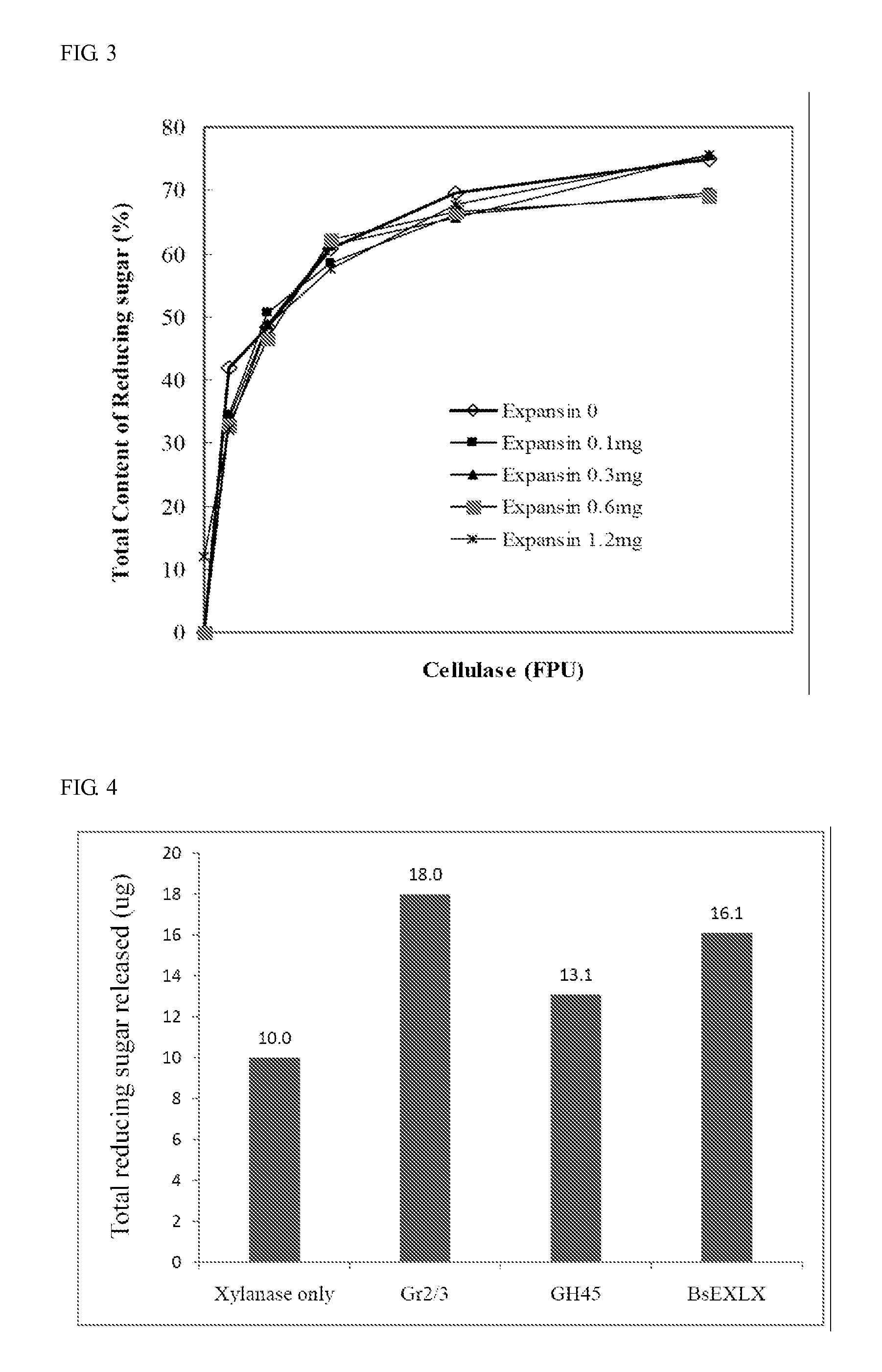
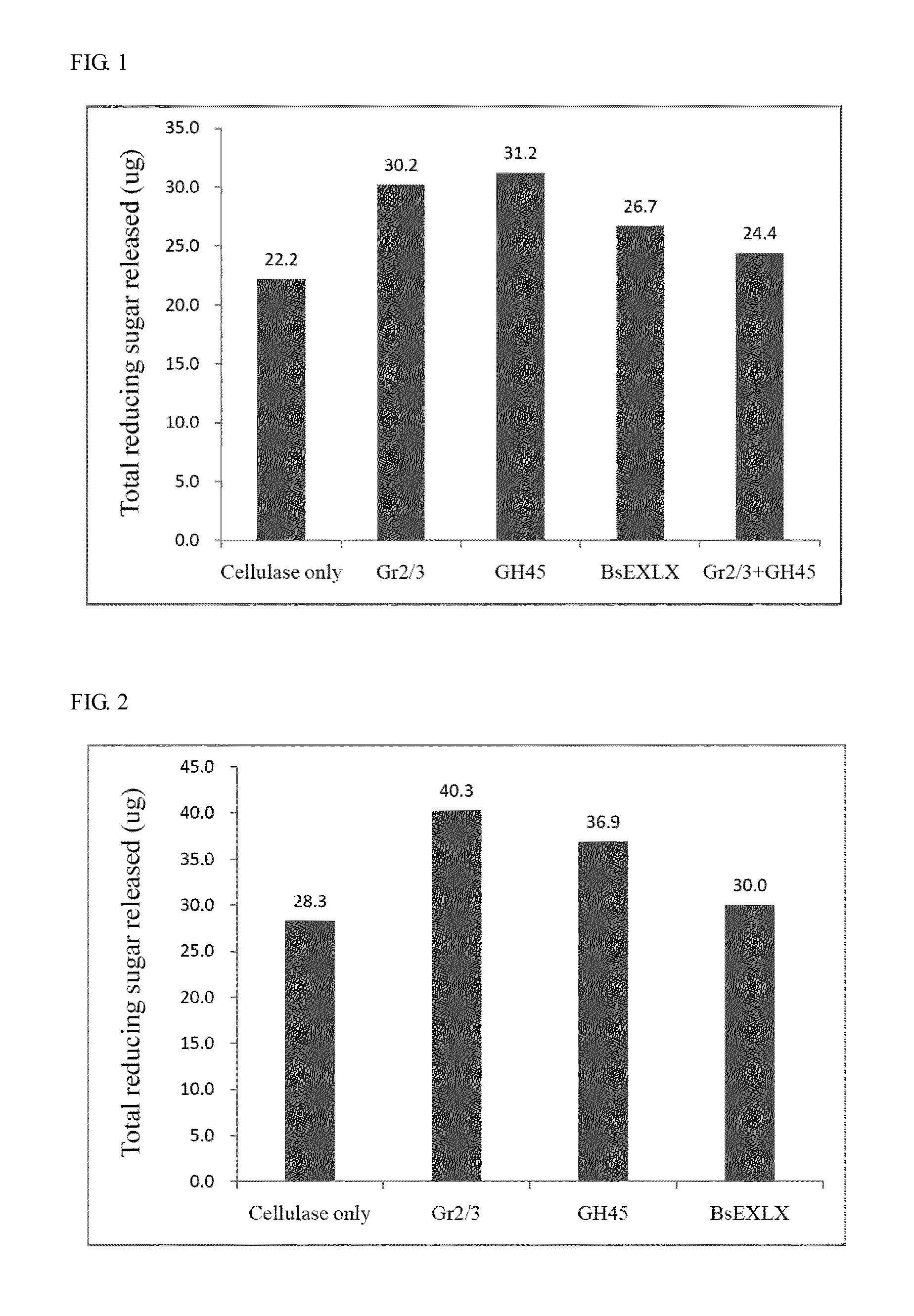
Isolated polypeptide for increasing activity of polysaccharide hydrolase and methods of use
InactiveUS20110177565A1Peptide/protein ingredientsLighting and heating apparatusPolysaccharide HydrolaseHydrolase
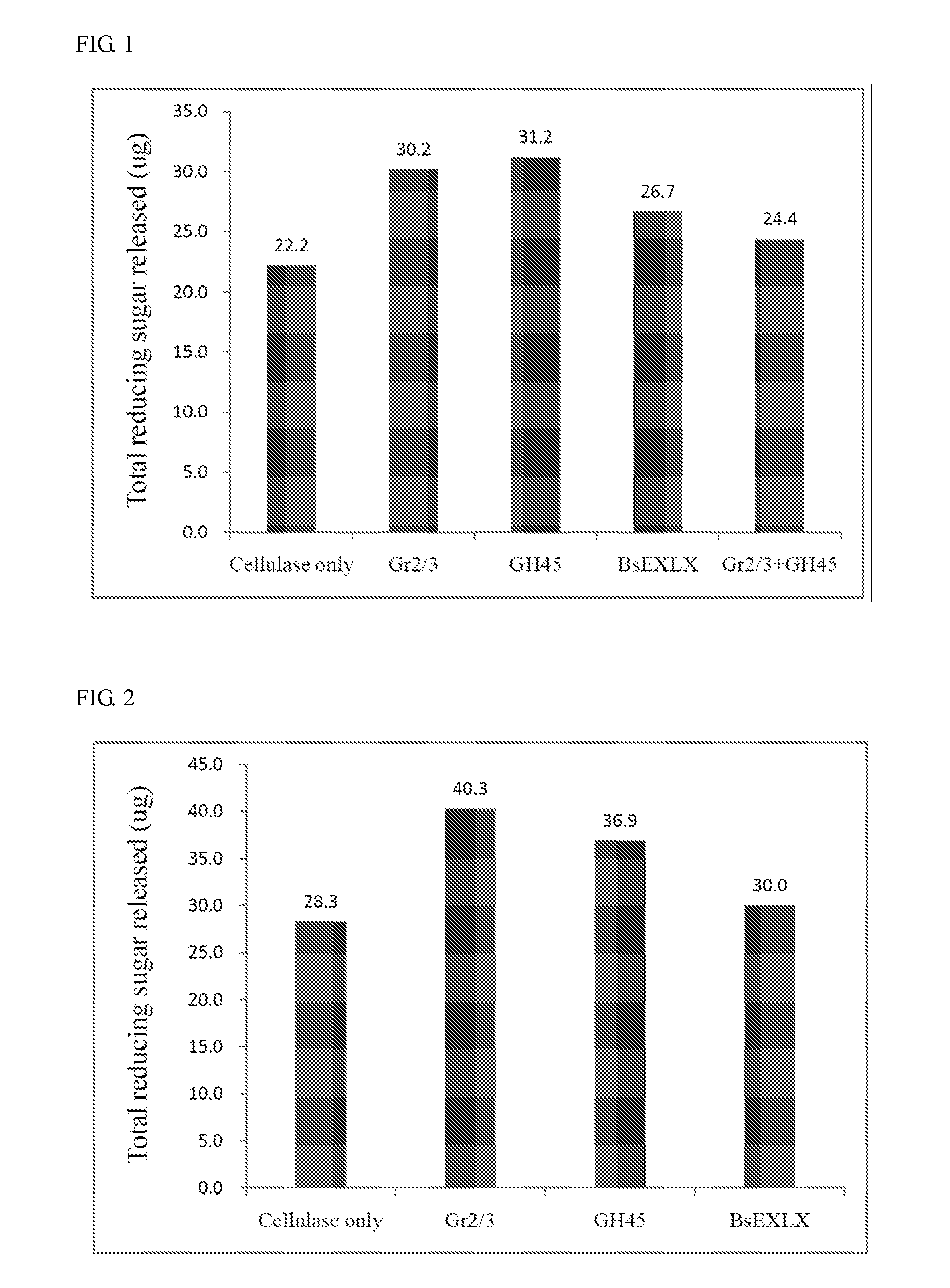
An isolated polypeptide is disclosed that improves the hydrolyzing capacity of a polysaccharide hydrolase such as cellulase, is capable of binding to a polysaccharide, is deficient in polysaccharide hydrolase activity, and includes a GH45 or a pollen-allergen domain. Methods of hydrolyzing polysaccharides using the isolated polypeptide are also disclosed.
Owner:SAMSUNG ELECTRONICS CO LTD
Isolated polypeptide for increasing activity of polysaccharide hydrolase and methods of use
InactiveUS8492126B2Peptide/protein ingredientsLighting and heating apparatusPolysaccharide HydrolaseHydrolase
An isolated polypeptide is disclosed that improves the hydrolyzing capacity of a polysaccharide hydrolase such as cellulase, is capable of binding to a polysaccharide, is deficient in polysaccharide hydrolase activity, and includes a GH45 or a pollen-allergen domain. Methods of hydrolyzing polysaccharides using the isolated polypeptide are also disclosed.
Owner:SAMSUNG ELECTRONICS CO LTD
A system and method for determining a risk level of a pollen-induced allergy of a user
The invention provides a system for determining a risk level of a pollen-induced allergy of a user. The system comprises an input for receiving information relating to a pollen level in a location, aninput for receiving information relating to a particulate matter level in the location and an input for receiving information relating to a sensitivity of a user to pollen allergen. The system further comprises a processor which is adapted to determine the risk level by taking account of the information relating to a pollen level, the information relating to a particulate matter level and the information relating to a sensitivity of the user to pollen allergen. A user interface communicates the risk level in the location to the user.
Owner:KONINKLJIJKE PHILIPS NV
Allergenic proteins and peptides from Japanese cedar pollen
InactiveUS20050152927A1Less baseDiagnosing, treating, and preventing Japanese cedar pollinosisPeptide/protein ingredientsSnake antigen ingredientsNucleic acid sequencingCedar pollinosis
The present invention provides nucleic acid sequences coding for the Cryptomeria japonica major pollen allergen Cry j I, Cry j II, Jun s I and Jun v I and fragments or peptides thereof. The present invention also provides purified Cry j I, Cry j II, Jun s I and Jun v I and at least one fragment thereof produced in a host cell transformed with a nucleic acid sequence coding for Cry j I, Cry j II, Jun s I and Jun v I or at least one fragment thereof, and fragments of Cry j I, Cry j II, Jun s I or Jun v I or at least one fragment thereof, and fragments of Cry j I, Cry j II, Jun s I or Jun v I prepared synthetically. Cry j I, Cry j II, Jun s I and Jun v I and fragments thereof are useful for diagnosing, treating, and preventing Japanese cedar pollinosis. The present invention also provides isolated peptides of Cry j I and Cry j II. Peptides within the scope of the invention comprise at least one T cell epitope, or preferably at least two T cell epitopes of Cry j I or Cry j II. The invention also pertains to modified peptides having similar or enhanced therapeutic properties as the corresponding naturally-occurring allergen or portion thereof but having reduced side effects. Methods of treatment or of diagnosis of sensitivity to Japanese cedar pollens in an individual and therapeutic compositions, and multipeptide formulations comprising one or more peptides of the invention are also provided.
Owner:MERCK PATENT GMBH
Platanus pollen allergen Pla a 3 and monoclonal antibody thereof
InactiveCN105481957AImmunoglobulins against plantsTissue cultureMonoclonal antibodyAllergy disorders
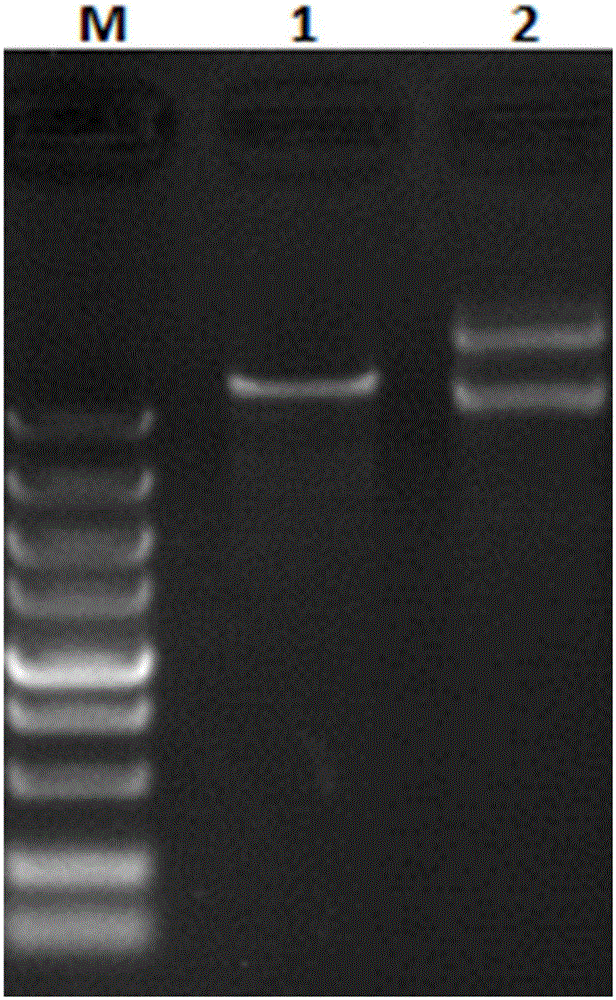
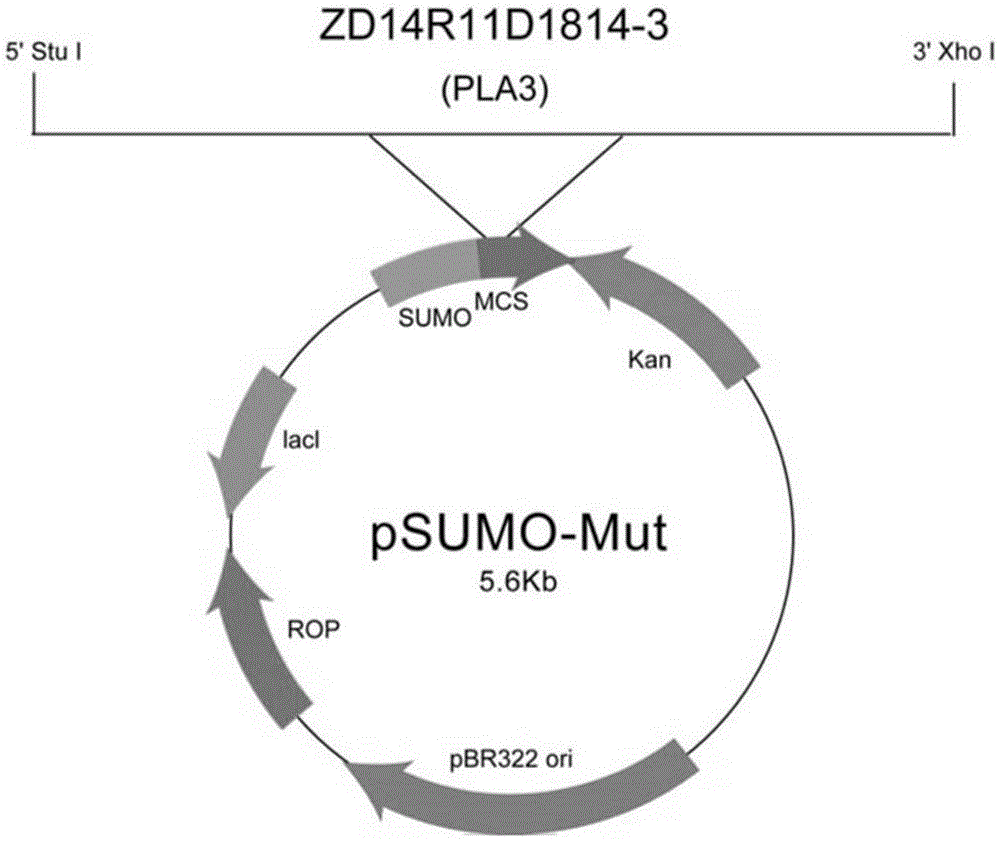
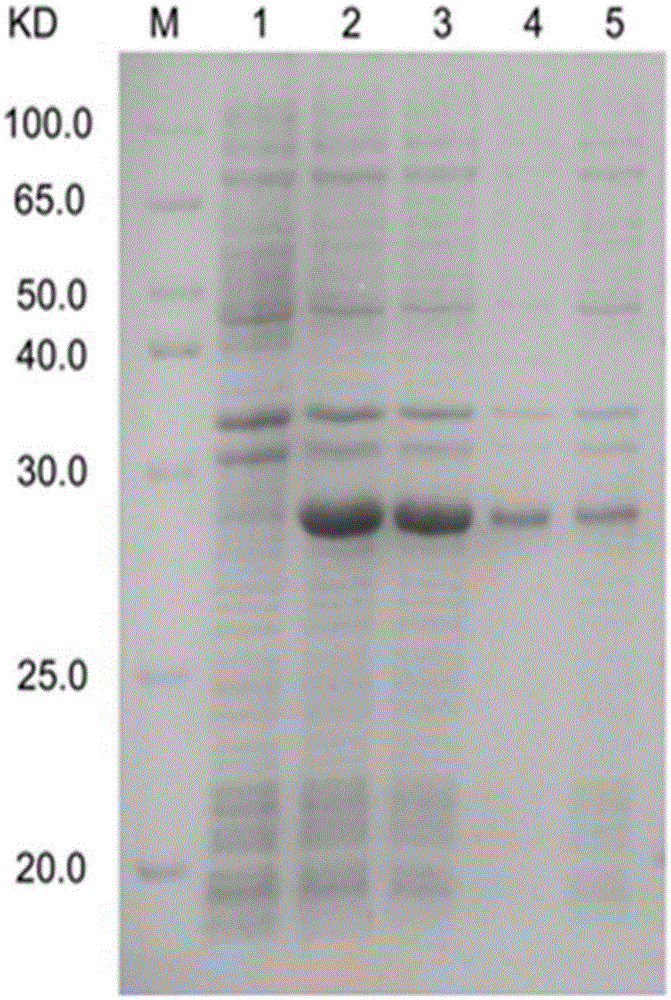
The invention discloses a platanus pollen allergen Pla a 3, a monoclonal antibody thereof and a preparation method of the monoclonal antibody. The platanus pollen allergen Pla a 3 is separated from phoenix tree pollen in a purification mode, and the apparent molecular weight of fusion protein SUMO-Pla a 3 of the platanus pollen allergen Pla a 3 is measured as 28 kDa through SDS-PAGE. The protein Pla a 3 is separated from the phoenix tree pollen in the purification mode, the molecular weight of the fusion protein SUMO-Pla a 3 of the platanus pollen allergen Pla a 3 is measured as 28 kDa through SDS-PAGE. The monoclonal antibody prepared through the platanus pollen allergen Pla a 3 is characterized in that the monoclonal antibody can be specifically combined with the platanus pollen allergen Pla a 3 and plays an important role in diagnosis and treatment of allergic skin diseases, asthmas and other I-type allergic diseases caused by phoenix tree pollen.
Owner:JIANGSU PROVINCE HOSPITAL
Ragweed pollen allergen extract, ragweed pollen allergen extraction liquid and preparation method of extract
ActiveCN109939227AEfficient diagnosis of allergic diseasesImprove effectivenessPowder deliveryAllergen ingredientsDiseaseBasophilic Granulocyte
The invention relates to a ragweed pollen allergen extract, ragweed pollen allergen extraction liquid and a preparation method of the extract. The ragweed pollen allergen extraction liquid includes ragweed pollen allergens containing the amino acid sequences shown in SEQIDNO.4 to SEQIDNO.9. The ragweed pollen allergen extraction liquid is prepared through ragweed pollen collection, drying, degreasing, extraction, ultra-filtration concentration, freeze drying, re-dissolving and the like and mainly includes ragweed pollen allergens, glycerinum, sodium chloride and the like. The ragweed pollen allergen extraction liquid has the advantage of being high in specificity, allergenic protein components of ragweed are fully extracted, the total biological value is stable, the service life is long, and the sterile effect is good. The stoste of the ragweed pollen allergen extraction liquid can be effectively used for skin prick test diagnosis of inspiratory allergosis, in-vitro basophilic granulocyte activation test diagnosis, intracutaneous test diagnosis and specific immunotherapy after appropriate dilution, and can effectively diagnose inspiratory allergosis induced by ragweed pollen and conduct specific immunotherapy on the inspiratory allergosis.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
Artemisia pollen allergen vaccine lozenge and preparation method thereof
ActiveCN102988979BImprove effectivenessEasy to transportAllergen ingredientsPill deliveryPollenArtemisia annua
Owner:北京新华联协和药业有限责任公司
Injectable artemisia pollen allergen vaccine and preparation method thereof
ActiveCN102512673BImprove effectivenessImprove securityAllergen ingredientsRespiratory disorderAllergic dermatitisAdjuvant
The invention relates to an injectable artemisia pollen allergen vaccine and a preparation method thereof. The injectable artemisia pollen allergen vaccine contains artemisia pollen allergens and an aluminum adjuvant, wherein the artemisia pollen allergens comprise artemisia sieversiana pollen allergens, sweet wormwood pollen allergens and argy wormwood pollen allergens. The injectable artemisia pollen allergen vaccine has obvious effects of treating hypersensitivity diseases such as allergic dermatitis, chronic urticaria, allergic rhinitis and allergic asthma.
Owner:北京新华联协和药业有限责任公司
Artemisia plant pollen allergen combination, application and kit
The invention discloses an artemisia plant pollen allergen combination for predicting an occurrence risk of asthma of an artemisia allergy patient, an application and a kit. The artemisia plant pollenallergen combination comprises Art v 1, Art ar 2, Art v 3 and Art an 7. The research on 240 artemisia pollen allergy cases discovers that when at least three kinds of allergen specific IgE in the Artv 1, the Art ar 2, the Art v 3 and the Art an 7 are positive in an artemisia pollen allergy case detection result, the occurrence rate of allergic asthma is 57%; when the positive number of the fourallergens is less than 3, the incidence rate of the allergic asthma is 24%; and the former is 2.4 times of the latter. By detecting the allergic patient, the patient with the high occurrence risk of the allergic asthma judged by detection can be possibly cured only by drug control and desensitization treatment, so that the occurrence of the allergic asthma is avoided.
Owner:杭州艾乐吉生物科技有限公司
T-cell epitope peptides
InactiveUS20060188446A1Simple wayUltrasonic/sonic/infrasonic diagnosticsSenses disorderGeneticsAllergy
Owner:MEIJI CO LTD
Application of acidic buffer in stable pollen allergen activity
ActiveCN101496901BAerosol deliveryInorganic non-active ingredientsBiotechnologyPharmaceutical medicine
The invention discloses application of an acid buffer solution with the pH value of between 3.0 and 6.0 (the preferred pH value is between 4.0 and 5.0) in stabilizing the activity of pollen allergen. The invention also discloses a pharmaceutical composition, which contains at least one allergen from pollen, the acid buffer solution with the pH value of buffer effective amount of between 3.0 and 6.0, and a pharmaceutically acceptable carrier. The pharmaceutical composition can be stored, used and transported at normal temperature.
Owner:ZHEJIANG WOLWO BIOTECH
London plane tree pollen allergen extract, extracting solution and preparation method thereof
ActiveCN110064052AEfficient diagnosis of allergic diseasesImprove effectivenessCompounds screening/testingPowder deliveryDiseaseFiltration
The invention relates to a London plane tree pollen allergen extract and an extracting solution and a preparation method thereof. The amino acid sequences of London plane tree pollen contained in theLondon plane tree pollen allergen extract are shown in SEQ ID NO.4 and SEQ ID NO.5, and the extracting solution can be prepared by London plane tree pollen collection, drying, degreasing, extracting,ultra-filtration concentrating, freeze-drying, re-dissolving and other process methods. The London plane tree pollen allergen extracting solution has the characteristic of high specificity, and the London plane tree pollen component is fully extracted, the total biological titer is stable, the validity period is long, and the aseptic effect is good. Its stock solution can be effectively used for skin prick test diagnosis of allergic reaction diseases, can be used for in-vitro basophil activation test diagnosis, intradermal test diagnosis and specific immunotherapy after proper dilution (10-2 to 10-20), and can effectively diagnose and carry out specific immunotherapy on allergic reaction diseases induced by London plane tree pollen.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
Preparation method and application of recombination ryegrass pollen allergen and mutant thereof
InactiveCN102675439AReduce the binding forceAllergenicity reductionAllergen ingredientsPlant peptidesBiotechnologyDisease
The invention specifically discloses a preparation method and application of a recombination ryegrass pollen allergen and a mutant thereof. The recombination ryegrass pollen allergen disclosed by the invention is a mutant which exists in a non-natural manner and is derived by an allergen naturally existing; and the amino acid sequence of the recombination ryegrass pollen allergen is shown as SEQIDNO.1. According to the preparation method and application disclosed by the invention, a coding gene of the recombination ryegrass pollen allergen is artificially synthesized by optimizing a codon by using a genetic engineering technology; an antigenic epitope of the coding gene is analyzed by adopting bioinformatics software; and the directional displacement for one or more loci with high allergen is realized by site-specific mutagenesis, and thus the allergic endogenous property of the loci is reduced and the mutant of the recombination ryegrass pollen allergen with low allergic endogenous property is constructed. The protein expression is carried out by artificial control, and thus the recombination ryegrass pollen allergen with high purity and a mutant protein of the recombination ryegrass pollen allergen are obtained. Compared with natural allergen, the recombination ryegrass pollen allergen has the characteristic that the combining capacity between the recombination protein mutant and specific IgE (Immunoglobulin E) is remarkably reduced; and the recombination ryegrass pollen allergen can be safely applied to diagnosis and treatment of allergic diseases even the judgment for the levels of other allergic endogenous properties.
Owner:THE SECOND AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV
Kochia scoparia(l.)schrad. pollen allergen extract and extract liquid and preparation method of extract liquid
ActiveCN110201188AEfficient diagnosis of allergic diseasesImprove effectivenessCompounds screening/testingAllergen ingredientsUltrafiltrationFreeze-drying
The invention provides an kochia scoparia(l.)schrad. pollen allergen extract and extract liquid and a preparation method of the extract liquid. The kochia scoparia(l.)schrad. pollen allergen extract contains a sensitizing protein Koc S1' containing an amino acid sequence shown in SEQ ID NO.4. The kochia scoparia(l.)schrad. pollen allergen extract liquid contains a therapeutic or diagnostic effective dose of the kochia scoparia(l.)schrad. pollen allergen. The extract liquid can also be prepared by process methods of kochia scoparia(l.)schrad. pollen collection, drying, degreasing, extraction, ultrafiltration concentration, freeze-drying and re-dissolution and the like. The kochia scoparia(l.)schrad. pollen allergen extract liquid has the advantages of high specificity, a kochia scoparia(l.)schrad. sensitizing protein component is extracted adequately, the total biological potency is stable, the period of validity is long, and the sterile effect is good; the kochia scoparia(l.)schrad. pollen allergen extract liquid can be effectively used in skin prick test diagnosis of allergic diseases, in-vitro basophil activation test diagnosis and specific immunotherapy, and can effectively diagnose allergic diseases induced by gkochia scoparia(l.)schrad. pollen and performs specific immunotherapy.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
A system and method for determining a risk level of a pollen-induced allergy of a user
PendingUS20200227171A1Facilitated releaseImprove the level ofHealth-index calculationMedical automated diagnosisRisk levelPhysical medicine and rehabilitation
The invention provides a system for determining a risk level of a pollen-induced allergy of a user. The system comprises an input for receiving information relating to a pollen level in a location, an input for receiving information relating to a particulate matter level in the location and an input for receiving information relating to a sensitivity of a user to pollen allergen. The system further comprises a processor which is adapted to determine the risk level by taking account of the information relating to a pollen level, the information relating to a particulate matter level and the information relating to a sensitivity of the user to pollen allergen. A user interlace communicates the risk level in the location to the user.
Owner:VERSUNI HLDG BV
Dna sequence, and recombinant preparation of the grass pollen allergen lol p4
InactiveUS20070154496A1Prevent adverse side effectsPeptide/protein ingredientsGenetic material ingredientsPollen AllergyA-DNA
The present invention relates to the provision of a DNA sequence of the major grass pollen allergen Lol p 4. The invention also encompasses fragments, new combinations of partial sequences and point mutants having a hypoallergenic action. The recombinant DNA molecules and the derived polypeptides, fragments, new combinations of partial sequences and variants can be utilised for the therapy of pollen-allergic diseases. The proteins prepared by recombinant methods can be employed for in vitro and in vivo diagnosis of pollen allergies.
Owner:MERCK PATENT GMBH
Method for removing pollen allergens from honey
The invention discloses a method for removing pollen allergens from honey. The method is as follows: subjecting honey to adsorption with 10# food-grade diatomite, pre-coating the 10# food-grade diatomite onto filter cloth to form compact filter medium, and performing plate-frame fine filtration to remove impurities which are 0.1 micron or above such as bacteria, yeast and pollen (pollen diameter is generally 30-50 microns) so as to effectively remove allergens and to produce desensitized honey without pollen allergen. The method of the invention is simple, reliable in effect and low in cost, and the eating trials by allergic people indicate that the prepared desensitized honey has no allergic phenomenon and enlarges the population who can eat the honey.
Owner:郑州港葡生物科技有限公司
Test strip for screening specific weedy pollen allergen IgE (Immunoglobulin E) and preparation method thereof
ActiveCN103995131ASimple and fast operationThe result is accurateDisease diagnosisBiological testingAntigenPollen allergen
The invention provides a test strip for screening specific weedy pollen allergen IgE (Immunoglobulin E). The test strip is coated by a weedy pollen antigen labeled by colloidal gold. The colloidal gold labelling technology is utilized in the invention; the specific weedy pollen allergen is labelled by using colloidal gold for the first time; then, the labelled allergen is uniformly sprayed on a cellulose acetate membrane or a nitrocellulose membrane, therefore, the test strip is prepared for detecting allergens; the test strip disclosed by the invention is simple and convenient to operate, accurate in result and low in cost; and a number of free tests can be realized.
Owner:BIOSINO BIO TECH & SCI
Antibody-mediated modulation of allergy
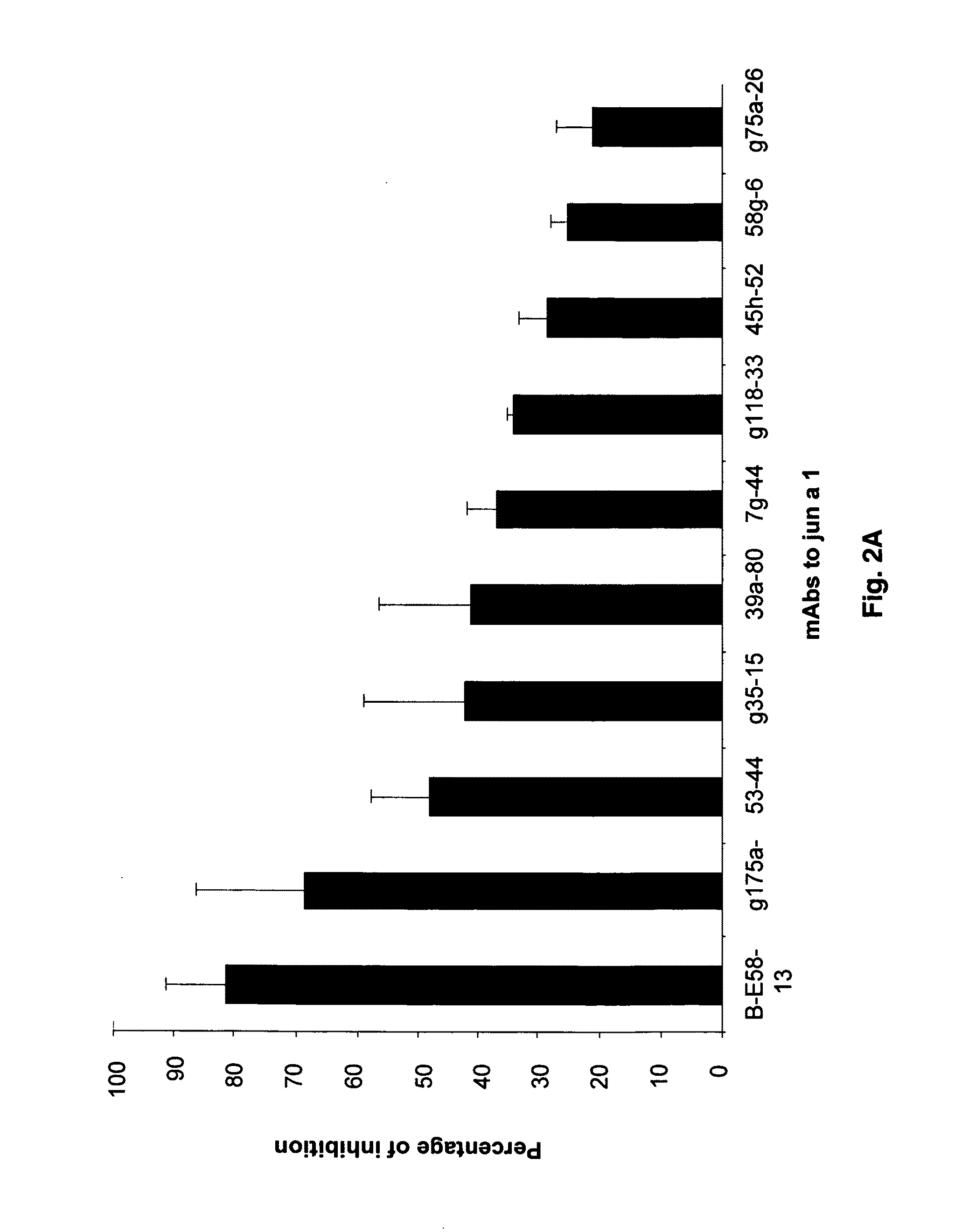
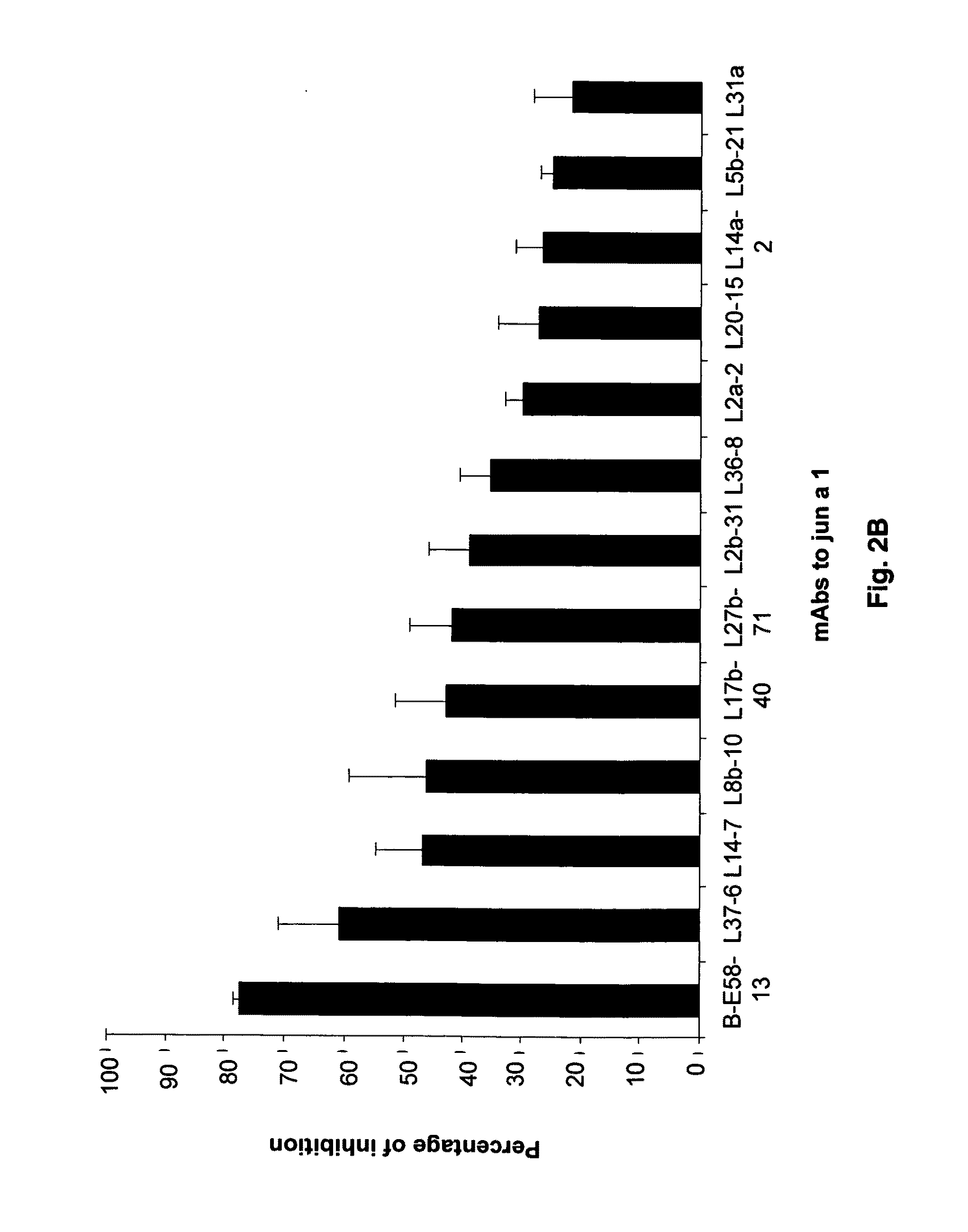
ActiveUS8883154B2Avoid developmentPeptide/protein ingredientsImmunoglobulins against plantsAntigenAntigen Binding Fragment
The present invention is drawn to antibody-mediated modulation of allergy. In this regard, the present invention discloses a monoclonal antibody, antigen-binding fragment or mimic thereof directed against Group 1 pollen allergens or homologues thereof. Also disclosed herein is the mechanism by which the disclosed monoclonal antibody, antigen binding fragment or mimic thereof will improve immunotherapy of allergic reactions in an individual. It is contemplated that herein that such a monoclonal antibody, antigen binding fragment or mimic thereof may also be useful in treatment of several microbial infections.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Humulus japonicas pollen allergen extract, infusion liquid and preparation method thereof
PendingCN110124028AEfficient diagnosis of allergic diseasesImprove effectivenessCompounds screening/testingPowder deliveryDiseaseBasophilic Granulocyte
The invention provides a Humulus japonicas pollen allergen extract, infusion liquid and a preparation method thereof. The Humulus japonicas pollen allergen extract contains an amino acid sequence of the Humulus japonicas pollen allergen protein shown as SEQ ID NO. 4-SEQ ID NO. 20. The infusion liquid is prepared by the processes of collecting of Humulus japonicas pollen, drying, degreasing, extracting, ultrafiltration concentrating, freeze drying, and redissolving. The Humulus japonicas pollen allergen infusion liquid has the characteristics of high specificity, the Humulus japonicas allergenic protein component is fully extracted, the total biological titer is stable, the effective period is long, and the sterilizing effect is good; an original solution can be effectively used for skin prick test diagnosis of allergic diseases, and can be used for in-vitro basophilic granulocyte activation test diagnosis, intradermal test diagnosis and specific immunotherapy after appropriate dilution(10-2-10-20), The infusion liquid can effectively diagnose allergic diseases induced by Humulus japonicas pollen and performs specific immunotherapy.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com