Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
63 results about "Adenovirus diseases" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Patients with compromised immune systems are especially susceptible to severe complications of adenovirus infection. Acute respiratory disease (ARD), first recognized among military recruits during World War II, can be caused by adenovirus infections during conditions of crowding and stress.
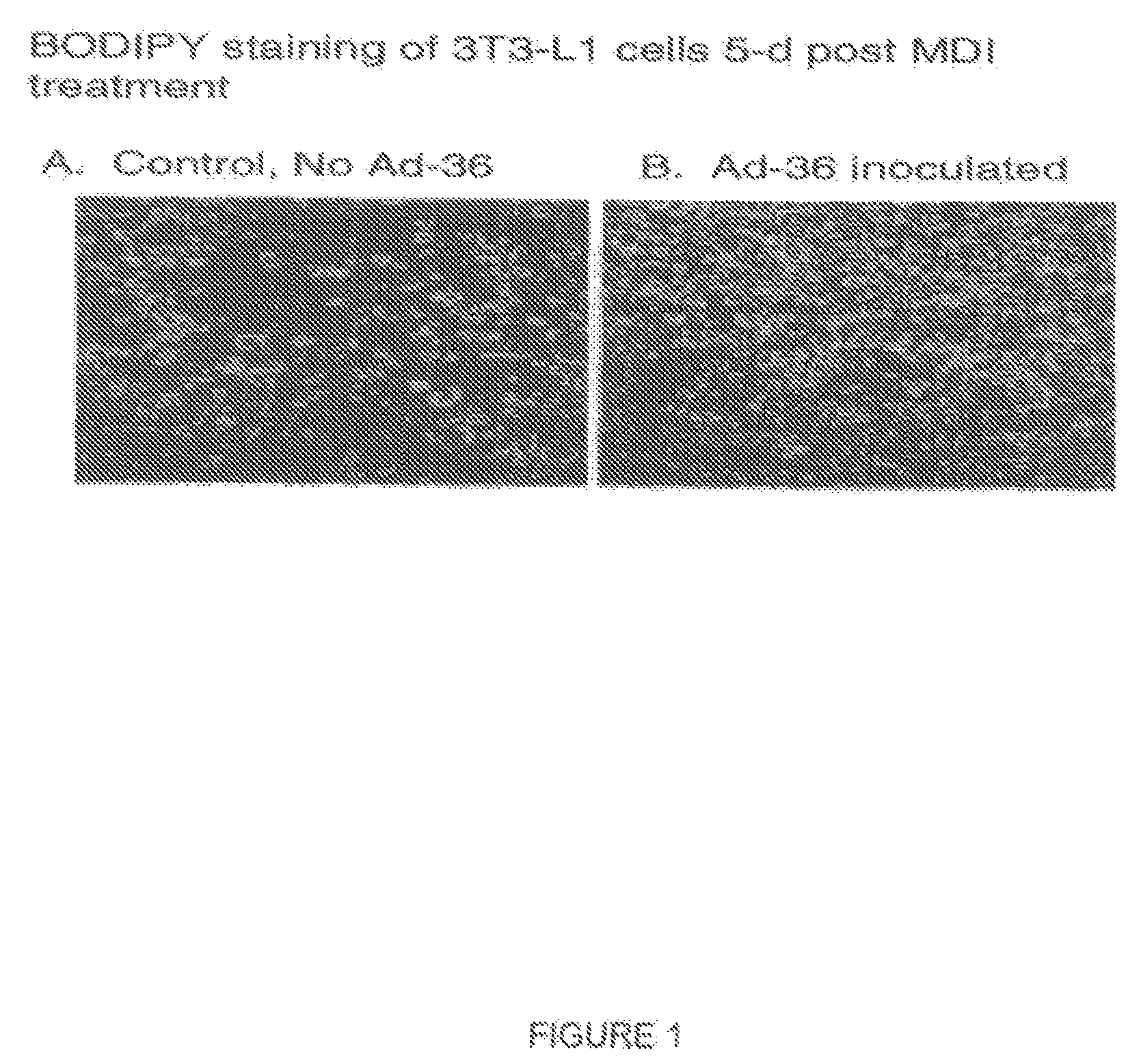
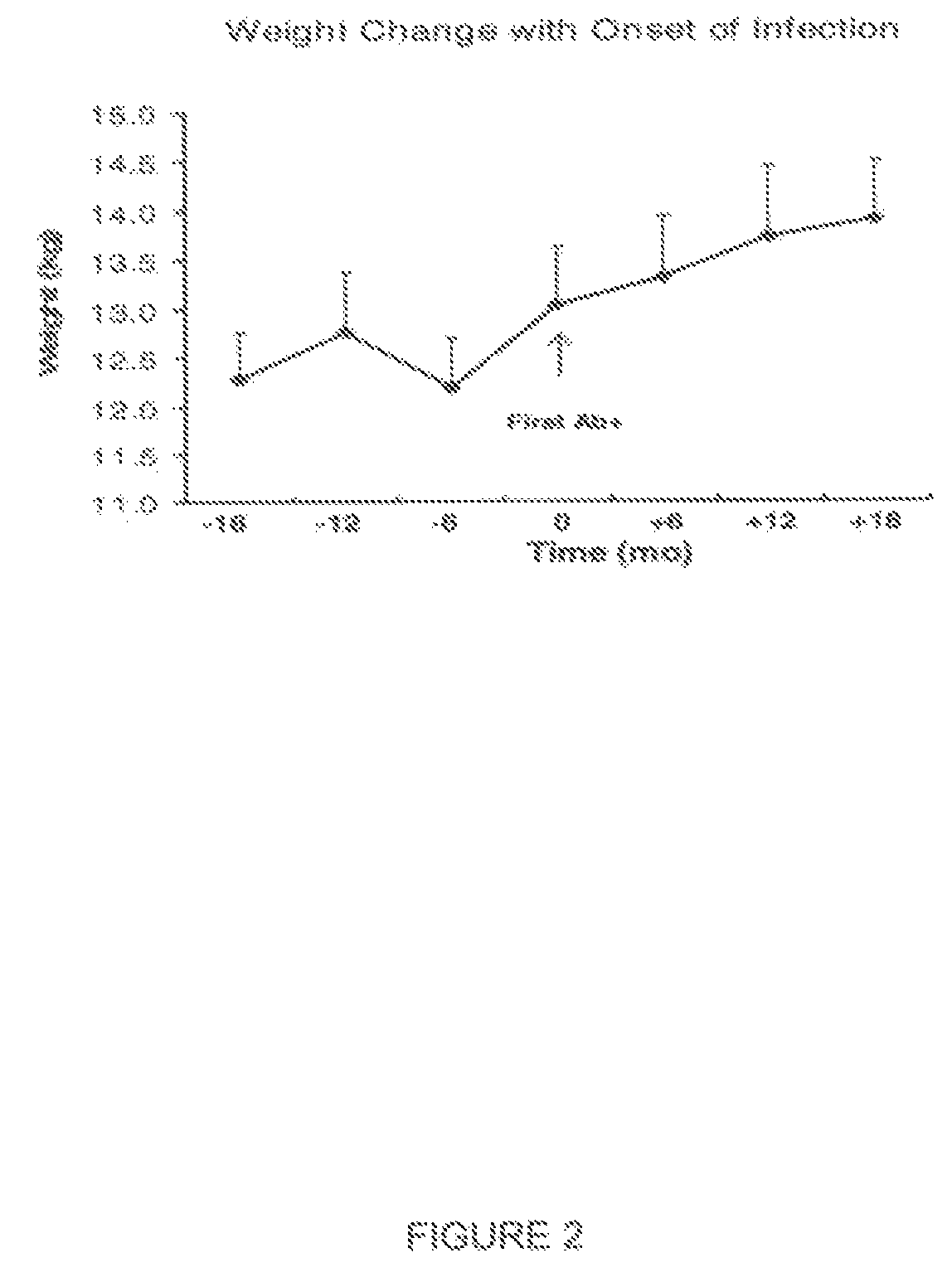
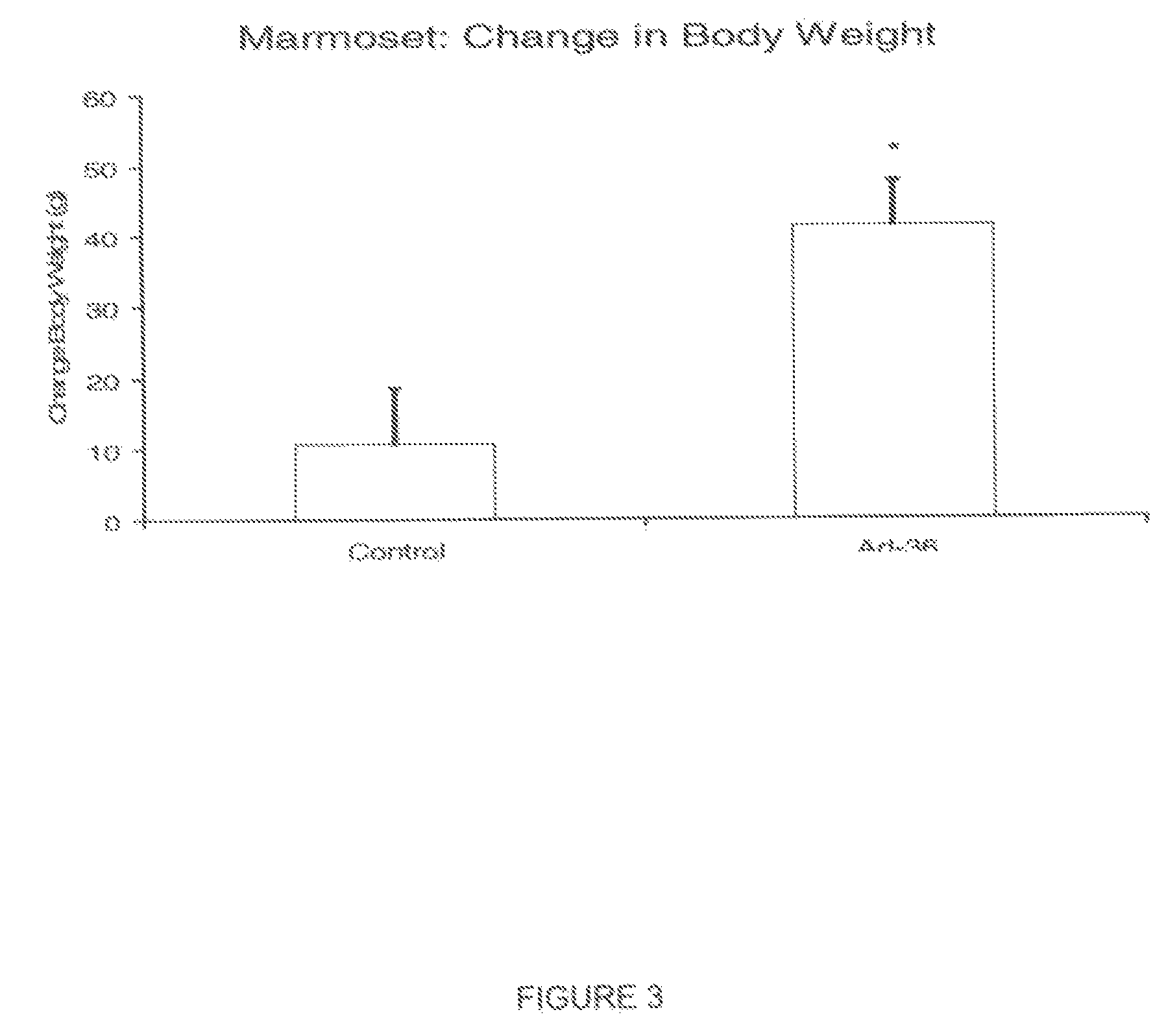
Method and system for preventing virus-related obesity and obesity related diseases
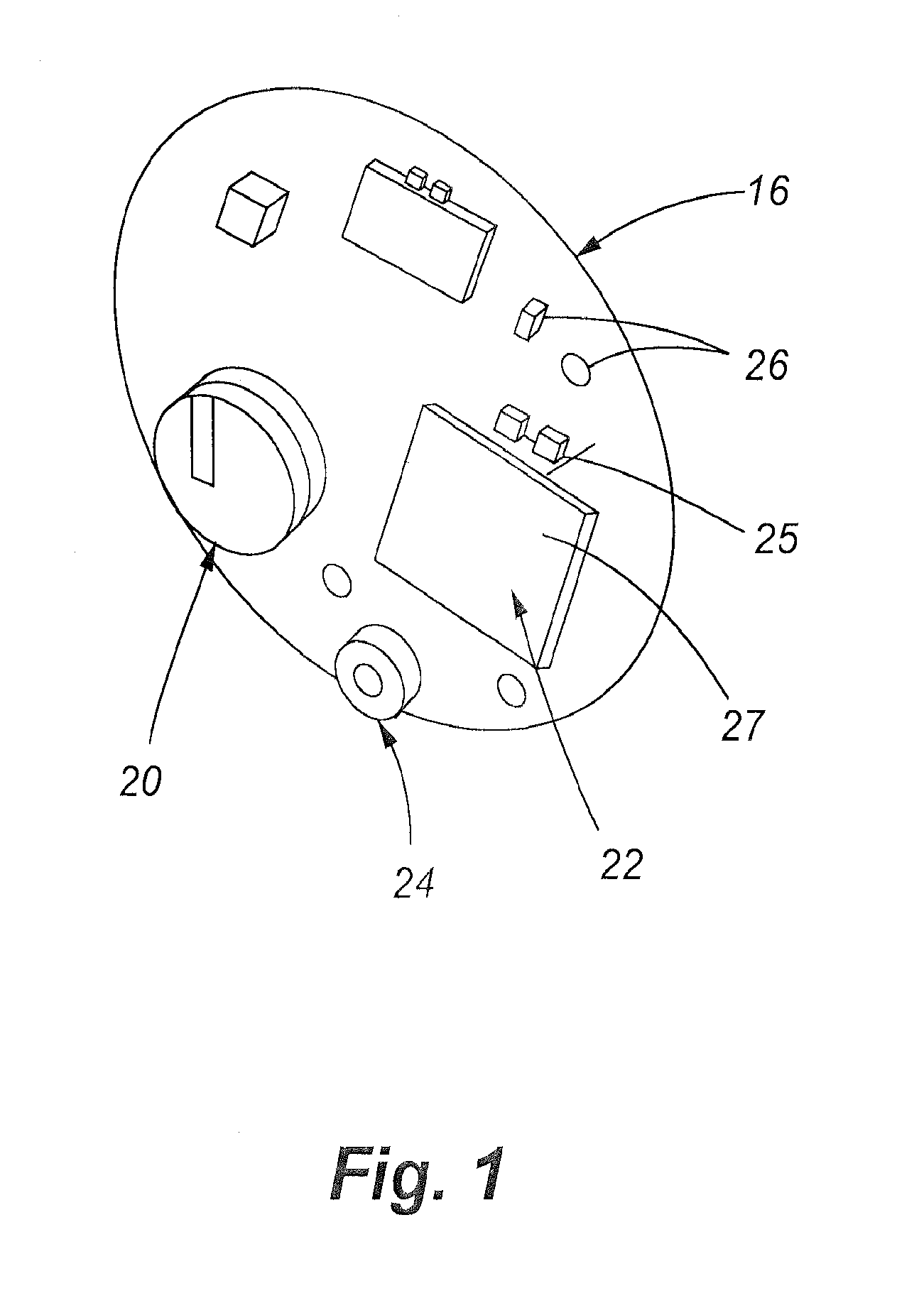
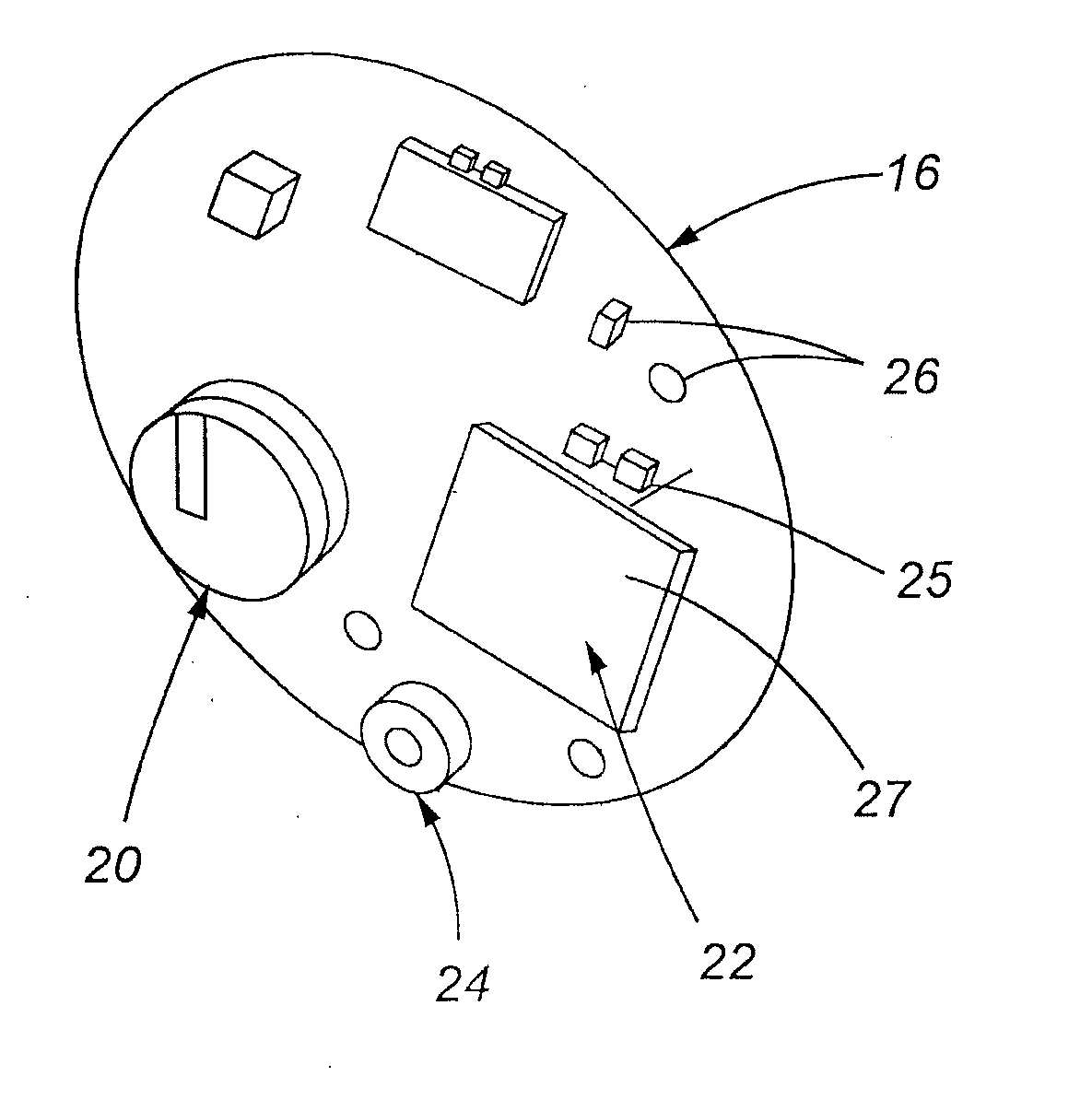
ActiveUS20120276525A1Promote hygiene habitsTransmission limitBioreactor/fermenter combinationsBiological substance pretreatmentsUses eyeglassesDisease
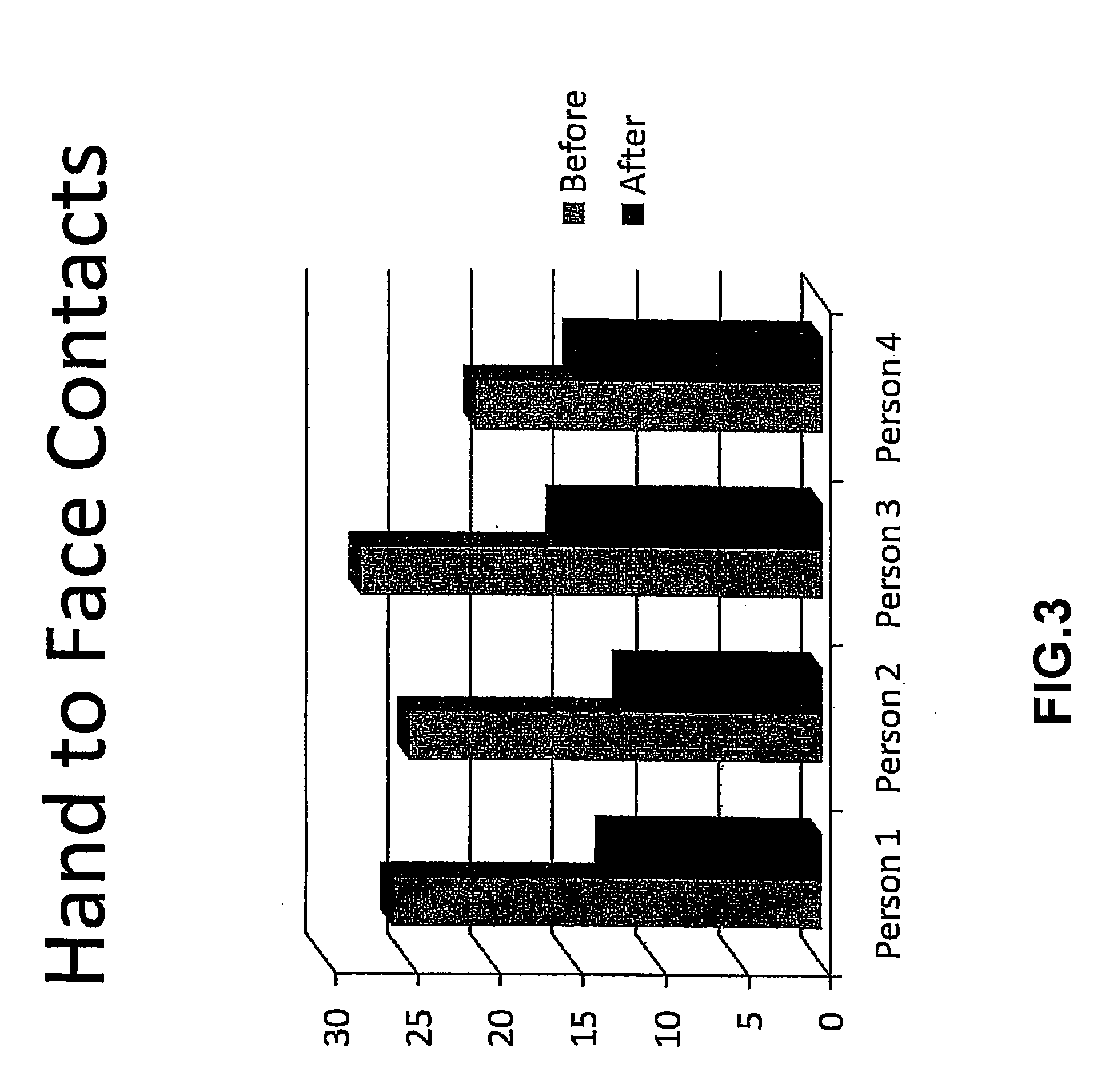
A method for preventing obesity related to infection by an adipogenic adenovirus includes assaying a sample from a person to determine whether the person has been previously infected with an adipogenic adenovirus, and if the person has not been previously infected, providing the person with at least one sensor positioned to detect when a person's hand approaches a predetermined distance from the person's face. By warning the person of undesired hand-to-face contacts, the person is able to reduce the incidence of obesity related infections. Other embodiments are directed to a kit for preventing obesity caused by infection with an adipogenic adenovirus, such kit including a container for assaying an agent indicating the presence of antibodies to Ad-36, and a sensor positioned on an item selected from the group consisting of one of a hat, a writing instrument, eye glasses, a belt, sunglasses, a bra, a shirt, and a tie.
Owner:NOHANDS +1
Method and System for Preventing Virus-Related Obesity and Obesity Related Diseases
ActiveUS20110144453A1Promote hygiene habitsTransmission limitVaccination/ovulation diagnosticsDisease diagnosisDiseaseUses eyeglasses
A method for preventing obesity related to infection by an adipogenic adenovirus includes obtaining a sample from a person, assaying the sample to determine whether the person has been previously infected with an adipogenic adenovirus, and if the person has not been previously infected, providing the person with at least one sensor positioned to detect when a person's hand approaches a predetermined distance from the person's face. By warning the person of undesired hand-to-face contacts, the person is able to reduce the incidence of obesity related infections. Other embodiments are directed to a kit for preventing obesity caused by infection with an adipogenic, adenovirus, such kit including a container for assaying an agent indicating the presence of antibodies to Ad-36, and a sensor positioned on an item selected from the group consisting of one of a hat, a writing instrument, eye glasses, a belt, sunglasses, a bra, a shirt, and a tie.
Owner:SEED HEALTH INC +1
Dual PCR detection probe and kit for duck adenovirus 2 and duck adenovirus A
InactiveCN107058634AEquivalent sensitivitySimplify operating proceduresMicrobiological testing/measurementDNA/RNA fragmentationDuplex pcrMicrobiology
The invention provides a dual PCR detection probe and kit for duck adenovirus 2 and duck adenovirus A, and belongs to the field of epizootiology. Two pairs of primers which are shown in SEQ ID NO.1-4 are adopted, a dual PCR detection method through which differential diagnosis can be conducted on duck adenovirus 2 and duck adenovirus A in a duck flock is built, a basis is laid for developing an epidemiological survey of adenovirus infection types and scientific prevention and control of relevant diseases in the duck flock, and thus the dual PCR detection probe and kit for duck adenovirus 2 and duck adenovirus A have very important study significance.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
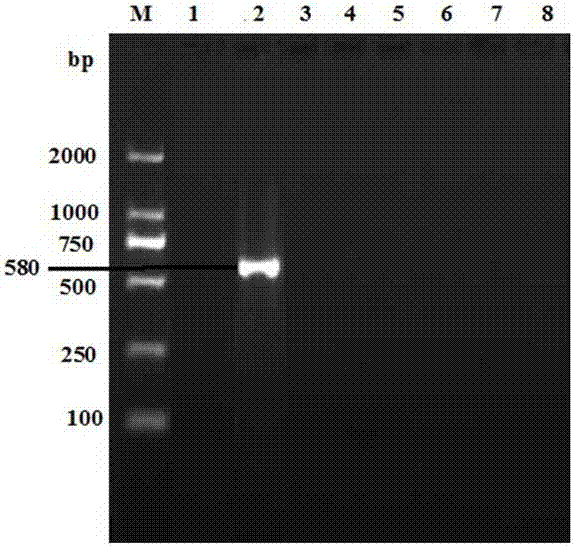
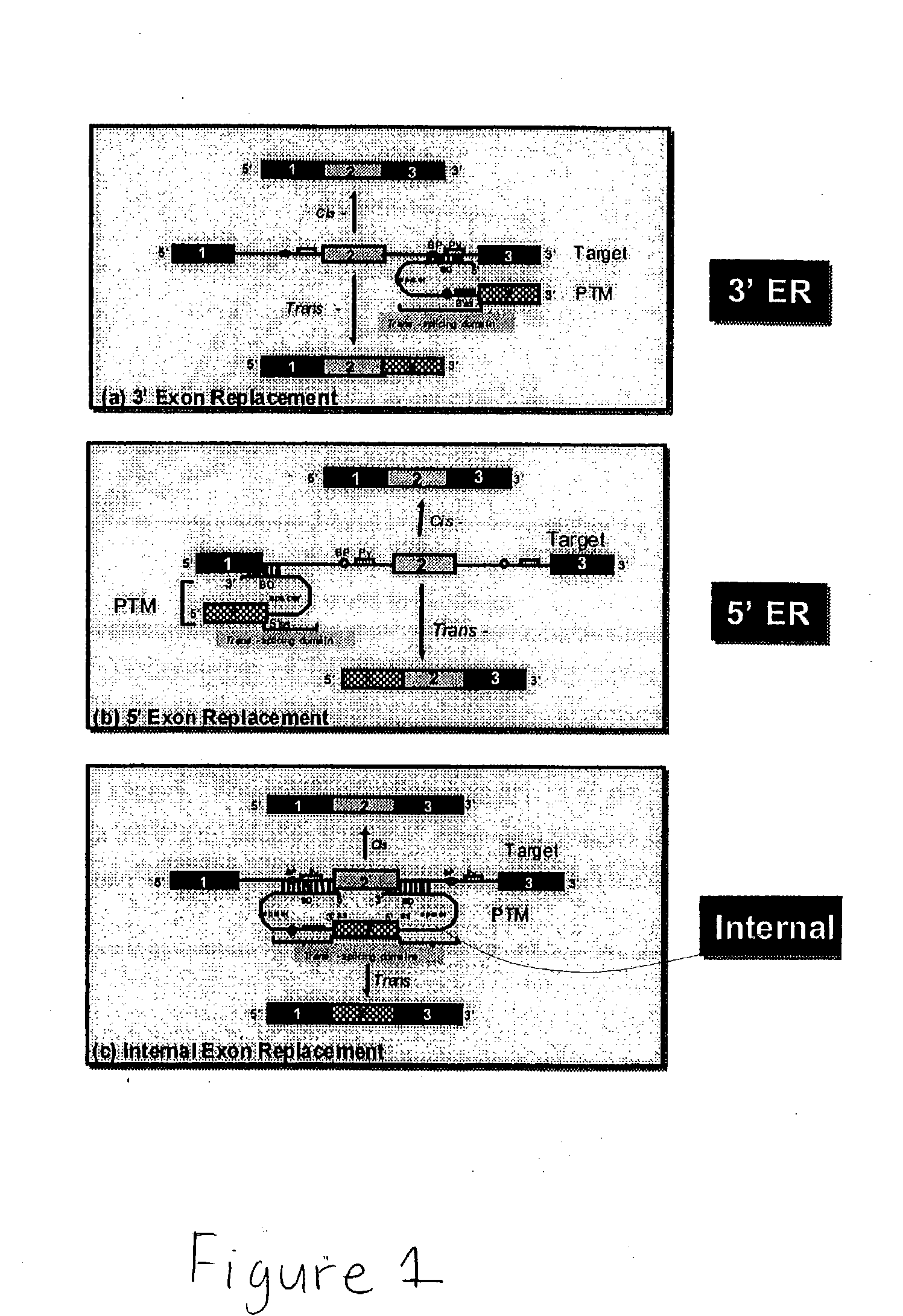
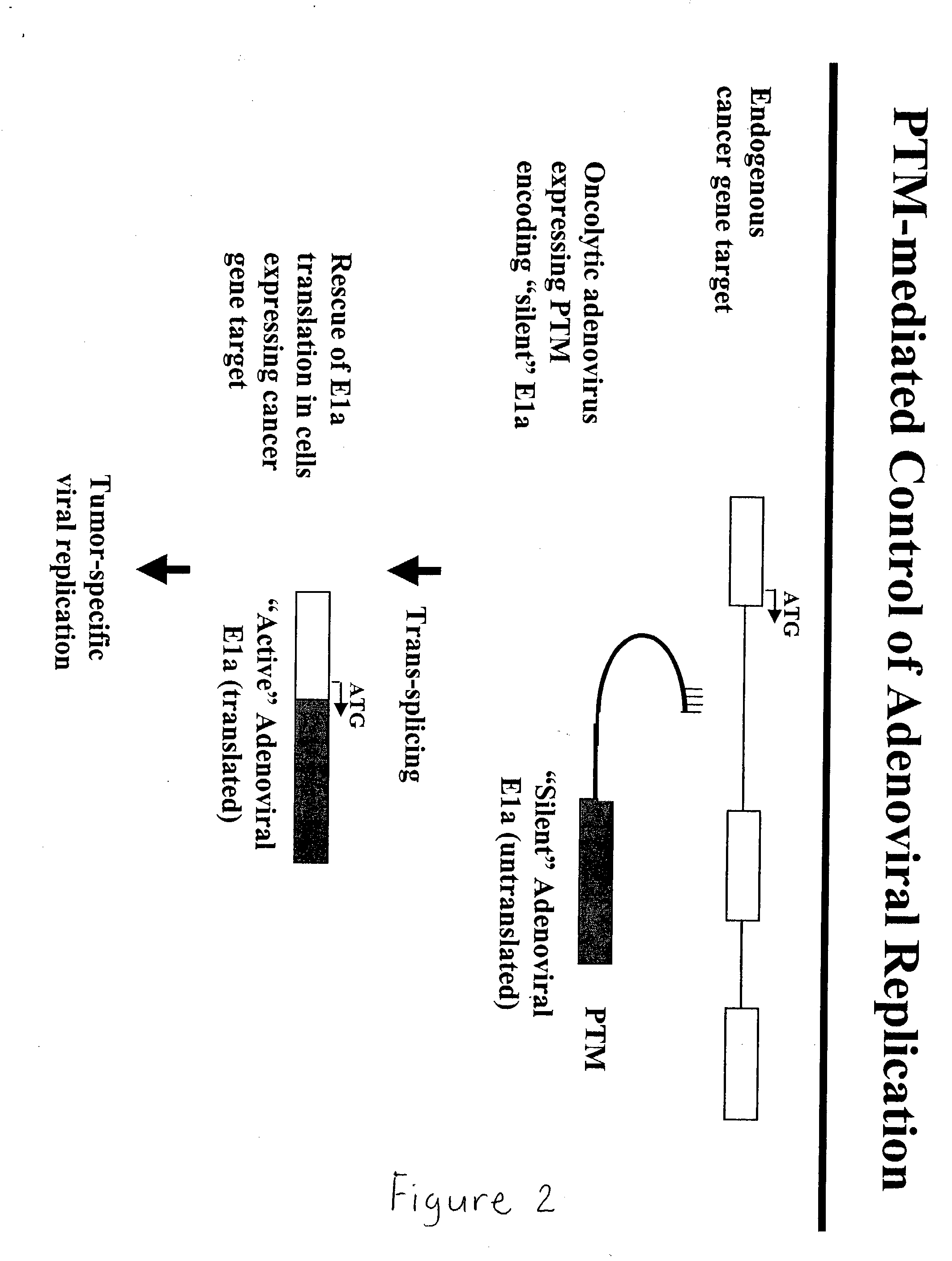
Use of spliceosome mediated RNA trans-splicing to confer cell selective replication to adenoviruses
The present invention provides methods and compositions for conferring tumor selective cell death on cancer cells expressing specific target precursor messenger RNA molecules (cancer cell selective target pre-mRNAs). The compositions of the invention include conditionally replicative adenoviruses that have been genetically engineered to express one or more pre-trans-splicing molecules (PTMs) designed to interact with one or more cancer cell target pre-mRNA and mediate a trans-splicing reaction resulting in the generation of novel chimeric RNA molecules (chimeric RNA) capable of encoding adenovirus specific protein(s). Adenovirus specific proteins include those proteins complementing an essential activity necessary for replication of a defective adenovirus. The methods and compositions of the invention may be used to target a lytic adenovirus infection to cancer cells thereby providing a method for selective destruction of cancer cells. In addition, the adenoviruses of the invention may be engineered to encode PTMs designed to interact with target pre-mRNAs encoded by infectious agents within a cell, thereby targeting selective destruction of cells infected with such agents.
Owner:VIRXSYS
Preparation and application of adenovirus parting gene chip
InactiveCN105671212AImprove throughputStrong specificityNucleotide librariesMicrobiological testing/measurementOligonucleotide chipEpidemiologic survey
The invention relates to preparation and application of a gene chip capable of detecting adenoviruses in a parting mode.A preparation method comprises the steps of preparing specific primers and probes for different types of adenoviruses, preparing oligonucleotides chips, establishing a plurality of PCR systems, and establishing a hybridization system.The gene chip prepared through the method can screen different types of adenoviruses including a 3-type adenovirus, a 7-type adenovirus, a 14-type adenovirus, an 11-type adenovirus and a 55-type adenovirus.The gene chip has the advantages of quickness, accuracy, high throughput and high specificity.A new detection means is provided for clinical diagnoses of different types of adenovirus infections and epidemiological surveys.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Use of spliceosome mediated RNA trans-splicing to confer cell selective replication to adenoviruses
The present invention provides methods and compositions for conferring tumor selective cell death on cancer cells expressing specific target precursor messenger RNA molecules (cancer cell selective target pre-mRNAs). The compositions of the invention include conditionally replicative adenoviruses that have been genetically engineered to express one or more pre-trans-splicing molecules (PTMs) designed to interact with one or more cancer cell target pre-mRNA and mediate a trans-splicing reaction resulting in the generation of novel chimeric RNA molecules (chimeric RNA) capable of encoding adenovirus specific protein(s). Adenovirus specific proteins include those proteins complementing an essential activity necessary for replication of a defective adenovirus. The methods and compositions of the invention may be used to target a lytic adenovirus infection to cancer cells thereby providing a method for selective destruction of cancer cells. In addition, the adenoviruses of the invention may be engineered to encode PTMs designed to interact with target pre-mRNAs encoded by infectious agents within a cell, thereby targeting selective destruction of cells infected with such agents.
Owner:VIRXSYS
Detection test paper strip of colloidal gold method for adenovirus IgM and IgG antibodies, kit and preparation method thereof
The invention provides a detection test paper strip of a colloidal gold method for adenovirus IgM and IgG antibodies, a kit and a preparation method thereof. The invention can determine the IgM and IgG antibodies by an immunocapture method principle, and can co-detect specific anti-Adv IgM and IgG antibodies by once operation, thereby simplifying an operation process. The kit is used for simple, rapid and accurate detection, reduces a lot of complex programs, needs neither a special instrument nor special training, is particularly suitable for a primary department, and can make an auxiliary diagnosis effect for early stage or mid-stage adenovirus infection.
Owner:JIANGSU KEYGEN BIOTECH CORP LTD
Adipogenic adenoviruses as a biomarker for disease
Owner:OBETECH LLC
Preparation method of spleen byproducts for producing homology anti-serum blood and transfer factor from fox, raccoon dog, mink
InactiveCN101422485AEasy to manufactureControl spreadAntibacterial agentsPeptide/protein ingredientsDiseaseBlood collection
The invention relates to a preparation method for obtaining blood used for preparing homologous antiserums and a spleen byproduct used for preparing transfer factors from the bodies of fur bearing animals such as foxes, raccoon dogs and minks; the furs of the foxes, the raccoon dogs and the minks are taken concentratedly in December and confirmed according to the fur-taking dates of different areas; healthy individuals are picked up 45 days before a predicated fur-taking date; the booster immunization of univalent vaccines or polyvalent vaccines of canine distemper, Canine parvovirus enteritis, adenovirus disease, corona virus laxness, parainfluenza, and pasteurellosis is carried out for 3 times by purified and condensed antigens on the foundation of carrying out immunization for once in summer; when the furs are taken, aseptic anticoagulation blood collection is carried out to a heart to improve the blood serum yield; and the spleens of the animals are collected for extracting specific or common transfer factors simultaneously. The blood can be conveniently prepared into the blood serums which resist communicable diseases, and the transfer factors can be extracted the spleen, thus providing guarantee for the healthy development of the breeding in the next year; the invention is used for curing the corresponding communicable diseases of the wild animals of the same species; and when a small amount of communicable disease cases appear in a breeding crowd, the corresponding pathogeny hyper-immune serums and transfer factors are mainly injected to a supposed healthy crowd after the pathogeny is confirmed so as to cure the affected animals in the early period of disease and control the spreading of the epidemic situations.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS
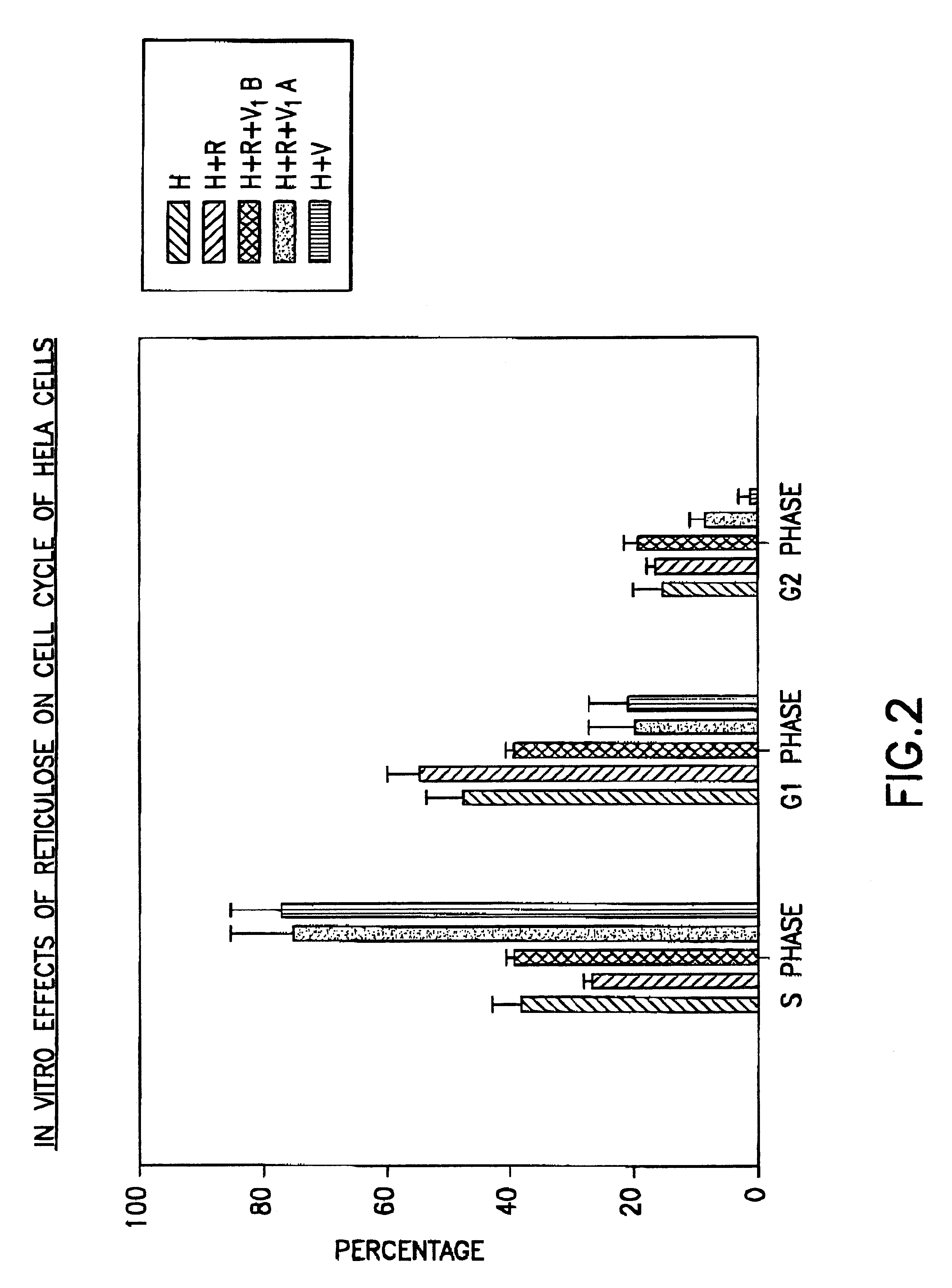
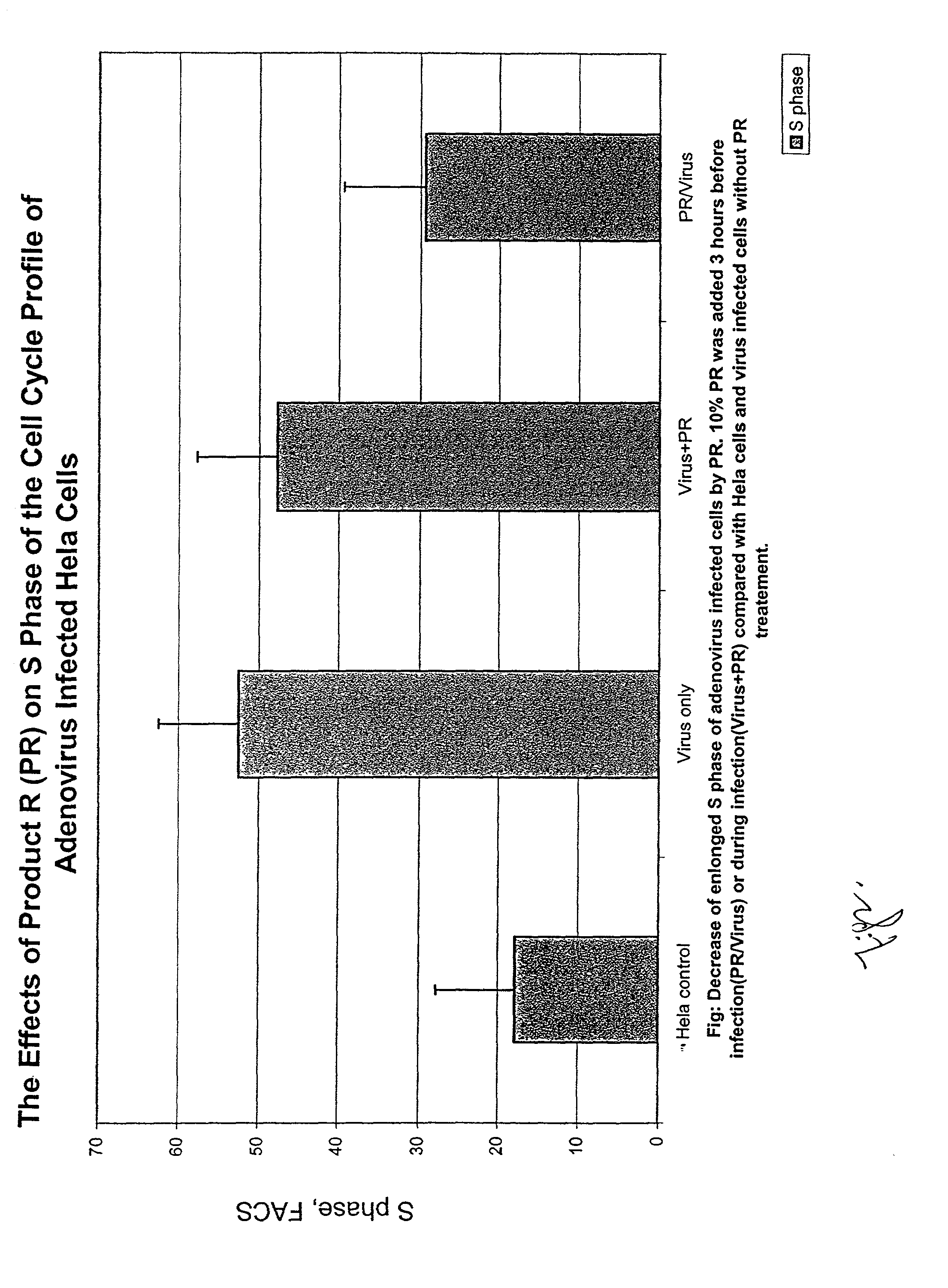
Assay method for determining Product R's effect on adenovirus infection of Hela cells
InactiveUS6440658B1Avoid virus infectionMicrobiological testing/measurementBiological testingDNA fragmentationAdenovirus diseases
An assay method for determining the effect of Product R on virus infection of Hela cells. The method comprising the following step: (1) dividing Hela cells into several groups, (2) treating one group with Product R prior to infecting the cells with a virus and treating another group with Product R after the cells being infected with the virus, and (3) determining the effects of Product R on virus infection by comparing the changes in the cell cycle, DNA fragmentation and p53 protein in cells undergone the different treatments in step (2).
Owner:BBM HLDG
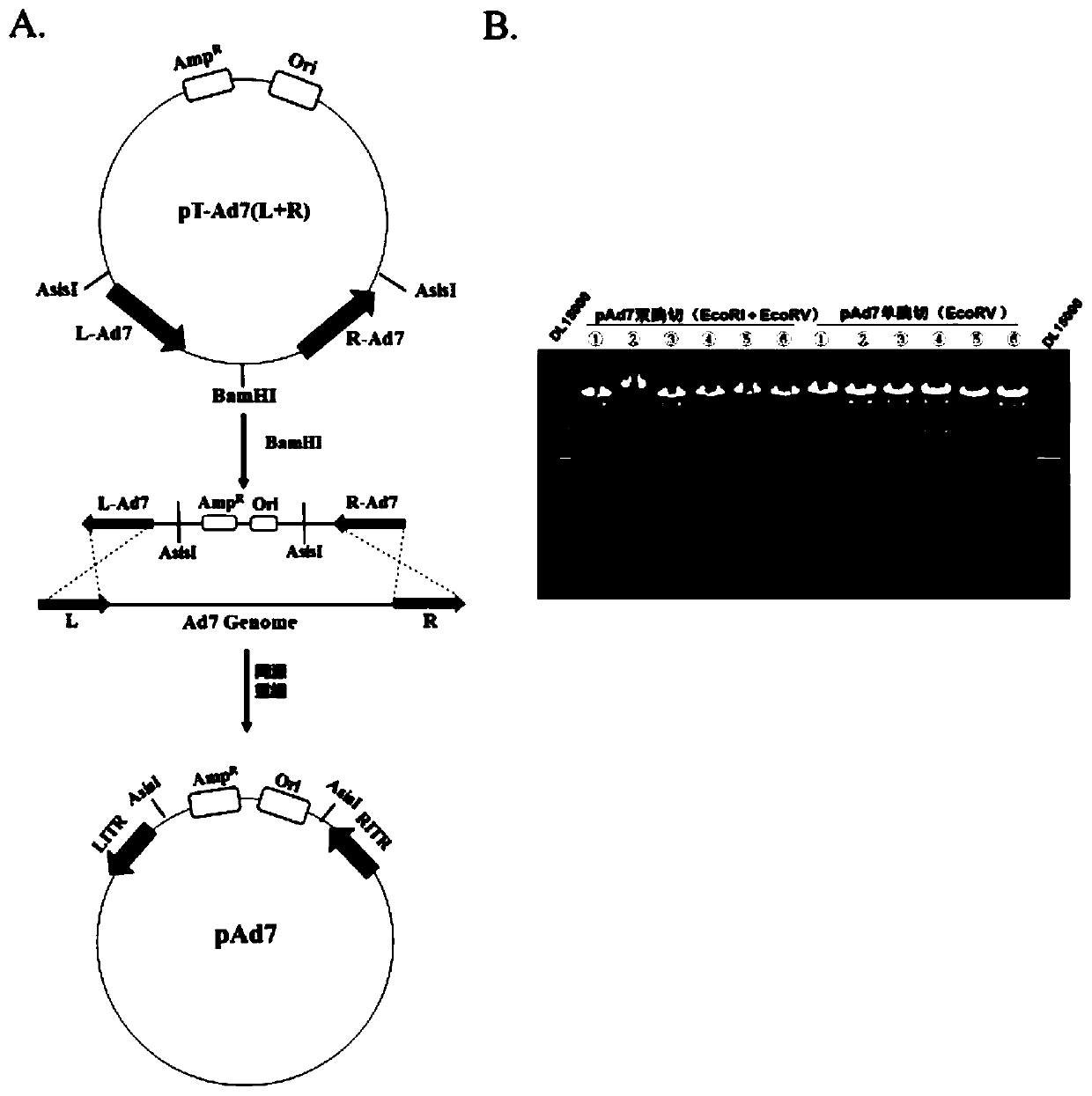
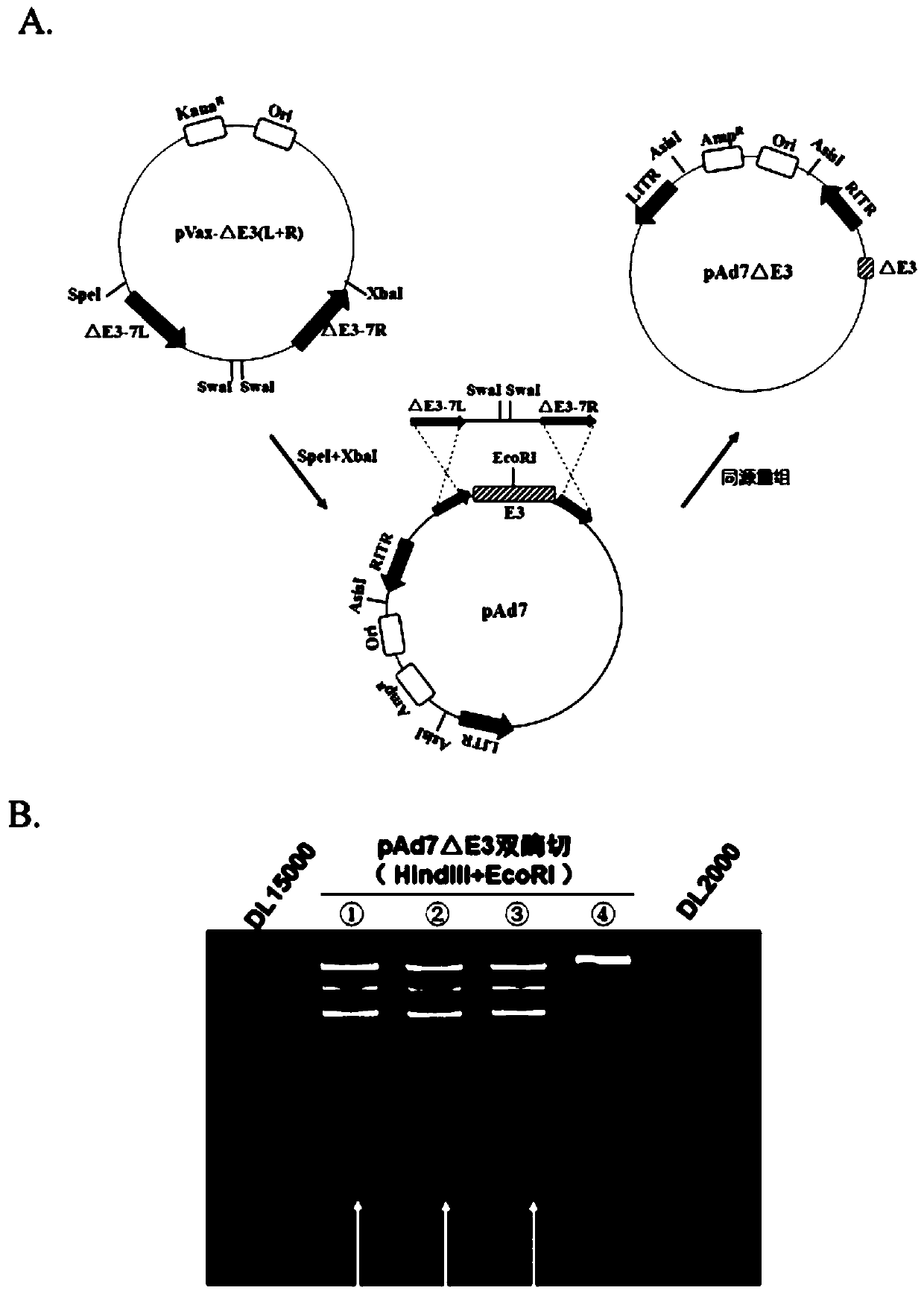
Replication-deficient type recombinant human type 7 adenovirus, preparation method and application thereof
InactiveCN110616199AIncrease production capacityViral antigen ingredientsVirus peptidesHuman typeNeutralizing antibody
The present invention relates to the field of biotechnology and specifically discloses a replication-deficient type human type 7 adenovirus and a preparation method and an application thereof. In thereplication-deficient type human type 7 adenovirus, E1 gene and E3 gene are deleted, E4 gene open reading frames 2, 3, 4, 6 and 6 / 7 are replaced into a corresponding reading frame of Ad5 genome, and aE1 gene region can integrate foreign gene expression box. The replication-deficient type 7 adenovirus can be successfully rescued and mass-produced in HEK293, but does not have replication ability inhuman normal cell lines; and after the replication-deficient type 7 adenovirus carrying foreign genes infects cells, efficient expression of the foreign genes can be realized. The replication-deficient type 7 adenovirus can potentially be applied in development of preventive vaccines against human type 7 adenovirus infection; neutralizing antibodies and drug screening against human type 7 adenovirus infection; application as a gene carrier for development of vaccines for other pathogens; and report tracking systems for biological research, etc.
Owner:GUANGZHOU N BIOMED LTD
Inhibition of adenovirus replication by product R, A peptide nucleic acid immunomodulator
The invention relates to a method of inhibiting adenovirus replication. The method comprises the step of contacting Product R with cells that are infected with adenovirus. The invention provides a basis for the treatment of adenovirus infections in human such as pink eye disease.
Owner:HIRSCHMAN SHALOM Z
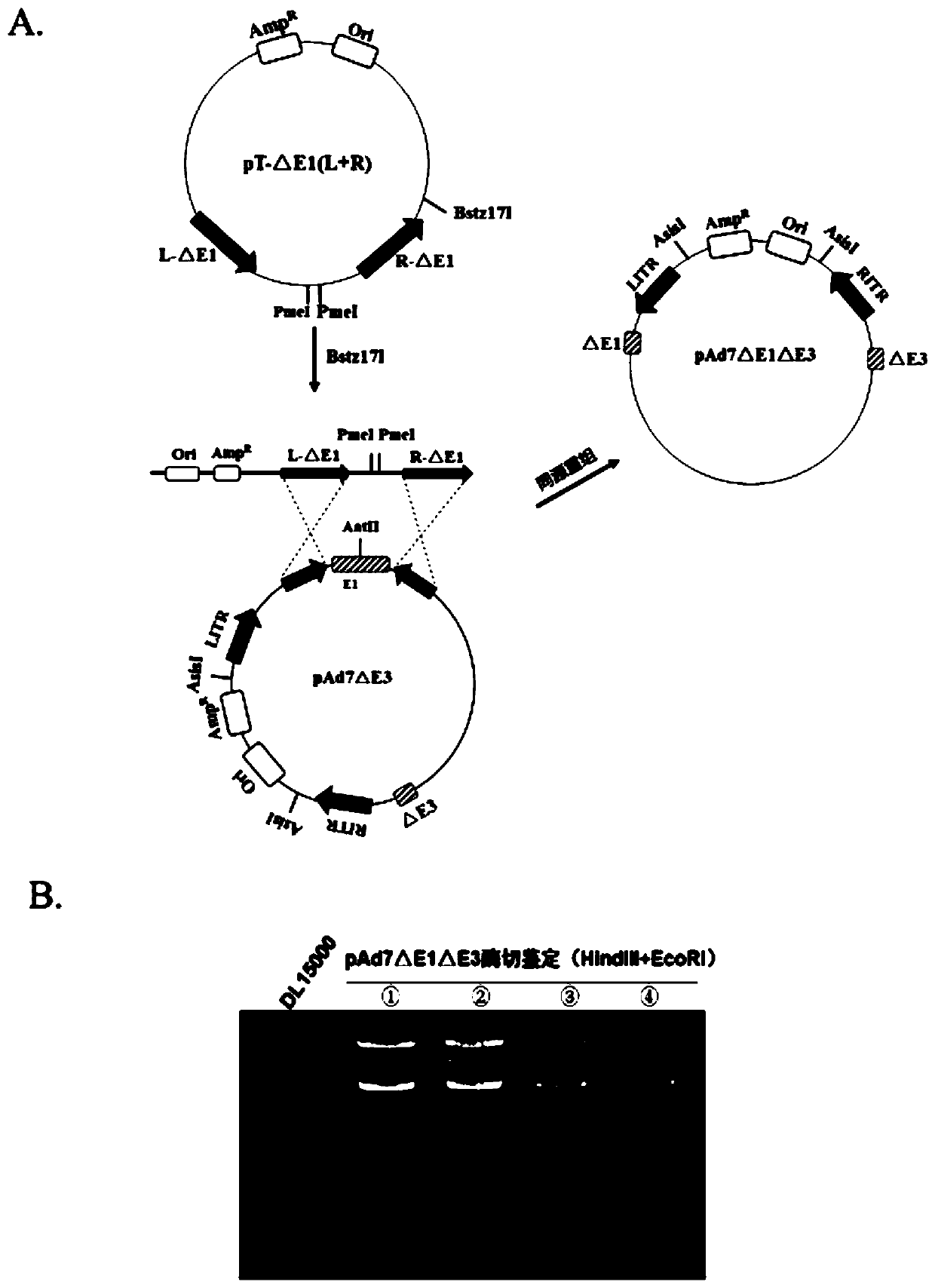
Replication-defective human 14-type adenovirus vector, and preparation method and applications thereof
InactiveCN106916851AViral antigen ingredientsGenetic material ingredientsBiological studiesAdenovirus diseases
The invention relates to the technical field of biology and particularly relates to a replication-defective human 14-type adenovirus vector, and a preparation method and applications thereof. The preparation method includes the steps of plasmidizing Ad 14 genome to knock-out E3 and E1 genes therefrom, and further changing open reading frames 2, 3, 4, 6, and 6 / 7 of an E4 gene into corresponding reading frames of Ad5 genome. The replication-defective human 14-type adenovirus vector can be potentially applied in research on anti-human 14-type adenovirus infection vaccines, screening of neutralizing antibodies and medicines for anti-human 14-type adenovirus infection, research on other pathogenic vaccines as a gene vector, a report tracing system for biological research, etc.
Owner:GUANGZHOU N BIOMED LTD
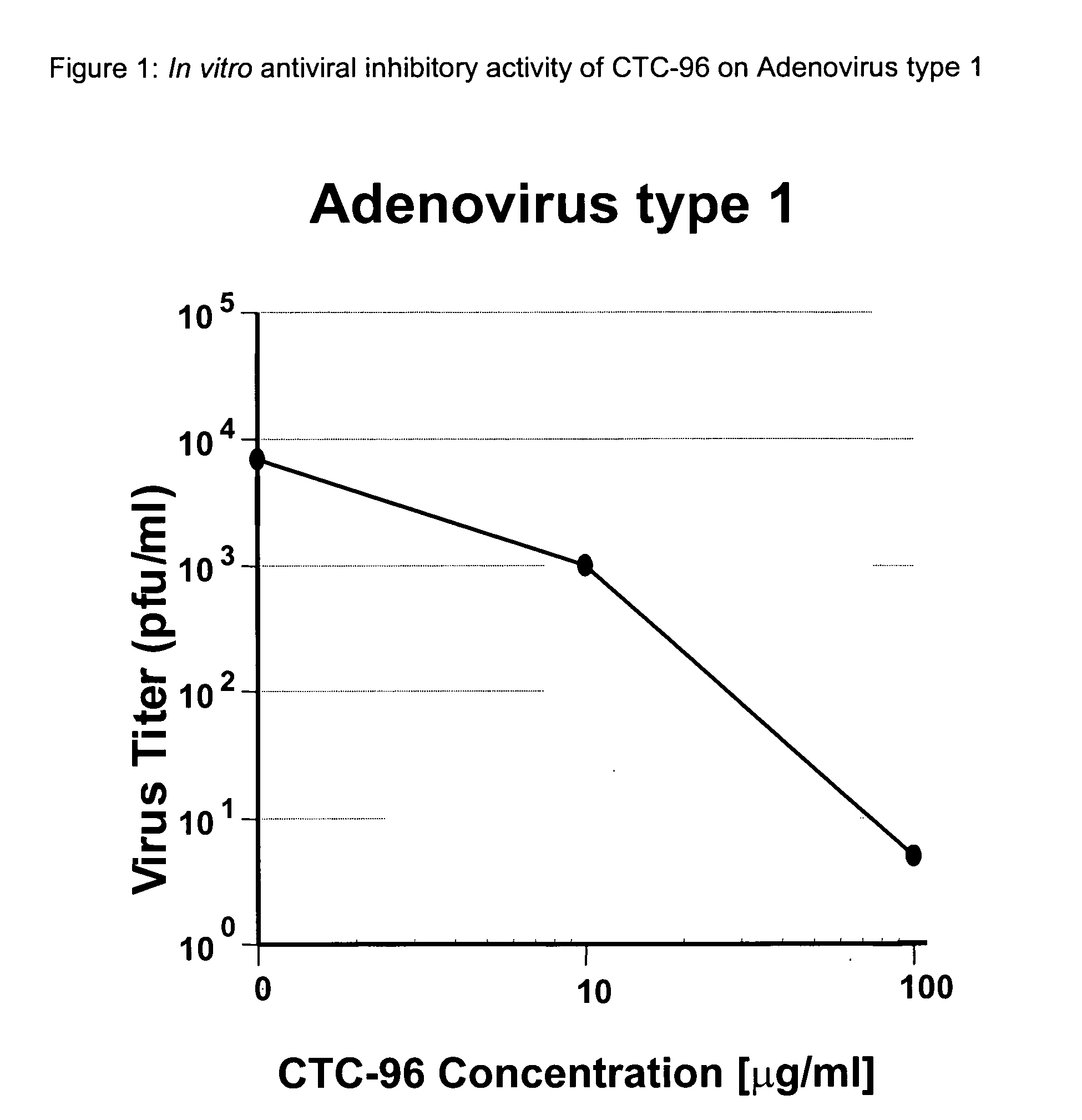
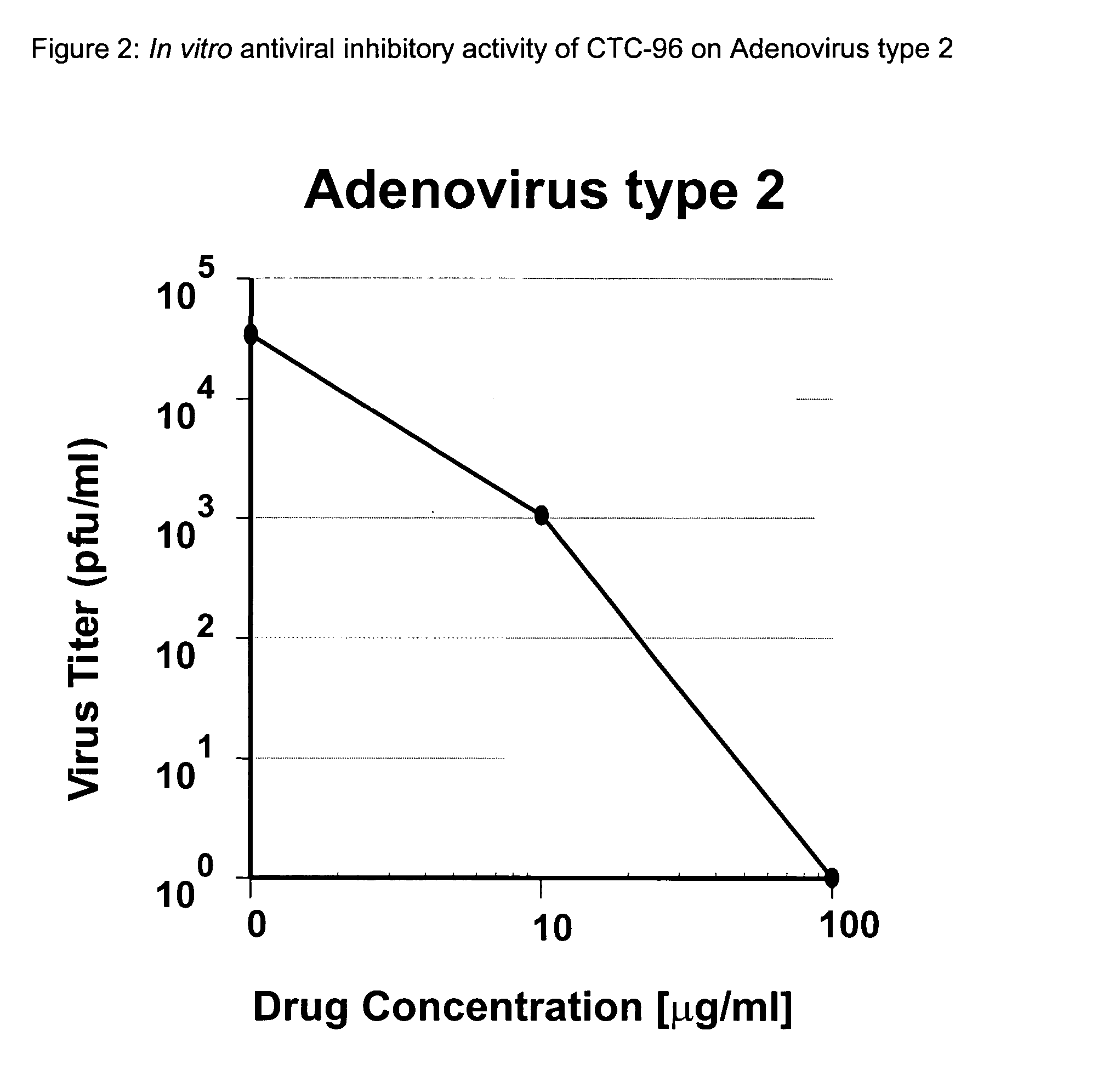
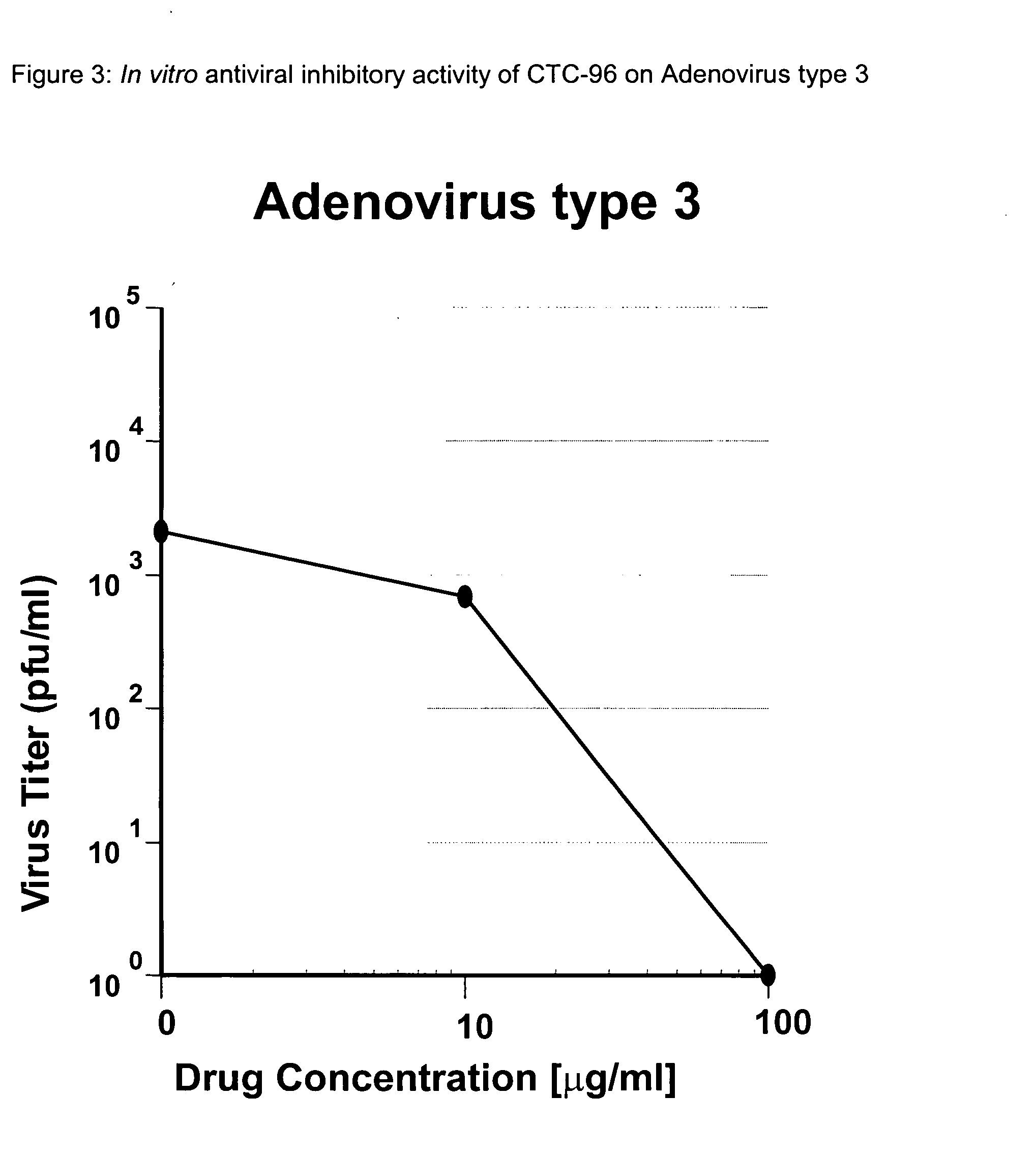
Topical antiviral therapeutic and prophylactic treatment of adenoviruses and their associated diseases
InactiveUS20050032739A1Protect and decrease likelihoodBiocideSenses disorderTopical antiviralProphylactic treatment
A method for the therapeutic and prophylactic treatment of adeviruses, More specifically, a method for the therapeutic treatment of adenovirus in a subject by topically administering an antiviral effective amount of CTC-96 to the subject. In addition, a method for the prophylactic treatment against an adenovirus infection in a subject by topically administering a prophylactically anti-adenovirus effective amount of CTC-96 to the subject to minimize the likelihood of the subject veing infected by the adenovirus.
Owner:REDOX PHARMA
Recombinant adenovirus carrier for expressing African swine fever virus B646L gene, construction method and preparation method of recombinant adenovirus
InactiveCN108342414ATo achieve the purpose of normal expressionVirus peptidesFermentationD'Aguilar virusVector vaccine
The invention provides a recombinant adenovirus carrier for expressing an African swine fever virus B646L gene, a construction method and a preparation method of the recombinant adenovirus, belongingto the technical field of genetic engineering. According to the method, adenovirus shuttle vectors pKO-FH and pAD-EF1a-GFP are utilized to be subjected to a series of intermediate processes to obtaina recombinant adenovirus expression plasmid pAD-B646L. An HEK293 cell is transfected to an obtained linear recombinant adenovirus vector plasmid; according to cytopathy caused by adenovirus infection,a recombinant virus is screened to finally realize an adenovirus packaging process, the recombinant adenovirus capable of directly infecting eukaryocyte can be obtained through amplification, concentration and authentication, so that the purpose of normally expressing a B646L gene in the eukaryocyte is realized, and the foundation is laid for researching an adenovirus vector vaccine based on expression of B646L.
Owner:YANGZHOU UNIV
Methods and compositions for the production of adenoviral vectors
InactiveUS7419808B2Maximize productionProduced in advanceBiocideGenetic material ingredientsPurification methodsMicrobiology
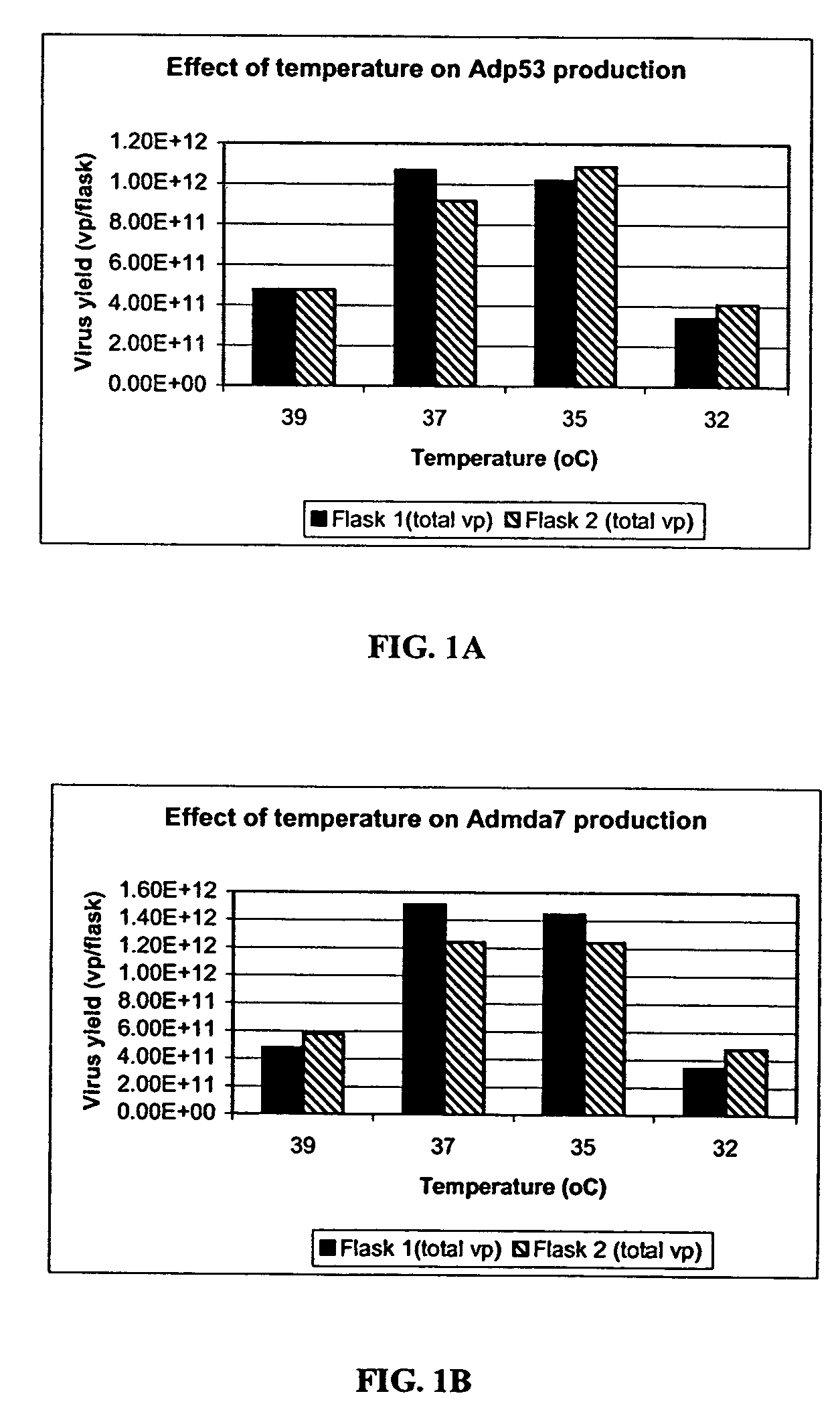
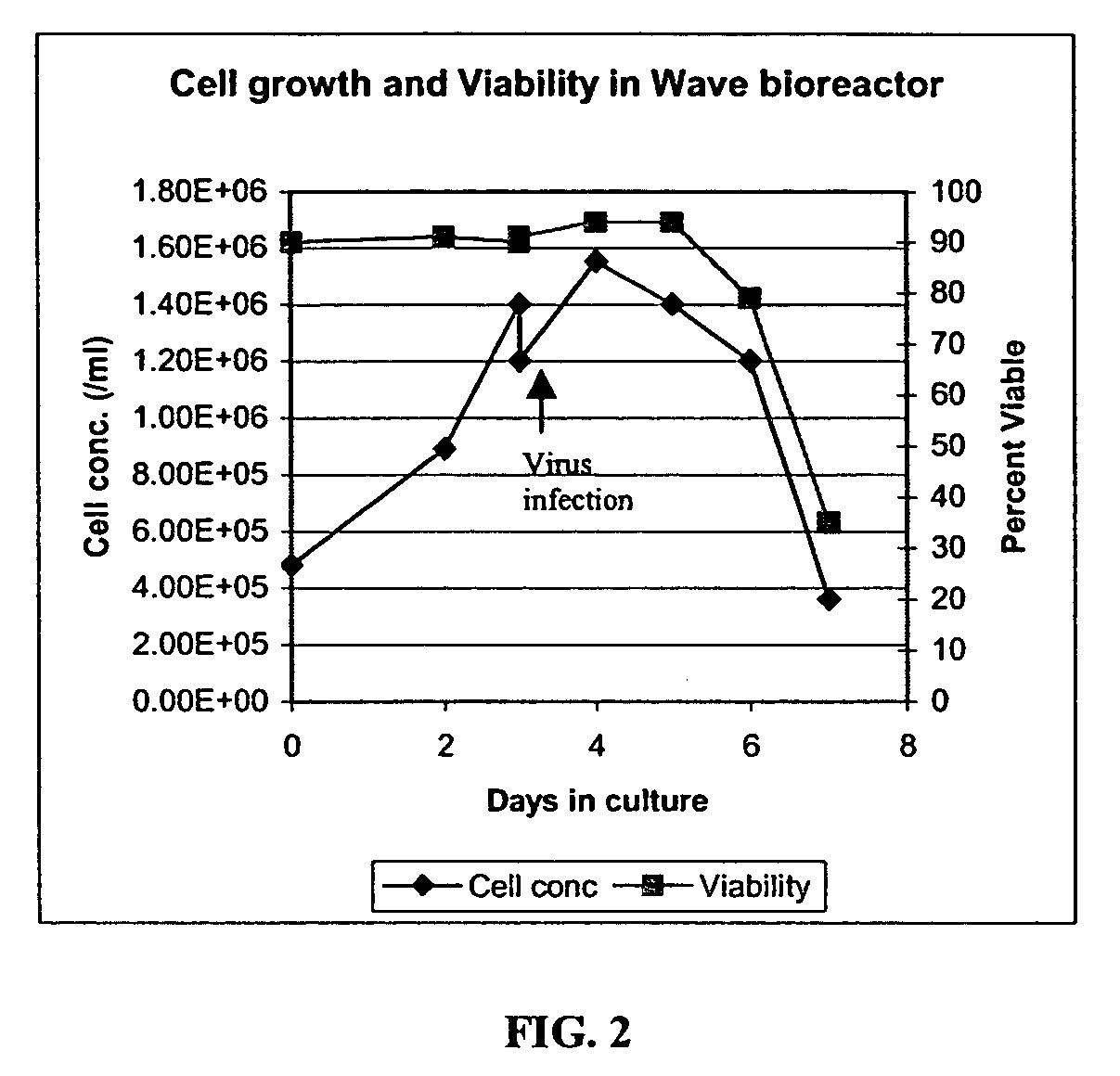
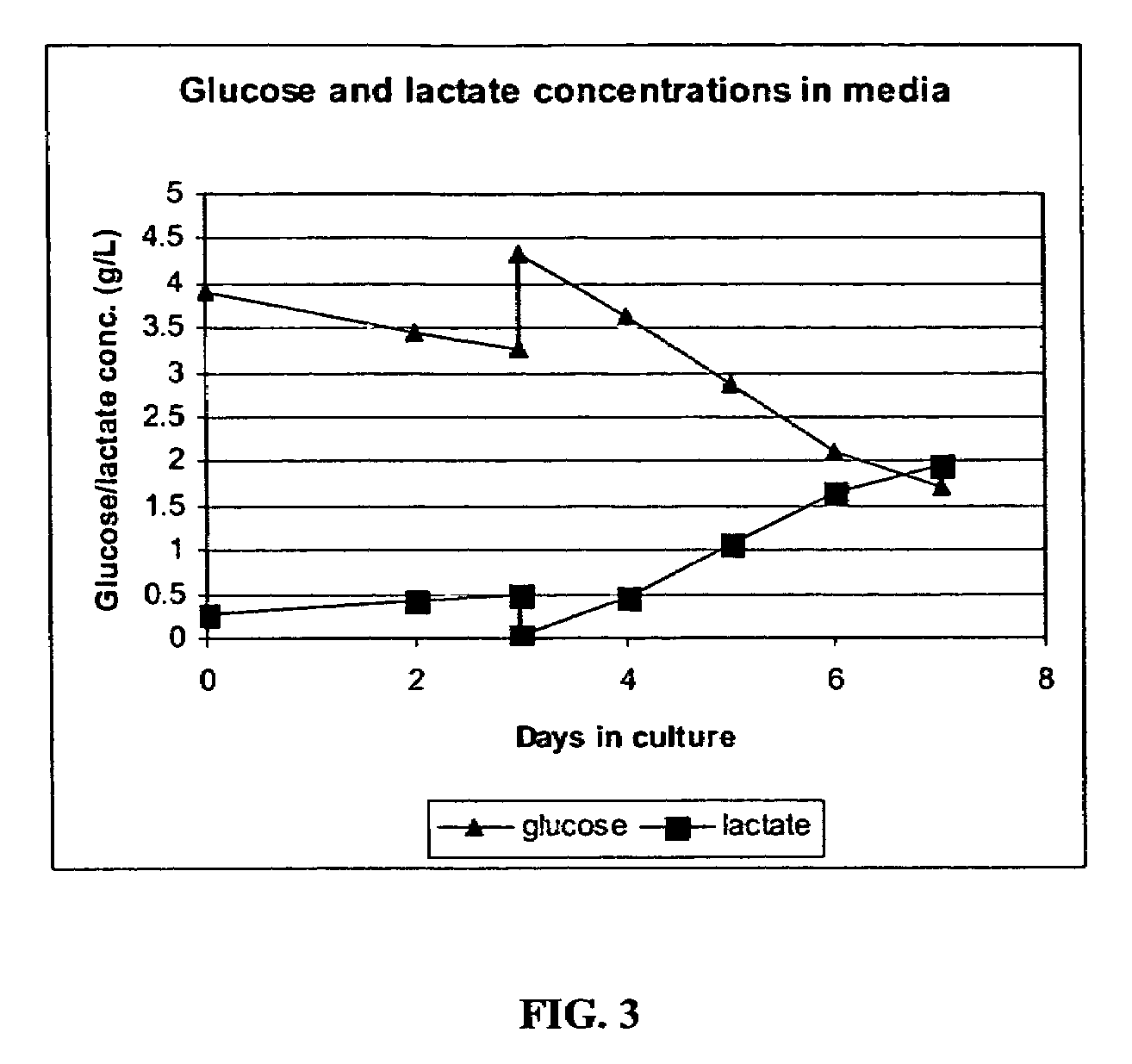
The present invention addresses the need to improve the yield of adenovirus when grown in cell culture systems. In particular, it has been demonstrated that for adenovirus, the use of infection temperatures lower than 37° C. in a cell culture system results in improved yields of adenovirus. In addition, it has been demonstrated that when host cells are grow in a bioreactor, initiating adenovirus infection by diluting the host cells with fresh media and adenovirus results in improved yield of adenovirus. Methods of adenoviral production and purification using infection temperatures less than 37° C. are disclosed. Methods of adenoviral production and purification wherein the host cells are grown in a bioreactor and adenovirus infection is initiated by diluting the host cells with fresh media and adenovirus are also disclosed.
Owner:JANSSEN VACCINES & PREVENTION BV
Egg yolk antibody of type-2 duck adenovirus infection and preparation method
ActiveCN107365382AAvoid infectionImprove securityEgg immunoglobulinsImmunoglobulins against virusesAntigenWhole body
The invention provides an egg yolk antibody of type-2 duck adenovirus infection. An antigen used in a preparation process of the egg yolk antibody is a vaccine prepared by inactivated GD strain viruses. The type-2 duck adenovirus GD strain has been deposited in China Center for Type Culture Collection in Wuhan University on June 5, 2016 with the accession number of CCTCC No: V201633. The egg yolk antibody of type-2 duck adenovirus infection is good in safety, and any local or systemic adverse reactions caused by the egg yolk antibody do not exist. By identification, safety test and analysis of potency test data, various indexes are stable and effectively; and the use effect of the egg yolk antibody is estimated by a method for injecting after counteracting toxic substances and a method for counteracting toxic substances after injecting. A result shows that the egg yolk antibody of type-2 duck adenovirus infection can effectively prevent and treat the type-2 duck adenovirus infection, and has good commercialization development prospect.
Owner:SHANDONG SINDER TECH +1
Monoclonal antibody 3-3E for human adenovirus type 7 and application of monoclonal antibody 3-3E
The invention discloses a monoclonal antibody 3-3E for a human adenovirus type 7 and an application of the monoclonal antibody 3-3E. The monoclonal antibody comprises a heavy chain variable region anda light chain variable region, wherein the heavy chain variable region comprises three complementary determining regions HCDR1, HCDR2 and HCDR3; the light chain variable region comprises three complementary determining regions LCDR1, LCDR2 and LCDR3; HCDR1, HCDR2 and HCDR3 are sequentially represented as 26-33-posiition, 51-58-poisiton and 97-110-position from the N end of a sequence 2 in a sequence table; LCDR1, LCDR2 and LCDR3 are sequentially represented as 27-32-posiition, 50-52-poisiton and 89-96-position from the N end of a sequence 4 in a sequence table. On the basis of the clinical demand, the antibody 3-3E resistant to the human adenovirus type 7 is discovered, can be used for preventing and treating adenovirus infection and has great biological and medical significance.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Immunofluorescence reagent applied to detection of adenovirus IgM antibody and application of immunofluorescence reagent
InactiveCN104357407AMeet the needs of clinical diagnosis of infectionStrong specificityMicroorganism based processesViruses/bacteriophagesImmunofluorescenceIgm antibody
The invention relates to an adenovirus antigen prepared by culturing an adenovirus strain with the preservation number of CGMCC No.9596. The invention also relates to an adenovirus IgM antibody detecting kit. The kit comprises the adenovirus antigen. Compared with the prior art, the diagnostic kit applied to preparation of the adenovirus antigen has the advantages of high specificity, high sensitivity, low cost and the like; the requirements on clinical diagnosis of adenovirus infection are well met.
Owner:北京英诺特生物技术股份有限公司
Anti-human adenovirus type 7 antibody 2-1H and application thereof
The invention discloses an anti-human adenovirus type 7 antibody 2-1H and an application thereof. The invention provides a monoclonal antibody, and the monoclonal antibody comprises a heavy chain variable region and a light chain variable region; the heavy chain variable region comprises three complementary determining regions HCDR1, HCDR2 and HCDR3; the light chain variable region comprises threecomplementary determining regions LCDR1, LCDR2 and LCDR3; the HCDR1, the HCDR2 and the HCDR3 are sequentially shown as a 26th-33th position, a 51th-58th position and a 97th-117th position of a sequence 2 of a sequence table from an N end; and the LCDR1, the LCDR2 and the LCDR3 are sequentially shown as a 27th-32th position, a 50th-52th position and a 89th-97th position of a sequence 4 of the sequence table from the N end. Based on clinical needs, the anti-human adenovirus type 7 antibody 2-1H is found, is used for prevention and treatment of adenovirus infection, and has important biologicaland medical significance.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Methods and reagents for the detection of antibodies to adenovirus
InactiveUS6964843B1Viral antigen ingredientsMicrobiological testing/measurementAssayAdenovirus infection
The present invention provides methods and reagents for detecting antibodies to adenovirus. In a preferred embodiment, the present invention is useful for detecting antibodies to adenovirus serotype 5. In a further preferred embodiment, the present invention can be used in a biosensor-based assay. It is contemplated that the present invention is useful to detect antibodies for ascertaining adenovirus infection, evaluating patient response to gene therapy using adenovirus vectors, developing vaccines to adenovirus infection, developing therapeutics for inducing passive immunity to adenovirus infection, as well as other uses.
Owner:SCHERING CORP
Culturing method of adenovirus gene-modifying tumor specificity cytotoxic T lymphocyte
InactiveCN105602900AAvoid restrictionsReduce the burden onBlood/immune system cellsCell culture active agentsLymphocyte cultureCytotoxicity
The invention relates to a lymphocyte culture method, and in particular discloses a culturing method of adenovirus gene-modifying tumor specificity cytotoxic T lymphocyte. The method comprises: taking 150-200ml of bleeding of the umbilicus or autologous peripheral blood for single karyocyte separation, separately culturing DC cells by adhering to a wall for 2h, and performing T lymphocyte preculture on the non-adhered cells in an IL-2-contained culture medium; collecting mature DC after the DC cells are mature through induction of iAPA factor adenovirus infection, stimulating the autologous primary T lymphocyte to differentiate into cytotoxic T lymphocyte according to an effect target ratio of DC to T being 1 to 10, and observing and recording multiplication capacity of CTL and detecting the cytotoxicity of the CTL. The limitation of a blood component single sampling machine is solved, collected blood volume is less, burden of a patient is reduced, and iAPA-DC can be observed in vitro to stimulate and differentiate the primary T lymphocyte into tumor specificity cytotoxic T lymphocyte.
Owner:山东省齐鲁细胞治疗工程技术有限公司
Novel cairna moschata adenovirus strain, novel cairna moschata adenovirus inactivated vaccine, and preparation method for inactivated vaccine
ActiveCN110564699AEffective preventionEffective therapeuticViral antigen ingredientsMicroorganism based processesDiseaseEffective solution
The invention disclose a novel cairna moschata adenovirus strain, a novel cairna moschata adenovirus inactivated vaccine, and a preparation method for the inactivated vaccine. The adenovirus strain iscollected in the China Center for Type Culture Collection in the Wuhan University on the July 30th, 2019 with a collection number of CCTCC No:V201952. The invention establishes a preparation method for a corresponding inactivated vaccine on the basis of the novel cairna moschata adenovirus. The inactivated vaccine prepared with the method can effectively prevent and treat a novel cairna moschataadenovirus disease, has a good commercial development prospect, solves the problem that no effective solutions can be provided when the novel cairna moschata adenovirus begins to spread on a large scale at present, and greatly reduces the economic loss of cairna moschata breeding industry due to the novel cairna moschata adenovirus disease.
Owner:SHANDONG SINDER TECH +1
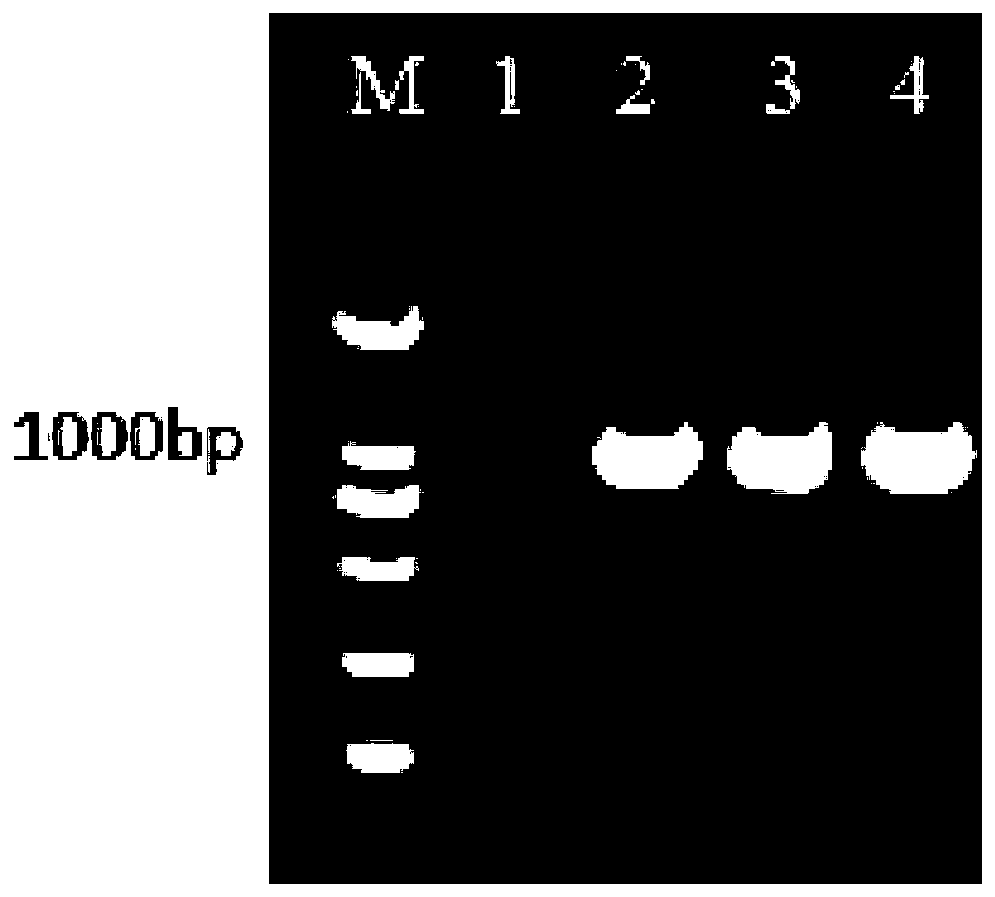
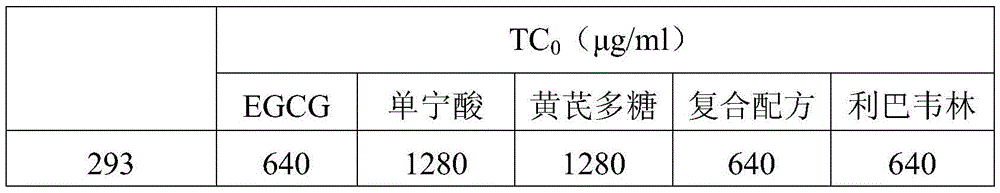
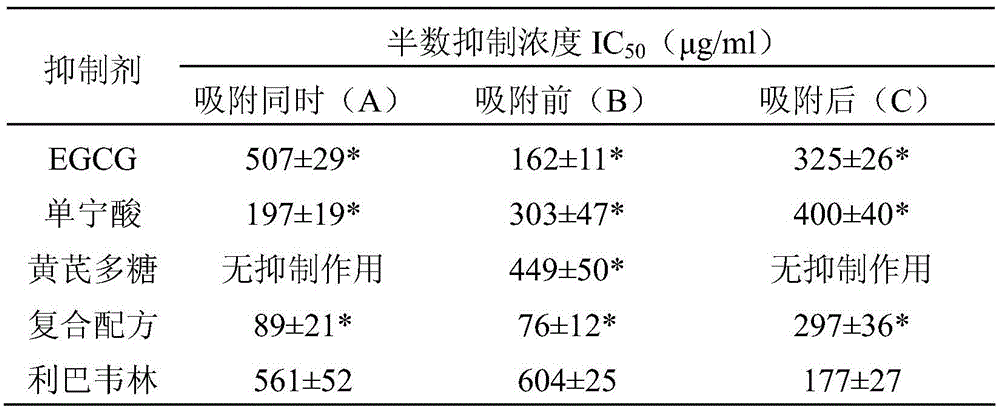
Preparation for restraining adenovirus infection
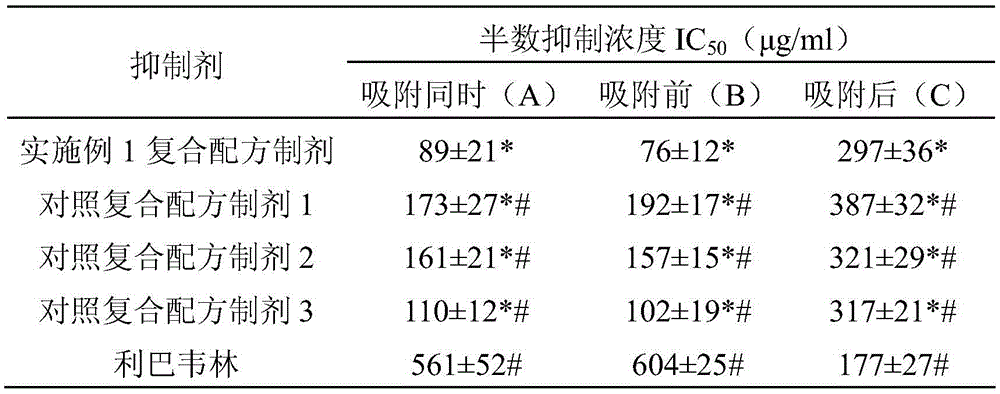
ActiveCN105560351AAvoid infectionLow cytotoxicityOrganic active ingredientsAntiviralsBiotechnologyAstragalus polysaccharide
The invention discloses a preparation for restraining adenovirus infection. The preparation is applied to 1 preparing a product for restraining adenovirus infection and 2 restraining adenovirus infection. The preparation is mainly formed by mixing epigallocatechin gallate, tannin and astragalus polysaccharide. The mass ratio of epigallocatechin gallate to tannin to astragalus polysaccharide is (0.5-1.0):(0.5-1.0):(0.5-1.5). The cytotoxicity of the complex formula preparation is not higher than that of a control sample ribavirin obtaining security permission, and the preparation is safe. Under safe use concentration, type 5 adenovirus infection can be effectively restrained through preventive use of the preparation, and the preparation has commercial value for being further developed into an adenovirus infection inhibitor.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
Islet cells differentiated from stem cells, method, compound and application
PendingCN113174408AConvenient treatmentImprove efficiencyPancreatic cellsPeptidesIslet cellsPancreatic hormone
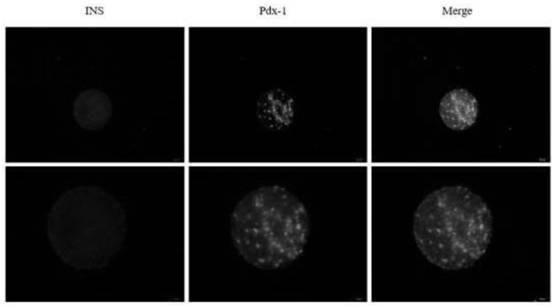
The invention is applicable to the technical field of biomedicine, and provides islet cells differentiated from stem cells, a method, a compound and application. The method for differentiating the stem cells into the islet cells comprises the following steps: conducting extracting and separating to obtain high-purity adipose-derived mesenchymal stem cells with differentiation capacity; transferring the PDX-1 gene into the adipose-derived mesenchymal stem cells by adopting an adenovirus infection method; and directionally differentiating the adipose-derived stem cells into islet cells by using an inducer. The adipose-derived mesenchymal stem cells are directionally differentiated into the islet cells by combining an adenovirus infection method with an inducer, the insulin (INS) recombinant protein is highly expressed in the obtained islet cell cluster, and the PDX-1 protein is still highly expressed in most cells after induction is finished, so that the induced cells have the capability of secreting the insulin (INS) recombinant protein and are high in efficiency; and an islet cell carrying cytoskeleton compound is transplanted to a skeletal muscle part, and diabetes can be well treated.
Owner:JILIN UNIV
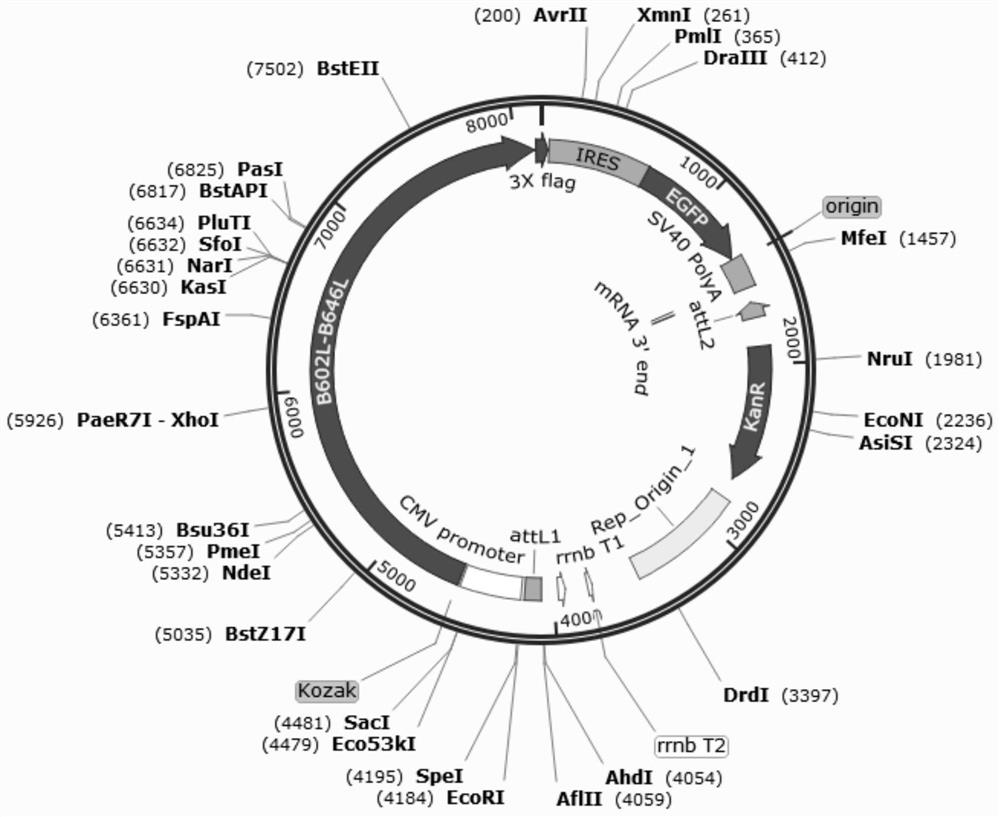
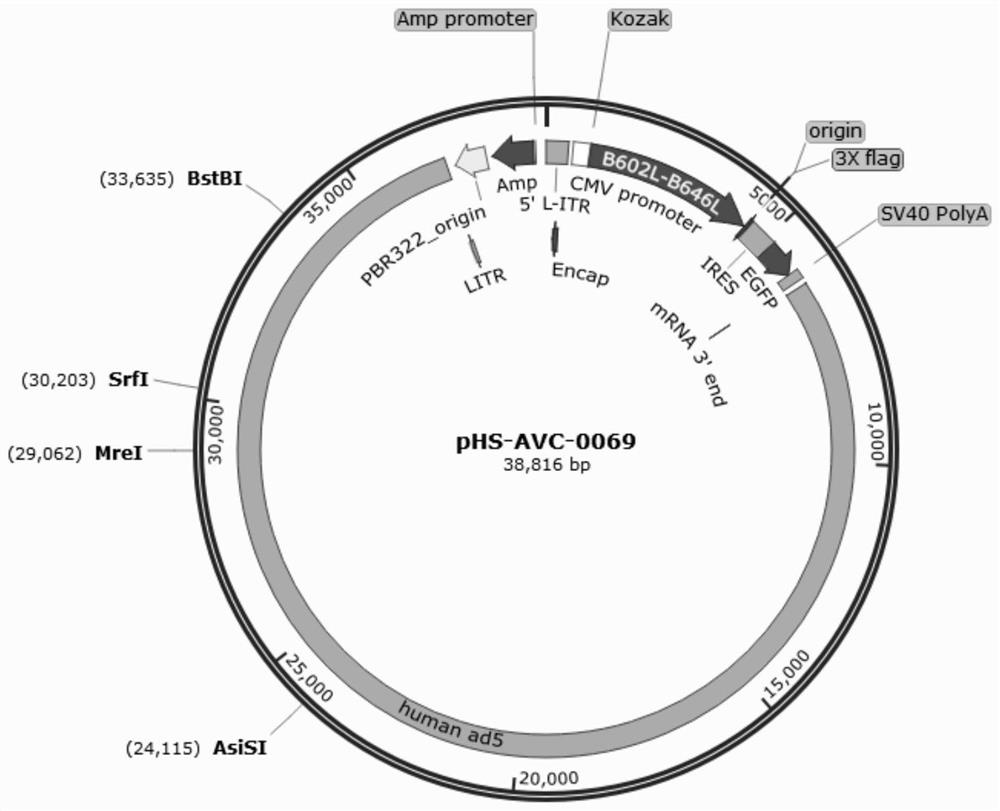
Recombinant adenovirus for expressing African swine fever virus B602L-B646L protein and construction method thereof
PendingCN114107389AViral antigen ingredientsVirus peptidesClassical swine fever virus CSFVCytopathic effect
The invention discloses a recombinant adenovirus for expressing African swine fever virus B602L-B646L protein and a construction method thereof, and belongs to the technical field of genetic engineering. A recombinant adenovirus shuttle vector pENTRE-EGFP-TOPO is utilized, and a recombinant adenovirus vector pAD-CMV-EGFP-B602L-B646L is obtained through a series of intermediate processes; according to the present invention, the African swine fever virus B602L-B646L protein expression recombinant adenovirus is constructed, the African swine fever virus B602L-B646L protein expression recombinant adenovirus is linearized and transfected with AD293 cells, the recombinant virus is screened according to the cytopathic effect formed by adenovirus infection, the adenovirus packaging process is achieved, the recombinant adenovirus expressing the African swine fever virus B602L-B646L protein is obtained, and the foundation is laid for the construction of the recombinant adenovirus vaccine expressing the African swine fever virus
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Monoclonal antibody 10G12 and application thereof
The invention discloses a monoclonal antibody 10G12 and application thereof. The monoclonal antibody comprises a heavy chain variable region and a light chain variable region, wherein the heavy chainvariable region comprises three complementary determining regions HCDR1, HCDR2 and HCDR3; the light chain variable region comprises three complementary determining regions LCDR1, LCDR2 and LCDR3; theHCDR1, HCDR2 and HCDR3 are sequentially shown as the positions 26-33, 51-66 and 97-109 from the N end of a sequence 2 in a sequence table; the LCDR1, LCDR2 and LCDR3 are sequentially shown as the positions 27-37, 55-57 and 94-102 of the N end of a sequence 4 in the sequence table. Based on clinical requirements, an inventor of the invention finds that the anti-human adenovirus type 7 antibody 10G12 is used for preventing and treating adenovirus infection and has important biological and medical significance.
Owner:ACADEMY OF MILITARY MEDICAL SCI
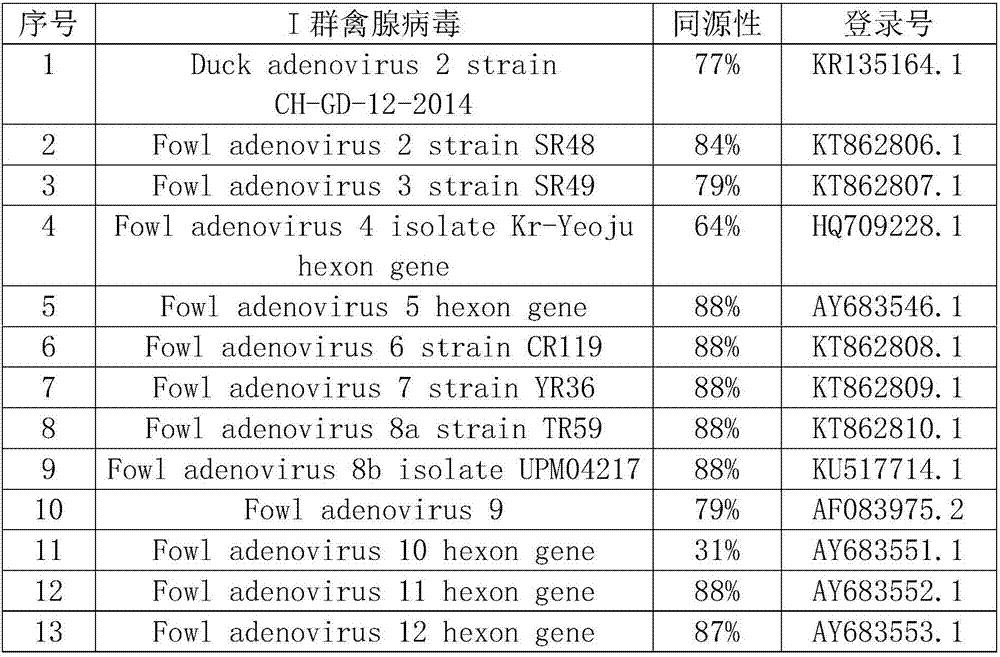
Group I type 4 fowl adenovirus fiber-2 protein antigen as well as method for preparing genetic engineering subunit vaccine and application of group I type 4 fowl adenovirus fiber-2 protein antigen
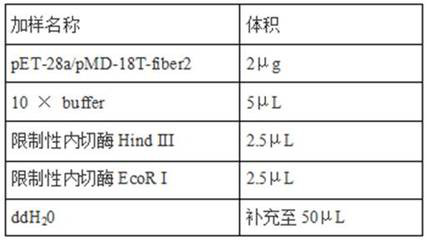
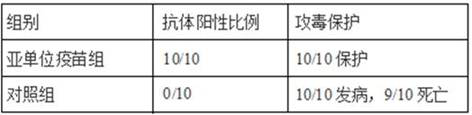
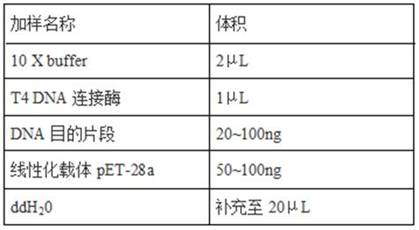
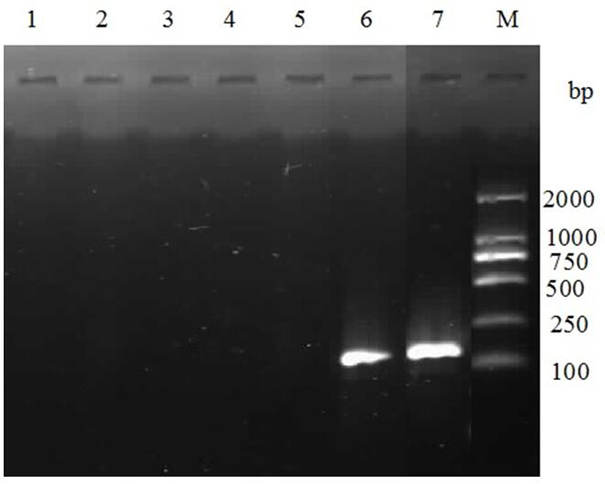
PendingCN114149493AGood prospects for commercial developmentAvoid infectionViral antigen ingredientsVirus peptidesNucleotideGenetic engineering
The invention relates to the technical field of genetic engineering vaccines, and particularly discloses an I-group 4-type fowl adenovirus fiber-2 protein antigen as well as a method for preparing a genetic engineering subunit vaccine and application of the I-group 4-type fowl adenovirus fiber-2 protein antigen. The method comprises the following steps: adding a non-coding gene in front of a fiber-2 gene of the fowl adenovirus FAdV-4, cloning the non-coding gene to a prokaryotic expression vector pET-28a, transforming the prokaryotic expression vector pET-28a to escherichia coli to construct an engineering bacterium, and inducing the engineering bacterium to express to obtain the soluble fiber-2 protein. Wherein the amino acid sequence of the aviadenovirus fiber-2 antigen protein is SEQ ID NO.2, the nucleotide sequence of a gene coded by the aviadenovirus fiber-2 antigen protein is SEQ ID NO.1, and soluble protein with high expression quantity is obtained through non-coding gene regulation and control. The fiber-2 protein of the fowl adenovirus is expressed through escherichia coli, a soluble expression product is obtained, the subunit vaccine is prepared, safety and effectiveness are achieved, and fowl I-group 4-type adenovirus infection can be prevented.
Owner:山东滨州沃华生物工程有限公司
Pigeon ttv and pigeon new adenovirus double Evagreen real-time fluorescent quantitative PCR detection kit
InactiveCN107604100BSimplify operating proceduresLow costMicrobiological testing/measurementMicroorganism based processesAdenovirus infectionAdenovirus diseases
The invention provides a double EvaGreen real-time fluorescent quantitative PCR detection kit for pigeon TTV and new pigeon adenovirus, the kit includes primers whose sequence is shown in SEQ ID NO.1‑4, and has high specificity and sensitivity. The established method can simultaneously detect pigeon TTV and pigeon new adenovirus infection in pigeon flocks, which simplifies operating procedures and saves costs. After the real-time fluorescent quantitative PCR reaction is completed, the result can be directly judged by observing the peak value of the melting curve (Tm value). The establishment of the present invention can fill in the gaps in related fields at home and abroad.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Method for detecting type-5, type-7 and type-55 adenoviruses by using mass spectrum multiple reaction monitoring technology
PendingCN113156133AThe result is accurateSimple and fast operationBiological material analysisBiological testingSerum samplesAdenovirus infection
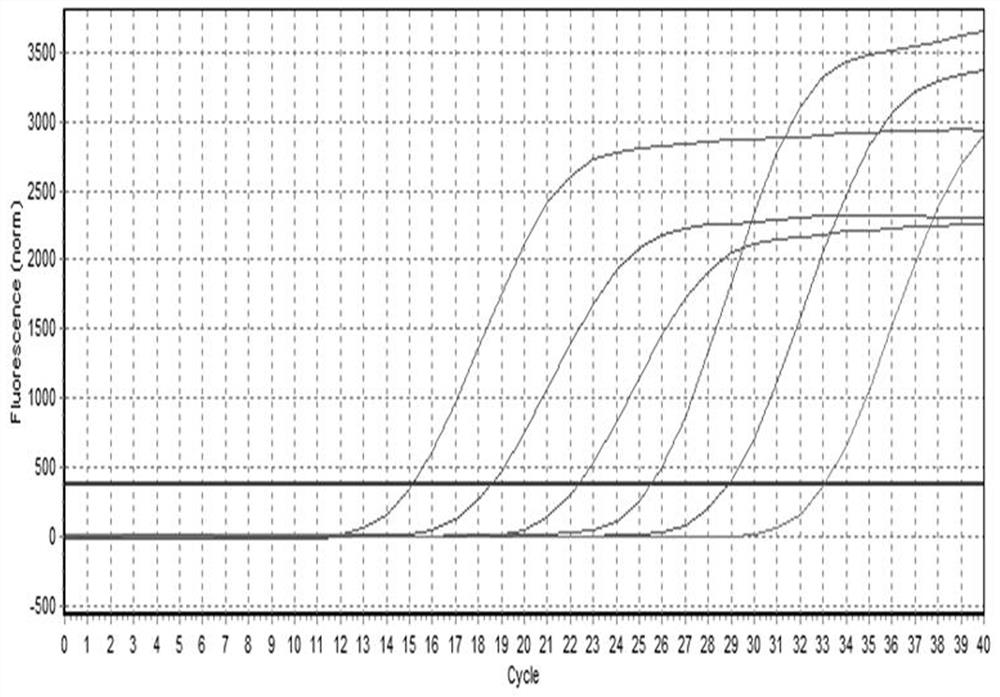
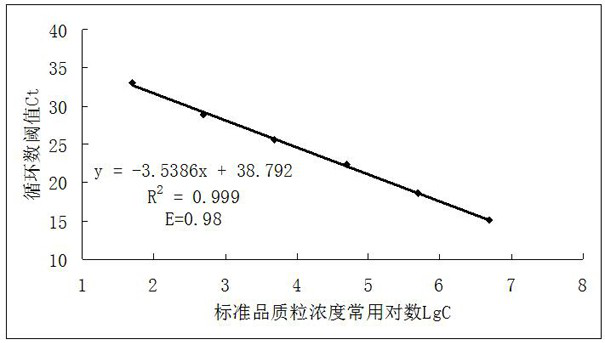
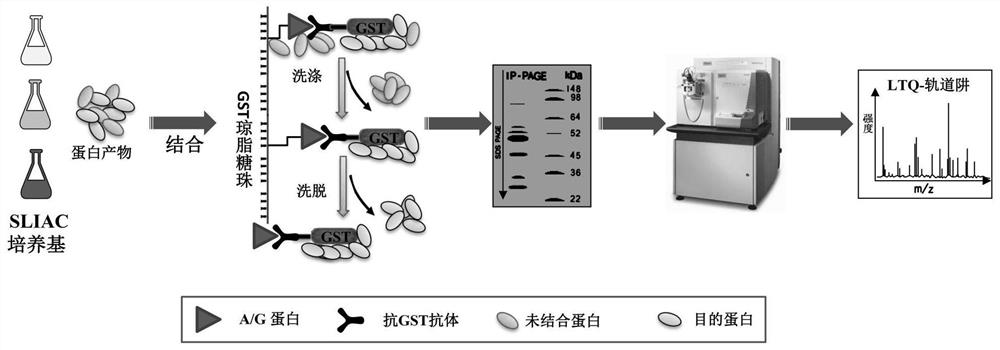
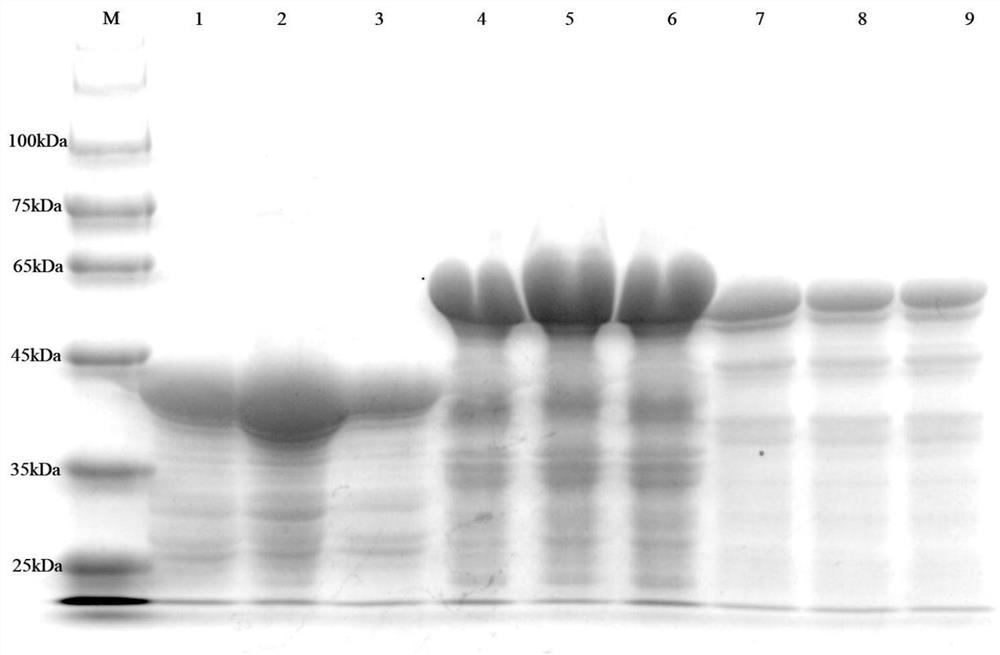
The invention relates to a method for detecting type-5, type-7 and type-55 adenoviruses by using a mass spectrum multiple reaction monitoring technology. The method comprises the following specific steps: synthesizing specific peptide fragment genes of hexon proteins of type-5, type-7 and type-55 adenoviruses; preparing a labeled peptide fragment mixed reagent; determining an ion peak value of the diagnostic marker; preparing the kit; and detecting the sample. The invention provides a new marker for early warning and diagnosis of adenovirus type, type 7 and type 55 infection, and has important clinical value. A mass spectrum result is utilized to confirm that mass spectrum ion peaks consistent with the standard and the heavy standard of preset adenovirus type 5, type 7 and type 55 hexon protein symbolic peptide fragments appear in a serum sample of a patient in the early stage of adenovirus infection. The SILAC-labeled winning and heavy-labeled hexon protein standard mixed reagent of adenovirus type 5, type 7 and type 55 can be used as an early warning and diagnosis marker for adenovirus type, type 7 and type 55 infection, and the result is accurate.
Owner:LOGISTICS UNIV OF CAPF
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com