Monoclonal antibody 3-3E for human adenovirus type 7 and application of monoclonal antibody 3-3E
A technology of monoclonal antibody and sequence, applied in the direction of application, antibody, antiviral agent, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1, the discovery of antibody
[0071] The peripheral blood of convalescent DENV-1 infected patients was collected, and peripheral blood mononuclear lymphocytes were separated and collected. Collected peripheral blood mononuclear lymphocytes were added with anti-cell surface marker antibodies (BD, #555332, #555415, #555441, #560677, #555622) or control antibodies (BD, #555748, #555742, #555751, # 555749, #557872) were labeled and flow sorted, and the sorted cells were used for antibody gene amplification. Using the isolated single B cell as a template, the antibody gene was amplified, and the amplified positive clone was sequenced and expressed, and the purified sample of adenovirus type 7 was used as the antigen. After a large number of screening, analysis, and verification, an antibody sequence was obtained, named as 3-3E antibody.
[0072] The amino acid sequence of the heavy chain variable region of the 3-3E antibody is shown in Sequence 2 of the Sequenc...
Embodiment 2、3-3
[0074] The preparation of embodiment 2, 3-3E antibody
[0075] 1. Construction of recombinant plasmids
[0076] 1. Replace the small fragment between the SalI and PmlI sites of the pTSE-G1n vector (Beijing Baite Meibo Biological Co., Ltd.) with the DNA molecule shown in Sequence 1 of the sequence listing to obtain a recombinant expression vector containing the heavy chain variable region (Already sequenced and verified).
[0077] 2. Replace the small fragment between the SalI and PmlI sites of the pTSE-K vector (Beijing Baite Meibo Biological Co., Ltd.) with the DNA molecule shown in sequence 3 of the sequence listing to obtain a recombinant expression vector containing the light chain variable region (Already sequenced and verified).
[0078] 2. Preparation of 3-3E antibody
[0079] 1. The day before transfection, FreeStyle TM HEK 293-F cells (Invitrogen, product number: R79007) were adjusted to a concentration of 1.0×10 6 / ml, inoculated into culture flasks, 37°C, 5% C...
Embodiment 3、3-3
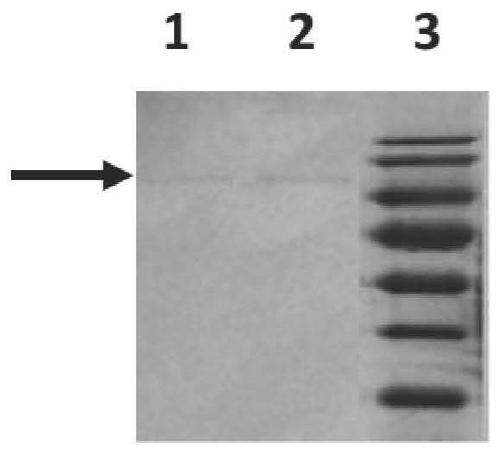
[0084] Example 3, the binding ability detection of 3-3E antibody
[0085] 1. Preparation of human adenovirus type 7 stock solution, virus concentrate and inactivated virus
[0086] 1. Preparation of adenovirus liquid
[0087] Adenovirus culture: conventional DMEM+10% (volume percentage) FBS medium to culture A549 cells (Beijing Concorde Cell Resource Center, article number: 25), and pass A549 cells to 75cm one day before virus inoculation. 2 In the cell flask, make the cell density reach 75% to 90% when the virus is inoculated the next day; on the day of inoculation, slowly suck out the cell culture medium in the culture flask, add 5ml DMEM to gently wash the cells and discard, and then add 3ml DMEM+2 % (volume percentage content) FBS; use a micropipette to draw an appropriate amount of HAdV7 virus into the cell bottle, and infect according to MOI ≈ 0.001, shake the bottle evenly several times to make the virus evenly dispersed, and place it at 37 ° C, 5% CO 2 Adsorb in an i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com