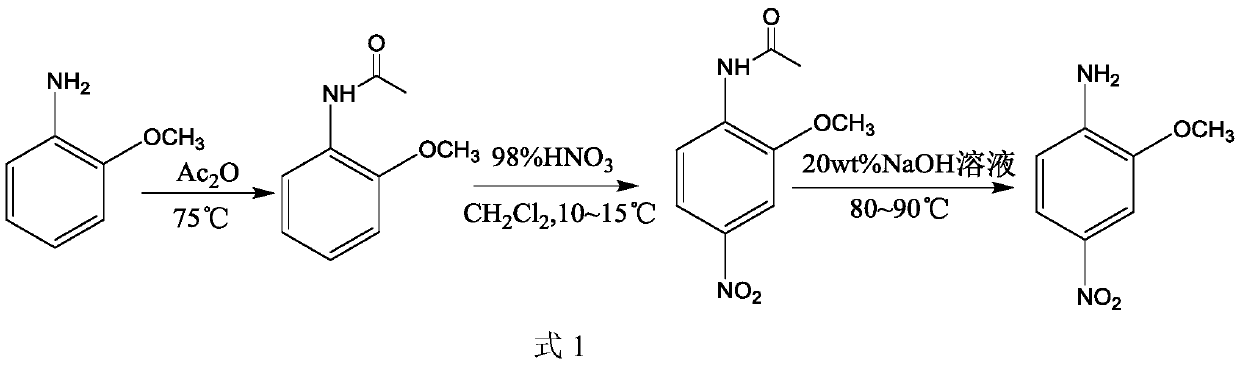
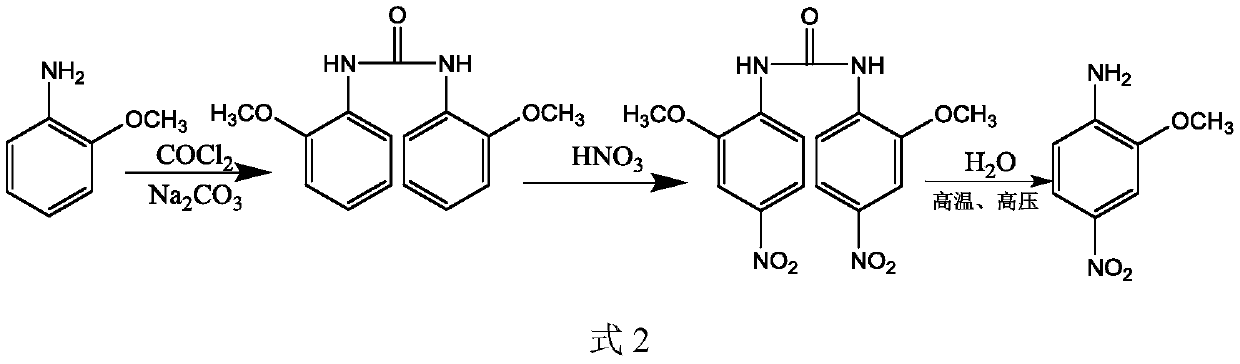
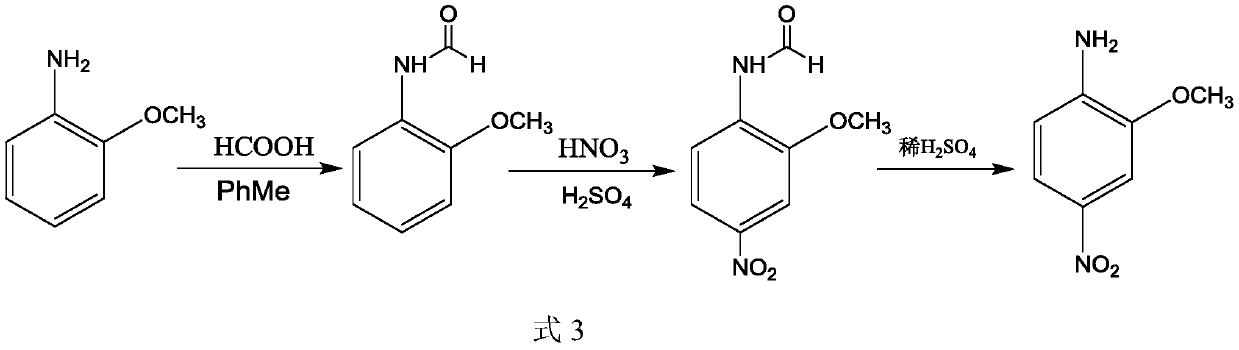
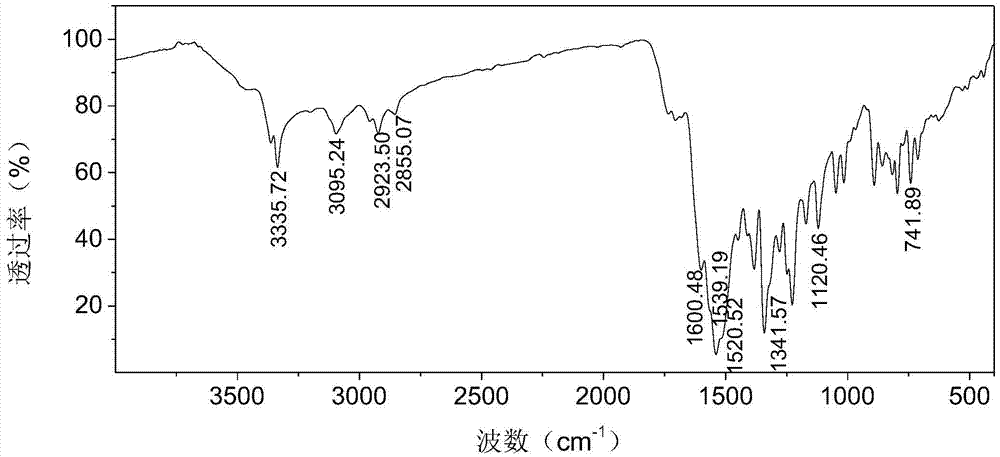
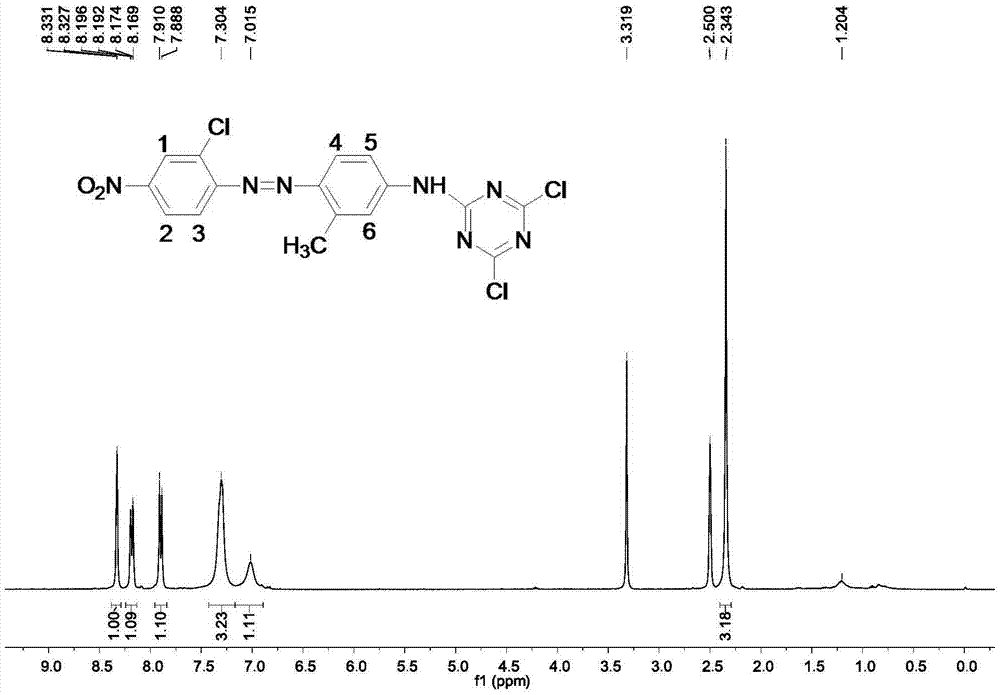
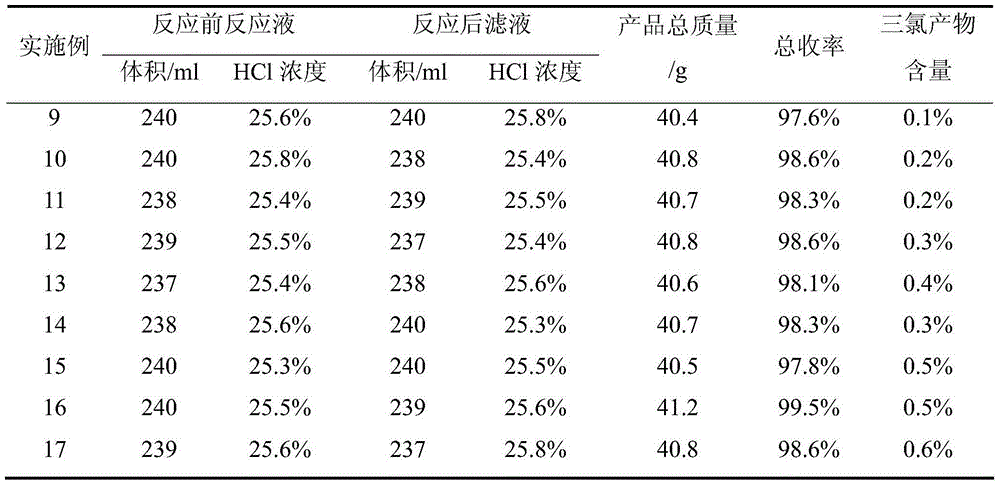
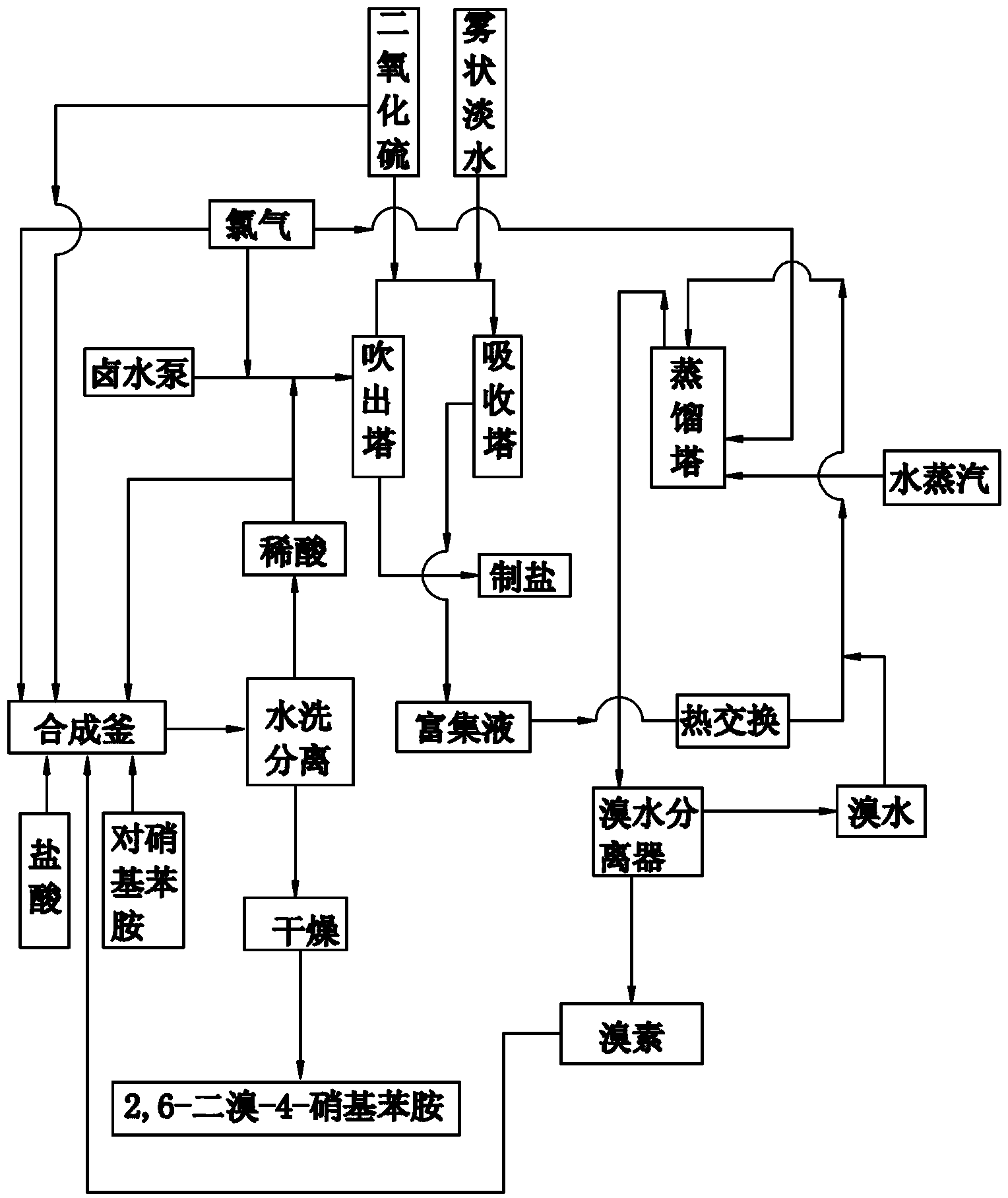
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
74 results about "4-Nitroaniline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
4-Nitroaniline, p-nitroaniline or 1-amino-4-nitrobenzene is an organic compound with the formula C₆H₆N₂O₂. It is an organic chemical compound, consisting of a benzene ring in which an amino group is para to a nitro group. This chemical is commonly used as an intermediate in the synthesis of dyes, antioxidants, pharmaceuticals, gasoline, gum inhibitors, poultry medicines, and as a corrosion inhibitor.
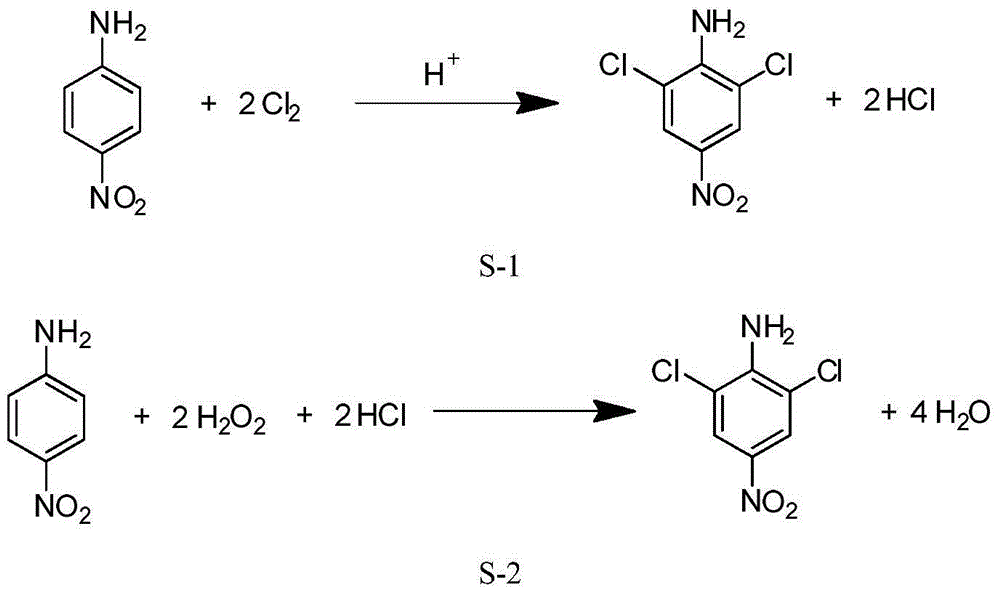
Method for preparing 2,6-dichloro-4-nitroaniline through direct chlorination of chlorine gas and oxidative chlorination of hydrogen peroxide
ActiveCN104610071AThe concentration of hydrochloric acid does not changeThe reaction process is stable and easy to controlOrganic compound preparationAmino compound preparationP-NitroanilineReaction system
The invention relates to a method for preparing 2,6-dichloro-4-nitroaniline through direct chlorination of chlorine gas and oxidative chlorination of hydrogen peroxide. The method comprises the following steps: (1) adding paranitroaniline into hydrochloric acid at the concentration of 5-35wt%, heating to 40-80 DEG C, stirring for uniformly mixing, slowly inflating the chlorine gas within 0.5-5h, slowly adding hydrogen peroxide dropwise, and after adding the chlorine gas and the hydrogen peroxide, continuing to preserve heat and react for 0.3-1.5h; (2) filtering a product obtained in the step (1), washing a filter cake to be neutral, and drying to obtain the 2,6-dichloro-4-nitroaniline. By regulating a dosage ratio of the chlorine gas to the hydrogen peroxide, the concentration of the hydrochloric acid in a reaction system can be kept unchanged, the reaction process is steady and easily controlled, and the product is high in yield and purity.
Owner:昌邑新澳化工有限公司
Preparation method of 2-methoxy-4-nitroaniline
InactiveCN109776337AHigh selectivityReduce usageOrganic compound preparationAmino-hyroxy compound preparationNitroacetanilideNitration
The invention discloses a preparation method of 2-methoxy-4-nitroaniline. The preparation method comprises the following steps of: carrying out acetylation reaction of o-methoxyaniline and acetic acid, discharging water generated in the acetylation reaction process from a reaction system, dropwisely adding fuming nitric acid into the prepared acetic acid solution of the o-methoxyacetanilide to carry out nitration reaction, adding deionized water after the nitration reaction is finished and then filtering, adding the prepared 2-methoxy-4-nitroaniline into an alkali solution to carry out hydrolysis reaction; after the hydrolysis reaction is finished, cooling the prepared reaction solution, and filtering to obtain 2-methoxy-4-nitroaniline. By adopting the method to synthesize 2-methoxy-4-nitroaniline, the acylation reaction cost is low, the nitration reaction selectivity is high, the discharge of three wastes is reduced, the production cost is reduced, the product purity is high, the yield is high, and the 2-methoxy-4-nitroaniline has a good industrial application value.
Owner:福建振新化学有限公司
Pigmentary azo composition, preparation method and use
The present invention is directed to a transparent pigmentary composition for use as a colorant in printing inks. The composition is manufactured by coupling a mixture of diazonium salts obtained from about 98 to about 85% in moles of 2-methoxy-4-nitroaniline and about 2 to about 15% in moles of 4-chloro-2-nitroaniline with acetylaceto-2-anisidide.
Owner:GEBR CAPPELLE +2
Supercritical CO2 dyeing special-purpose azo-type active disperse dye
InactiveCN105440726AHigh reactivityLower dyeing temperatureMonoazo dyesReactive dyesDisperse dyeNatural fiber
The invention belongs to the technical field of dye synthesis, and especially relates to a supercritical CO2 dyeing special-purpose azo-type active disperse dye. The supercritical CO2 dyeing special-purpose azo-type active disperse dye is a dichlorotriazine dye. In a preparation method, 2-chloro-4-nitroaniline and an aniline derivative are taken as raw materials to synthesize a dye precursor; the dye precursor and cyanuric chloride are subjected to nucleophilic substitution so as to obtain the supercritical CO2 dyeing special-purpose azo-type active disperse dye finally. The supercritical CO2 dyeing special-purpose azo-type active disperse dye possesses relatively high reaction activity, and low dyeing temperature, is suitable for supercritical CO2 fluid dyeing of natural fiber, and is green and friendly to the environment; the synthesis technology is simple; operation is convenient; and yield is relatively high.
Owner:SUZHOU UNIV
Method of preparing p-phenylenediamine
InactiveUS6245943B1Low production costHigh selectivityOrganic compound preparationOrganic chemistry methodsAlcoholNitrobenzene
A disclosed method of preparing p-phenylenediamine includes the steps of: reacting urea and nitrobenzene with a base in the presence of a polar solvent to yield 4-nitrosoaniline and 4-nitroaniline; and subsequently, diluting the resulting mixed solution in an alcohol and performing hydrogenation using a catalyst, thereby providing highly pure p-phenylenediamine destitute of an ortho- or meta-isomer as a byproduct. The method has some advantages in that: the process is simplified in such a manner that the hydrogenation is performed in the presence of the hydrogenation catalyst in a single reactor (i.e., one pot) without a need of isolating 4-nitrosoaniline or purifying the product; inexpensive urea and an alkali base are used to reduce the production cost; and 4-nitrosoaniline is formed as an intermediate to yield p-phenylenediamine with a high selectivity, thereby requiring no purification process after isolation of the product.
Owner:KOREA KUMHO PETROCHEMICAL CO LTD
Chitin deacetylase, and construction method and application thereof
The invention relates to a chitin deacetylase, and an expression gene and an application thereof The amino acid sequence of chitin deacetylase BcCDA is represented by SEQ ID NO.2; and the nucleotide sequence of the BcCDA expression gene is represented by SEQ ID NO.1. An engineering strain constructed by using the gene can efficiently secrete and express the chitin deacetylase BcCDA. A recombinant protein with biological activity is obtained after affinity chromatography one-step purification, and the recombinant protein can degrade solid plate generated yellow green 4-nitroaniline with 4-nitroacetanilide as a substrate. Cloning of the chitin deacetylase gene and the successful preparation of the recombinant protein with biological activity are of great theoretic and practical significance to deep development and high value utilization of the chitin resource.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Synthetic method of 2,6-dibromo-4-nitroaniline diazosalt
The invention relates to a synthetic method of 2,6-dibromo-4-nitroaniline diazosalt, solving the technical problems of simplified technique and operation, low requirement for production field and equipment, and energy consumption and 'three wastes' reduction. The invention comprises the following steps: using paranitroaniline as a raw material, pulping in sulphuric acid medium at the mass percentof 20% to 98%, adding brominated compound and oxidant for bromination, and directly diazotizing the mixture with the diazotizd agent after bromination to obtain product, wherein the molar ratio of paranitroaniline, sulphuric acid, brominated compound, oxidant and diazotized agent is 1:2.0 to 8.0:1.0 to 2.5:0.4 to 2.5:1.0 to 1.2.
Owner:HANGZHOU JIHUA JIANGDONG CHEMICAL CO LTD
Preparation method for 2-chloro-4-nitroaniline
ActiveCN101343232AImprove qualityLow costOrganic compound preparationAmino compound preparationWastewaterP-Nitroaniline
The invention relates to a preparation method of o-chloro-p-nitroaniline, which takes p-nitroaniline as a raw material, and prepares the o-chloro-p-nitroaniline by directly inputting chlorine gas for implementing the chlorination reaction in a diluted hydrochloric acid medium with a temperature of between -20 and 10 DEG C, wherein, the p-nitroaniline and the input chlorine gas are present in a molar ratio of 1:1-1.1. The preparation method can prepare the o-chloro-p-nitroaniline with best quality, and has advantages of simple process, high yield, low production cost and non wastewater discharge.
Owner:苏州市罗森助剂有限公司
Low-temperature synthesis method of 2,6-dichloro-4-nitroaniline
ActiveCN104592044AEasy to recycleNo emissionsOrganic compound preparationAmino compound preparationSynthesis methodsP-Nitroaniline
The invention discloses a low-temperature synthesis method of 2,6-dichloro-4-nitroaniline. The method comprises the following steps sequentially: (1) by taking 20-35% diluted hydrochloric acid or a filtrate obtained in the following step (2) as a solvent I, firstly adding p-nitroaniline into the solvent I, then, introducing chlorine gas for chlorination reaction, naturally standing a reaction solution at room temperature after the reaction ends, and filtrating so as to obtain 2,6-dichloro-4-nitroaniline and hydrochloric acid mother liquor; (2) by taking the hydrochloric acid mother liquor as a solvent II, adding the solvent II into a three-mouthed flask, then, adding p-nitroaniline, dropwise adding hydrogen peroxide, carrying out stirring reaction while carrying out heat preservation after dropwise adding is completed, naturally standing at room temperature after the reaction ends so as to precipitate solid, and filtrating, thereby obtaining 2,6-dichloro-4-nitroaniline and the filtrate. 2,6-dichloro-4-nitroaniline synthesized by adopting the method disclosed by the invention has advantages of low cost, high atomic economical efficiency, no wastewater discharge, environment friendliness, high efficiency and the like.
Owner:江苏艾科维科技股份有限公司
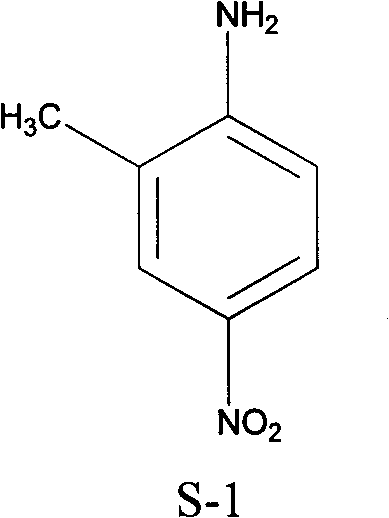
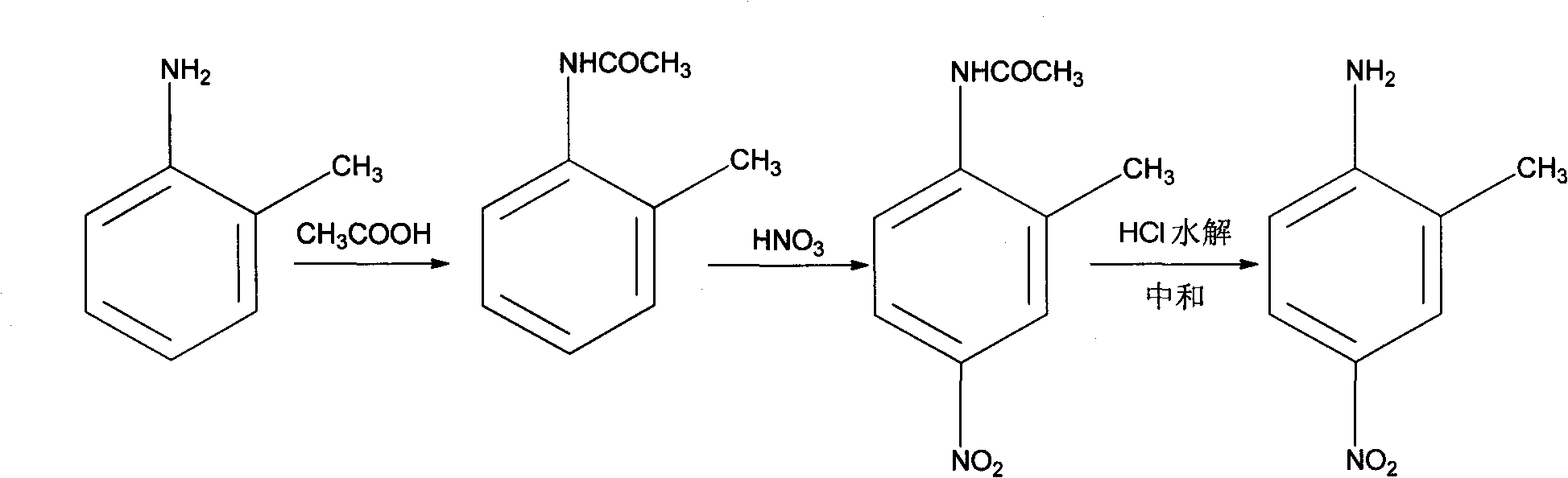
Preparation method of 2-methyl-4-nitrophenylamine
InactiveCN101774929ASolve the disadvantages of high costEmission reductionOrganic compound preparationAmino compound preparationNitrationHydrolysis
The invention discloses a preparation method of 2-methyl-4-nitrophenylamine, which sequentially comprises the following steps of: (1) protecting acylation amino group by using ortho-toludiene as a raw material and using acetic acid as an acylation agent; (2) carrying out nitrification reaction on the reaction liquid obtained in the step (1) by using concentrated nitric acid as a nitrifying agent to obtain a nitrified solid; (3) carrying out hydrolysis reaction on the nitrified solid by using concentrated hydrochloric acid as a hydrolysis reagent; and (4) regulating the pH of the reaction liquid obtained in the step (3) to1-2, and filtering and recrystallizing an obtained filter cake to obtain the 2-methyl-4-nitrophenylamine. The 2-methyl-4-nitrophenylamine prepared by using the method has the characteristics of concise process and low cost.
Owner:ZHEJIANG UNIV
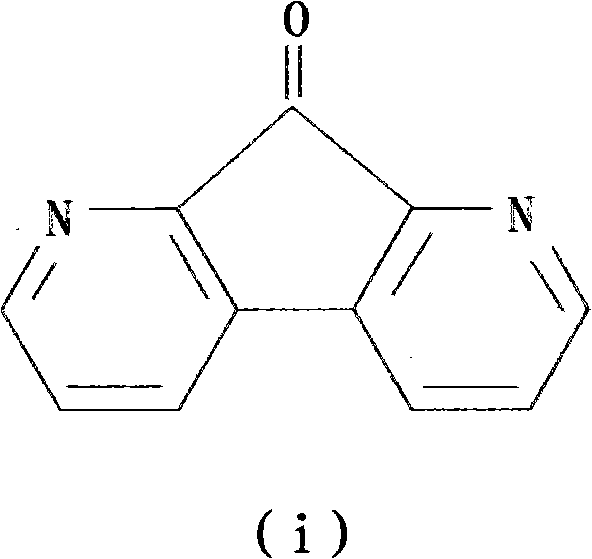
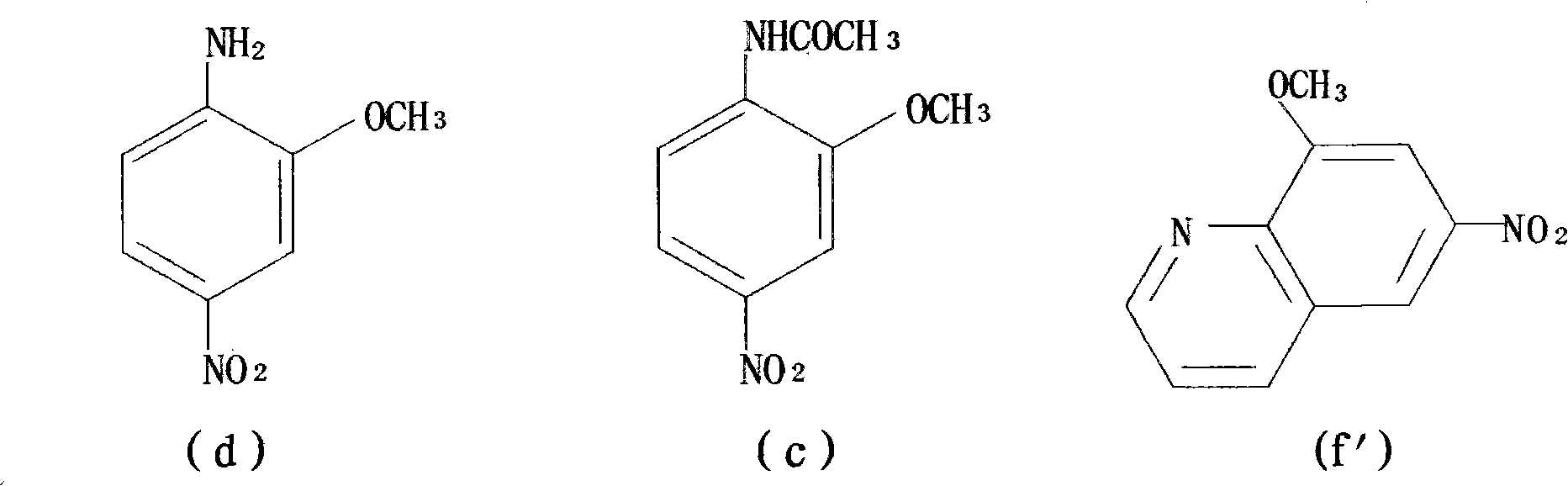
Preparation method of 1,8-dinitro-9-fluorenone
The invention discloses a preparation method of 1,8-dinitro-9-fluorenone (DFO). The preparation method of DFO comprises the following steps that 2-methoxy-4-nitroaniline is synthesized from 2-methoxyaniline; the 2-methoxy-4-nitroaniline is reduced into 2-methoxy-p-phenylenediamine sulfate; the 2-methoxy-p-phenylenediamine sulfate undergoes Skraup quinoline synthesis twice to produce 5(6)-methoxy-4,7-naphthisodiazine; the 5(6)-methoxy-4,7-naphthisodiazine undergoes an oxidation reaction to produce 4,7-naphthisodiazine-5,6-diketone; and the 4,7-naphthisodiazine-5,6-diketone is circulated in an alkaline aqueous solution to produce a product DFO, wherein an overall yield of the five DFO synthesis steps is 26.3%. The preparation method of DFO simplifies a preparation process and effectively reduces a production cost. Through the preparation method of DFO, DFO output can satisfy domestic demands on DFO.
Owner:SUZHOU BEC BIOLOGICAL TECH
New technology for separating nitride in production of 2-cyano-4-nitroaniline
InactiveCN105859582AReduce outputIncrease concentrationCarboxylic acid nitrile purification/separationChemical industryChlorobenzene
The invention belongs to the field of chemical industry, and relates to a new technology for separating nitride in a technology of using concentrated sulfuric acid and concentrated nitric acid to nitrify o-chlorobenzonitrile so as to generate 2-cyano-4-nitro chlorobenzene. An extraction technology is used for extracting the 2-cyano-4-nitro chlorobenzene from waste acid of nitration, and then a product 2-cyano-4-nitroaniline is obtained by an ammonolysis reaction. After the new technology is adopted, the nitride is separated from the waste acid of nitration; the new technology is simple in operation, overcomes the defect that a great deal of waste acid is produced in the traditional technology, and omits the operations of washing the nitride for a plurality of times and dissolving the nitride again after the nitride is separated out; nitride-containing organic liquid is stirred and washed in water, so that a small amount of sulfuric acid and water-soluble organic impurities in an organic phase are removed, and the purity of the nitride-containing organic liquid is higher.
Owner:HEJIAN YINGZHOU CHEM IND CO LTD
New technology for recycling sulfuric acid in production of 2-cyano-4-nitroaniline
ActiveCN105859580AEasy to useReduce enrichment costsOrganic compound preparationCarboxylic acid nitrile purification/separationChemical industryChlorobenzene
The invention belongs to the field of chemical industry, and relates to a new technology for recycling sulfuric acid in a technology of using concentrated sulfuric acid and concentrated nitric acid to nitrify o-chlorobenzonitrile so as to generate 2-cyano-4-nitro chlorobenzene. An extraction technology is used for extracting a product and nitration by-product impurities from waste acid of nitration, so that pure waste acid is obtained; dilute sulfuric acid is concentrated to 75% or more by using concentration equipment; therefore, the defects that the traditional technology is high in energy consumption, high in pollution and the like are overcome; the technology is free from generation of waste acid, thus being very environmentally friendly.
Owner:河北嘉泰化工科技有限公司
Nintedanib impurity and preparation method and application thereof
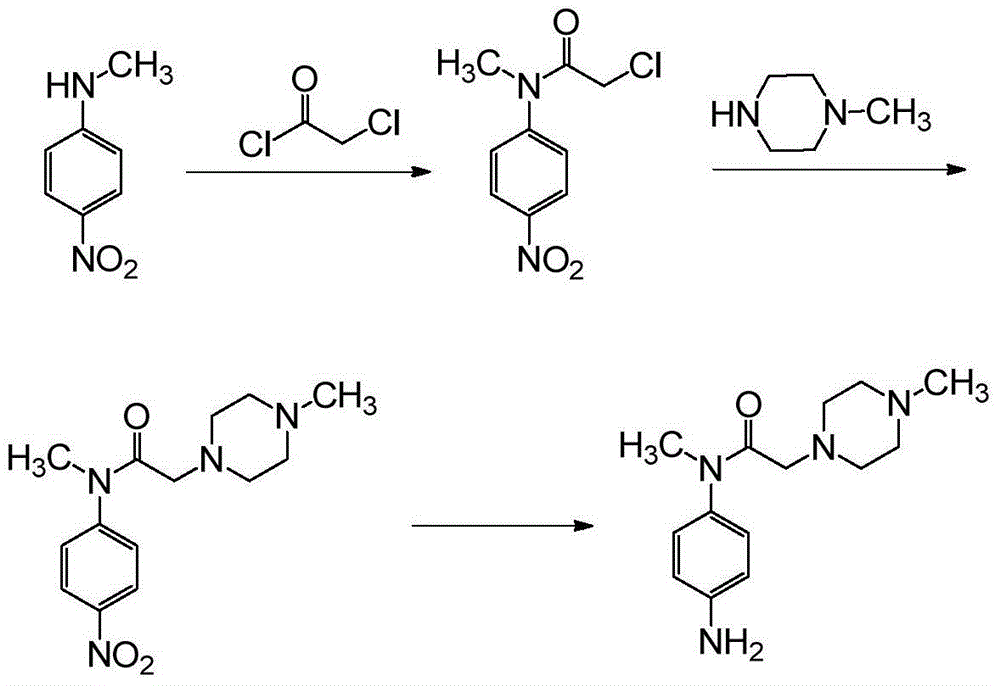
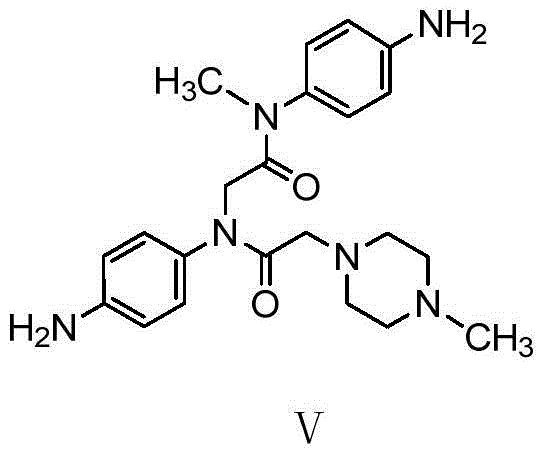
The invention relates to a nintedanib impurity represented by the formula VI and a preparation method thereof. The preparation method comprises that with N-methyl-4-nitroaniline as a starting material, the compound is obtained through the steps of acylation, substitution, acylation, substitution, reduction, substitution reactions and the like. The compound and an intermediate compound represented by the formula V can be used as reference substances for detection of nintedanib raw materials and preparation related substances.
Owner:HAINAN SIMCERE PHARMA CO LTD +1
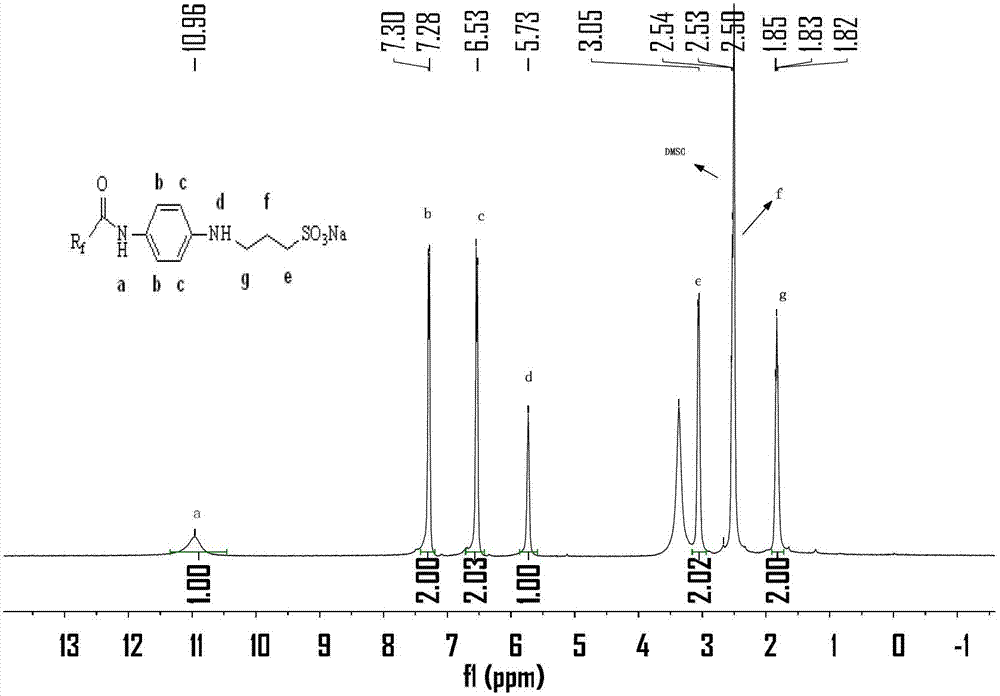
Sulfonate type and sulfonate inner salt type fluorocarbon surfactant as well as preparation and applications thereof
InactiveCN103240034AEasy to degradeSimple preparation processTransportation and packagingSulfonic acids salts preparationChemical industryPapermaking
The invention relates to a sulfonate type and sulfonate inner salt type fluorocarbon surfactant as well as a preparation method and applications thereof. The structure formula of the fluorocarbon surfactant is shown in the specification. The preparation method comprises the steps of: with hexafluoropropylene oxide polymer as a raw material, performing amidation on the raw material and 4-nitroaniline to obtain a product, carrying out nitroreduction on the product, and performing an open-loop alkylation reaction on the product subjected to nitroreduction and 1,3-propanesultone to obtain the sulfonate type and sulfonate inner salt type fluorocarbon surfactant. The sulfonate type and sulfonate inner salt type fluorocarbon surfactant is applied in the fields of textiles, chemical industry, papermaking, oil fields, leather and firefighting. The synthesized sulfonate type and sulfonate inner salt type fluorocarbon surfactant is more easily degraded by replacing the traditional perfluoroalkyl chain with a perfluoropolyether chain, belongs to novel environment-friendly fluorocarbon surfactants, and can be widely applied in multiple fields; and the sulfonate type and sulfonate inner salt type fluorocarbon surfactant is simple in preparation technique, and low in cost and has good application prospects.
Owner:DONGHUA UNIV +1
Organic amine desulfurizer for cyclically absorbing and removing SO2 from flue gas and application of organic amine desulfurizer
InactiveCN103894043AEfficient Absorptive CapacityStrong absorption capacityDispersed particle separationAbsorption capacityAntioxidant
The invention relates to an organic amine desulfurizer for cyclically absorbing and removing SO2 from flue gas. The organic amine desulfurizer comprises an absorbent and a solvent, wherein the absorbent is a water-soluble organic amine compound with the boiling point higher than 120 DEG C; the organic amine compound is selected from one of pyridylamine, N,N-dimethylacetamide, N,N-dimethyl-p-phenylenediamine, butyrolactam, polyacrylamide, hexamethylene tetramine, 2-aminoacetamide, 2-hydroxy-4-nitroaniline, or phenylenediamine, and also comprises an antioxidant, a corrosion inhibitor and an activator. The invention also relates to a regeneration application of the organic amine desulfurizer. The organic amine desulfurizer has a wide material resource; a processing method is simple; the organic amine desulfurizer disclosed by the invention has an efficient and high-specificity absorption capacity on sulfur dioxide when the organic amine desulfurizer is used for desulfurization; the influence of carbon dioxide in late-period sulphur dioxide recycle is eliminated; the recovery purity of the sulfur dioxide is improved. The desulfurizer can be recycled; the recycling and the low-energy-consumption regeneration of the desulfurizer are realized; the desulfurization cost is reduced; the desulfurization efficiency is improved.
Owner:山东金瑞达环保科技股份有限公司 +1
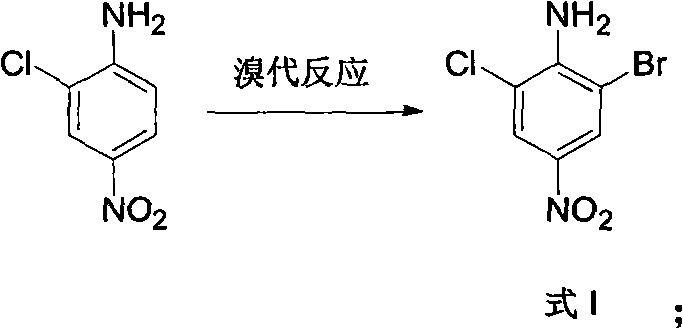
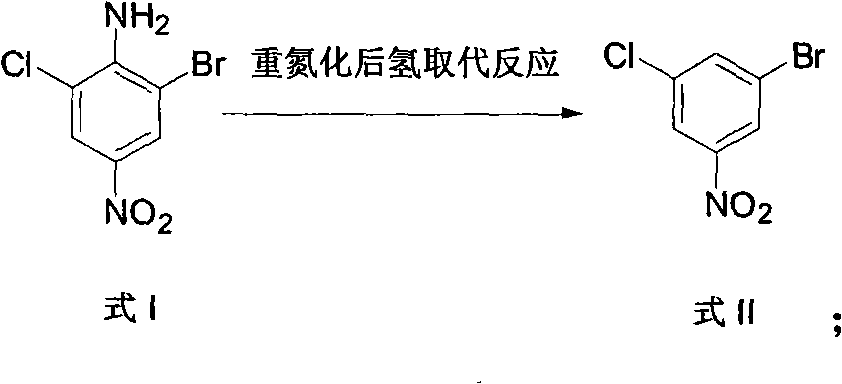
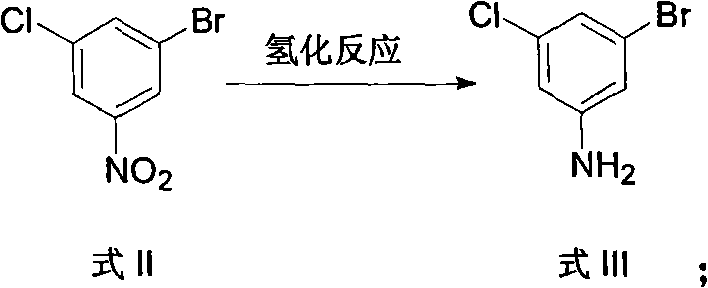
Method for preparing 3-chlorine-5-bromophenol
ActiveCN102146022ACheap and easy to getThe reaction steps are simpleOrganic chemistryOrganic compound preparationReaction stepRaw material
The invention relates to a method for preparing 3-chlorine-5-bromophenol. In the method, 2-chlorine-4-nitroaniline is used as the raw material; and the 3-chlorine-5-bromophenol is prepared according to the following process. In the preparation method, the raw material has low price and is easily obtained; the reaction steps are simple; the reaction condition is mild; the reaction yield is high; the post treatment is simple and is easy to operate; and the product has high content.
Owner:中国中化股份有限公司 +1
Green synthetic method of 2,5-diaminotoluene
ActiveCN101659620AReduce pollutionEasy to operatePhysical/chemical process catalystsOrganic compound preparationHydrazine compoundHydrotalcite
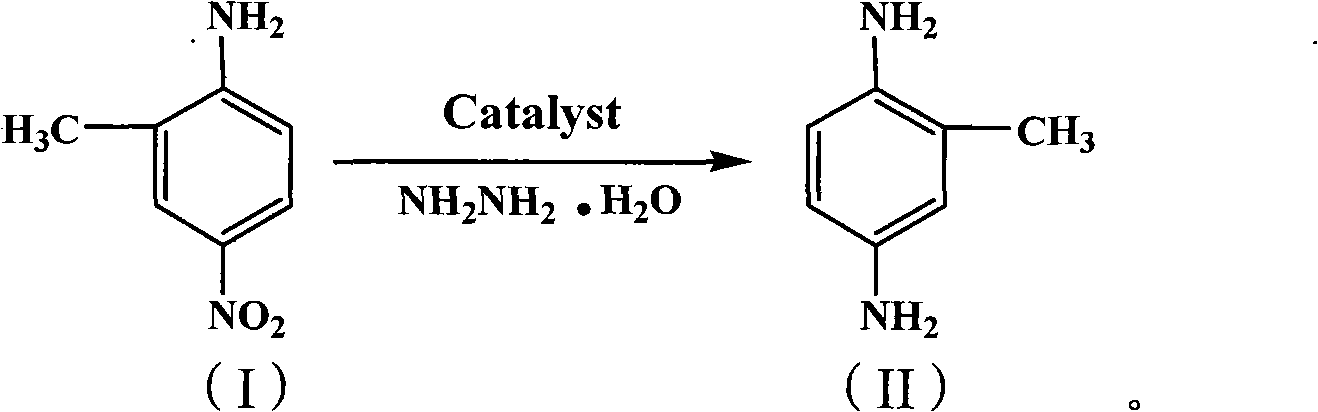
The invention provides a green synthetic method of 2,5-diaminotoluene shown as the formula (II). The method comprises the following steps: taking 2-methyl-4-nitroaniline as raw material, magnetic Mg / Al-hydrotalcite solid alkali as catalyst and hydrazine hydrate as reducing agent, reacting at the temperature of 0-100 DEG C for 1-20 hours, and separating and purifying the reaction solution to prepare the 2,5-diaminotoluene. Compared with the prior art, the green synthetic method has the beneficial effects that: the magnetic Mg / Al-hydrotalcite solid alkali taken as catalyst and the hydrazine hydrate taken as reducing agent are applied in the synthesis of the 2,5-diaminotoluene, so that compared with the prior art, the preparation of the 2,5-diaminotoluene is simpler in processing, easy in operation, high in reaction yield, and good in product purity, has no pollution to environment, is suitable for industrialized production with a certain scale, is a green and clean synthetic line, and has no application report of industrialized production at present.
Owner:山东兴安智慧科技有限公司
Soluble graphene nanoribbon as well as synthetic method and application thereof
InactiveCN105502351AEasy to gatherLower bandgapMaterial nanotechnologyGraphene nanoribbonsBoronic acidDehydrogenation
The invention belongs to the technical field of functional materials and discloses a soluble graphene nanoribbon as well as a synthetic method and an application thereof. The synthetic method comprises the steps that 2,6-dibromo-4-nitroaniline and alkyl / alkoxy arylboronic acid have a Suzuki coupling reaction, 2,6-bis(4-alkyl / alkoxy benzene)-4-nitroaniline is obtained and reduced, 2,6-bis(4-alkyl / alkoxy benzene)-1,4-phenylenediamine is obtained and has halogenation, 2,6-bis(4-alkyl / alkoxy benzene)-1,4-dibromo / iodobenzene is obtained and has an Miyaura borylation reaction, and 2,6-bis(4-alkyl / alkoxy benzene)-4-boronic acid pinacol ester-1-bromo / iodobenzene is obtained; then a poly-para-phenylene derivative is generated through the Suzuki coupling reaction, and a product is obtained through oxidative dehydrogenation. The soluble graphene nanoribbon can be applied to the fields of field effect transistors, photovoltaic cells, non-linear optics and sensing.
Owner:SOUTH CHINA UNIV OF TECH
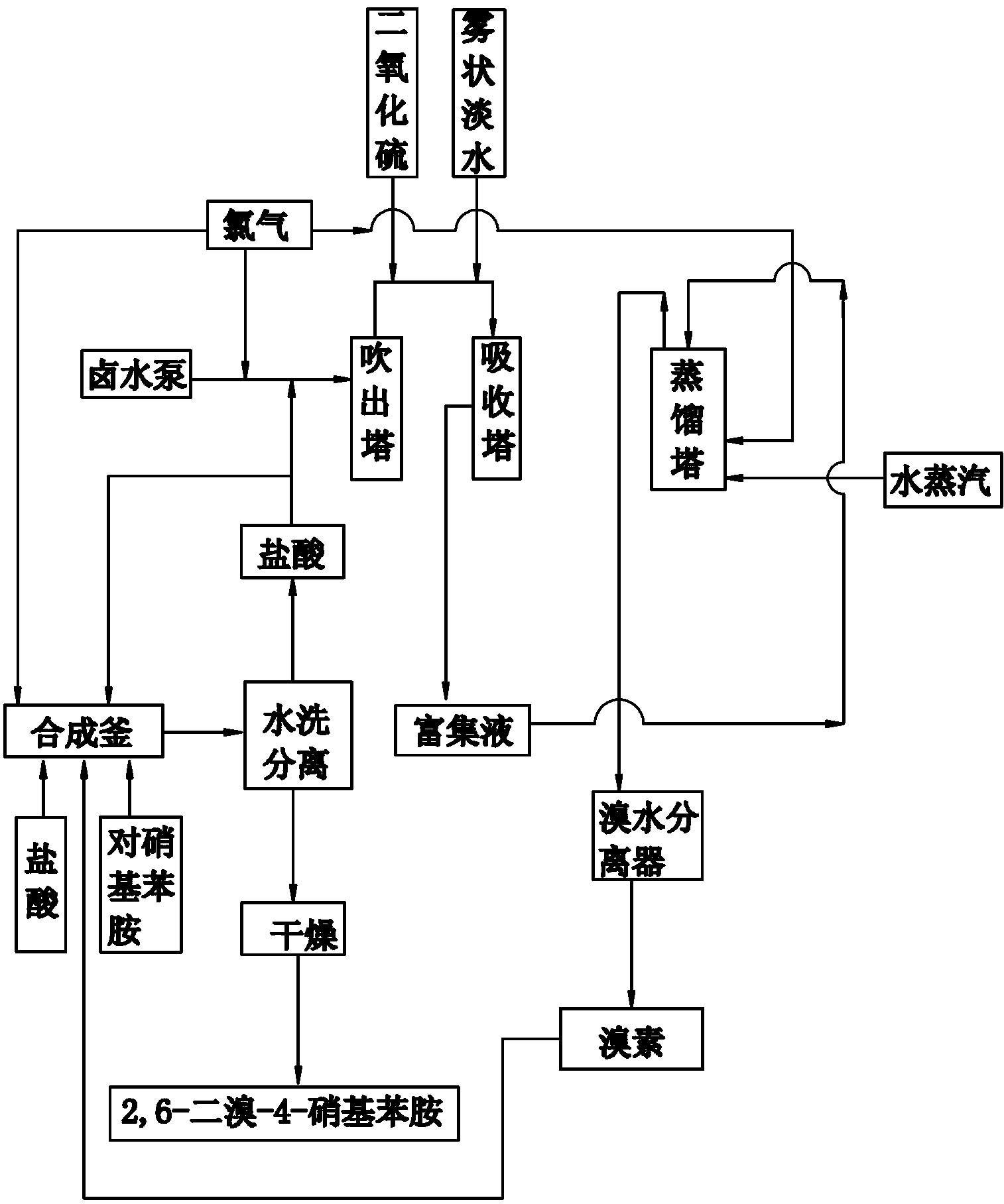
Co-production method and device for 2, 6-dibromo-4-nitroaniline and bromine
ActiveCN103073434AReduce consumptionLow costOrganic compound preparationAmino compound preparationDistillationP-Nitroaniline
The invention belongs to the technical field of synthesis of 2, 6-dibromo-4-nitroaniline and bromine, and particularly relates to a co-production method and device for 2, 6-dibromo-4-nitroaniline and bromine. The method includes the following steps: (1) reacting paranitroaniline with bromine in a hydrochloric acid medium at 40 DEG C-50 DEG C to generate 2, 6-dibromo-4-nitroaniline and hydrobromic acid, and reacting chlorine gas with hydrobromic acid to generate hydrochloric acid; (2) adding hydrochloric acid generated in the step (1) into brine, subjecting brine to acidification, blowout, absorption, distillation and condensation, and enabling brine to enter a bromine water separator for separation to prepare bromine; and (3) adding bromine obtained in the step (2) dropwise into a reaction kettle in the step (1) to prepare 2, 6-dibromo-4-nitroaniline. The device comprises a bromine producing unit and a 2, 6-dibromo-4-nitroaniline producing unit communicated with the bromine producing unit. The co-production method and device save the raw material cost and simplify the production technology.
Owner:山东鲁源化工科技有限公司
Synthesis method of dispersive dye diazonium salt
The invention provides a synthesis method of dispersive dye diazonium salt. In the method, a series compounds, including paranitroaniline, 2,4-dinitroaniline, 2-cyano-4-nitroaniline and the like, are employed as raw materials, which are then subjected to bromination reaction in an organic solvent to respectively prepare solutions or suspension liquids of 2,6-dibromo-4-nitroaniline, 2,4-dinitro-6-bromoaniline, 2-cyano-4-nitro-6-bromoaniline and the like. A certain amount of sulfuric acid is added and then a diazotization reagent is added, or that the diazotization reagent is directly added to perform diazotization; when the diazotization is finished, the reaction products are allowed to stand and are layered to obtain the corresponding diazonium salts and recycled organic solvent which is insoluble in water. Compared with the prior art, the method simplifies a large quantity of process and saves cost. Compared with references in whith bromination is carried out with sulfuric acid as the solvent, the synthesis method greatly reduces the use quantity of the sulfuric acid, thus reducing acidic wastewater emission and saving cost.
Owner:ZHEJIANG RUNTU INST
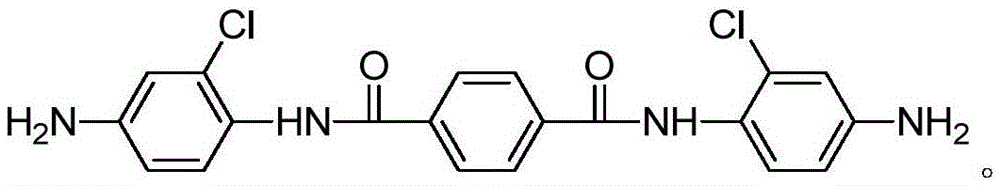
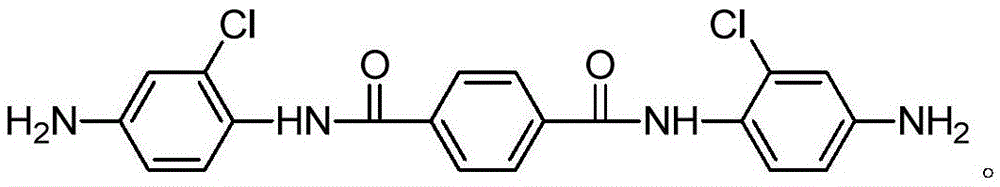
Aromatic diamine monomer and preparation method thereof
InactiveCN105646267AMaintain liquid crystal performanceLoose structureOrganic compound preparationMonocomponent copolyamides artificial filamentNitrobenzeneStructural formula
The invention relates to an aromatic diamine monomer and a preparation method thereof. The aromatic diamine monomer is N,N'-bis(4-amino-2-chlorphenyl) terephthalamide and has a structural formula shown in the specification. The preparation method comprises steps as follows: 2-chloro-4-nitroaniline, alkali and tetrahydrofuran are mixed, a tetrahydrofuran solution of paraphthaloyl chloride is dropwise added, the mixture reacts for 8-12 h at the temperature of 25-35 DEG C, N,N'-bis(2-chloro-4-nitrobenzene) terephthalamide is obtained, ethanol and Pd / C are added to N,N'-bis(2-chloro-4-nitrobenzene) terephthalamide under the protection of argon, the mixture reacts for 5-12 h at the temperature of 80-100 DEG C under the protection of hydrogen, and the aromatic diamine monomer is obtained. The aromatic diamine monomer is simple in structure, has good development prospect and is an important constituent part for PPTA (poly(p-phenylene terephthalamide)) copolycondensation modification in the future. The preparation method is simple, and the yield is high.
Owner:DONGHUA UNIV
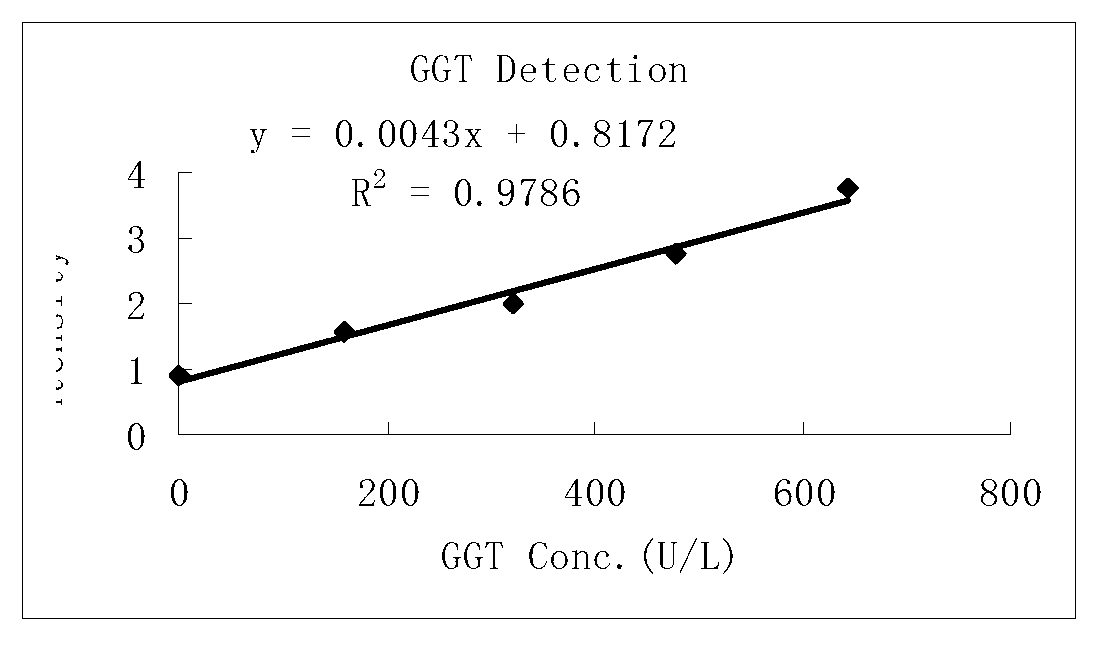
Gamma-glutamoyl transferase detection reagent
ActiveCN103290097AHigh sensitivityImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsVitamin CFreeze-drying
The invention discloses a gamma-glutamoyl transferase detection reagent which comprises a diluent and a reaction reagent, wherein the diluent is a buffer liquid, a surfactant, a preservative, a debilirubin and a vitamin C oxidase, and the reaction reagent is a buffer liquid, a bi-glycoside peptide, L-gamma-glutamoyl-3-hydroxyl-4-nitroaniline, a primer protector, a preservative and a freeze-drying protective additive. The detection reagent disclosed by the invention has good sensitivity, accuracy, precision and linearity, and can completely satisfy the clinical examination requirement.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
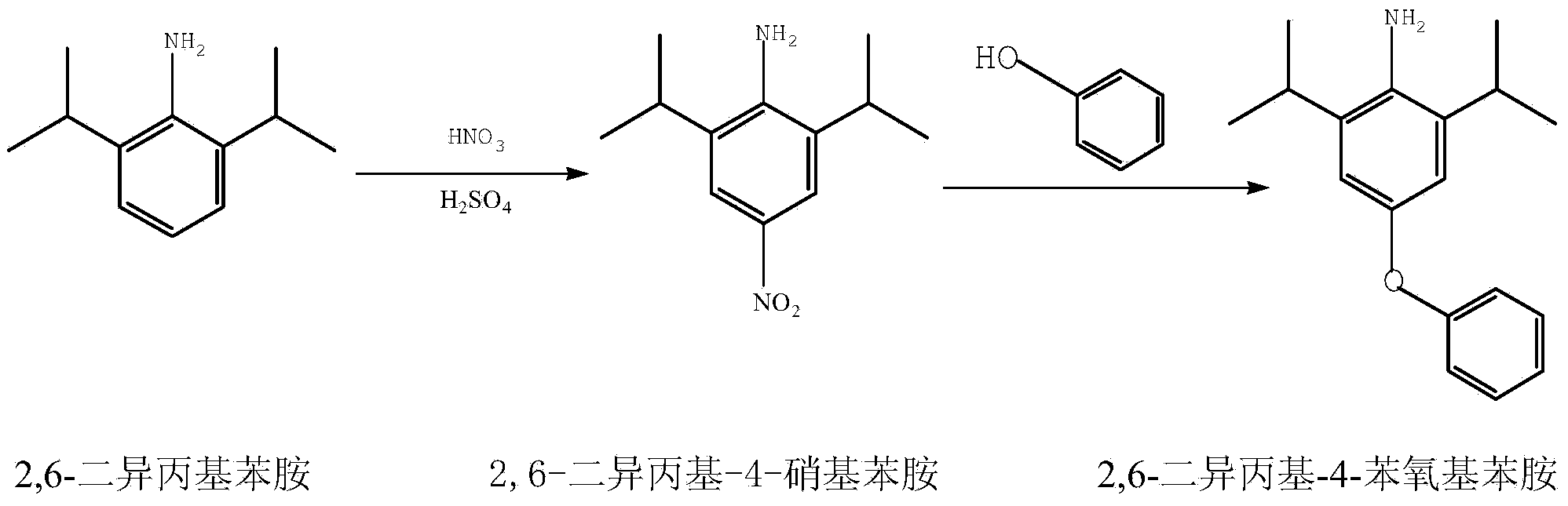
Synthetic method for 2,6-diisopropyl-4-phenoxy aniline
ActiveCN103724213AGood effectReduce usageOrganic compound preparationAmino-hyroxy compound preparationReaction temperatureSolvent
The invention relates to a synthetic method for 2,6-diisopropyl-4-phenoxy aniline. According to the method, 2,6-diisopropyl aniline is used as a raw material and is dissolved into a solvent; sulfuric acid is added and nitric acid is dropwise added to react to generate 2,6-diisopropyl-4-nitryl aniline; the 2,6-diisopropyl-4-nitryl aniline is not separated and is directly subjected to the next step of a condensation reaction to prepare the 2,6-diisopropyl-4-phenoxy aniline. According to the synthetic method, a quaternary ammonium salt is used as a catalyst and a reaction temperature is reduced to 110 DEG C from 140 DEG C; the requirements on reaction equipment are reduced and the period of a technological process is shortened. The synthetic method is simple to operate, environment-friendly and low in cost, is high in product selectivity, yield and purity, and is suitable for large-scale industrial production.
Owner:XINFA PHARMA
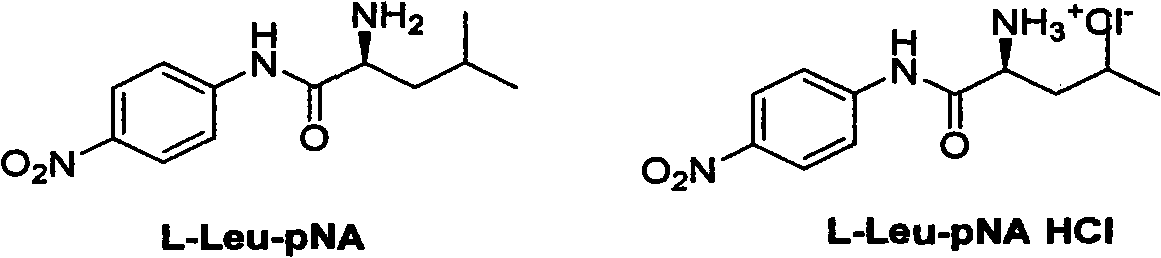
New synthesis method of important biochemical reagent L-leucine-4-nitroaniline hydrochloride
ActiveCN102936207ARaw materials are easy to getEasy to operateOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsP-Nitroaniline
The invention relates to a new synthesis method of an important biochemical reagent L-leucine-4-nitroaniline hydrochloride. The method includes: under the action of sodium hydroxide, reacting an L-Leu-OH solution with BOC anhydride to obtain Boc-L-Leu-OH; subjecting the Boc-L-Leu-OH and a pyridine solution of p-nitroaniline to condensation under the action of phosphorus oxychloride so as to generate the key intermediate Boc-L-Leu-pNA, with the condensation method being novel and not reported in the literature; and reacting a glacial acetic acid solution of the Boc-L-Leu-pNA with a hydrogen chloride gas so as to generate Boc-L-leucine-4-nitroaniline hydrochloride. The method has the advantages of easily available starting raw materials, short reaction steps, convenient operation, high yield, and low cost.
Owner:SUZHOU BAILINGWEI HYPERFINE MATERIAL
Detection method for 2-methyl-4-nitroaniline in waste water
InactiveCN106053420AImprove crystal structureHigh purityFluorescence/phosphorescenceFluorescenceMetal-organic framework
The invention provides a detection method for 2-methyl-4-nitroaniline in waste water, belonging to the technical field of analytical chemistry. According to the method, a one-dimensional metal-organic framework coordination polymer {[Pb(asba)(obix)].2H2O}n is synthesized by using a solution method at first; the method is simple; and the prepared coordination polymer has a better crystal structure and high in purity. A crystal material with a periodic network structure is formed through molecule self-assembling of metal salts and organic ligand, inherits the advantages of inorganic and organic matters and can be applied to a detection environment based on a fluorescence quenching mechanism, especially to detection of a toxic pollutant 2-methyl-4-nitroaniline in waste water.
Owner:YANGZHOU UNIV
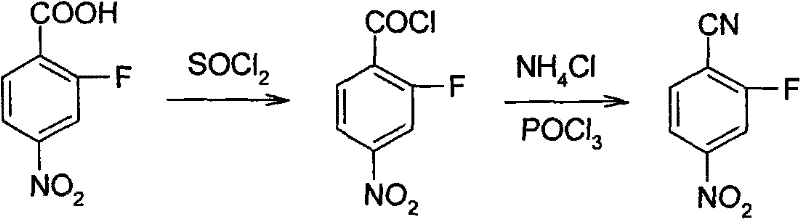
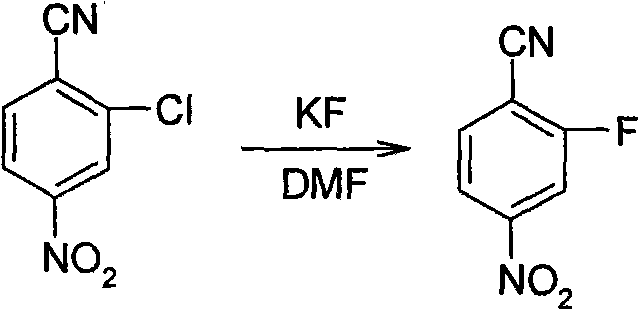
Synthesis method of 2-fluoro-4-nitrobenzonitrile
ActiveCN101648890BReduce manufacturing costEase of industrial productionPreparation by cyanide reaction4-nitrobenzonitrileSynthesis methods
The invention relates to a synthesis method of 2-fluoro-4-nitrobenzonitrile and the method comprises the following steps: adopting 2-fluoro-4-nitrophenylamine as raw material, using diazotization bromination to convert -NH2 in 2-fluoro-4-nitrophenylamine to -Br and then using NMP as solvent to perform cyaniding and obtain 2-fluoro-4-nitrobenzonitrile. The invention has the advantage of cheap and accessible raw material, lower toxicity of raw material and capability of industrialized production.
Owner:CHANGZHOU XIAOGUO INFORMATION SERVICES
Method for preparing 2,4-dinitroaniline diazonium salt
InactiveCN101619030ASafe to dryImprove stabilityOrganic chemistrySimple Organic CompoundsAqueous solution
The invention provides a method for preparing 2,4-dinitroaniline diazonium salt. The method comprises the following steps: (1) diazotization: slowly adding dry NaNO2 to anhydrous sulfuric acid, then adding 2,4-nitroaniline, cooling the solution after a reaction and diluting the solution in a mixture of ice and water to obtain a sulfuric acid aqueous solution of the 2,4-dinitroaniline diazonium salt; (2) crystal separation: adding an anionic organic compound to the sulfuric acid aqueous solution of the 2,4-dinitroaniline diazonium salt; stirring the solution to crystallize and separate; filtering crystals to obtain a filter cake of the 2,4-dinitroaniline diazonium salt; and (3) drying: drying the filter cake of the 2,4-dinitroaniline diazonium salt to obtain 2,4-dinitroaniline diazonium salt powder, i.e. solid 2,4-dinitroaniline diazonium salt powder.
Owner:TIANJIN DEK CHEM
Process for preparing 3-chloro-5-nitrotoluene
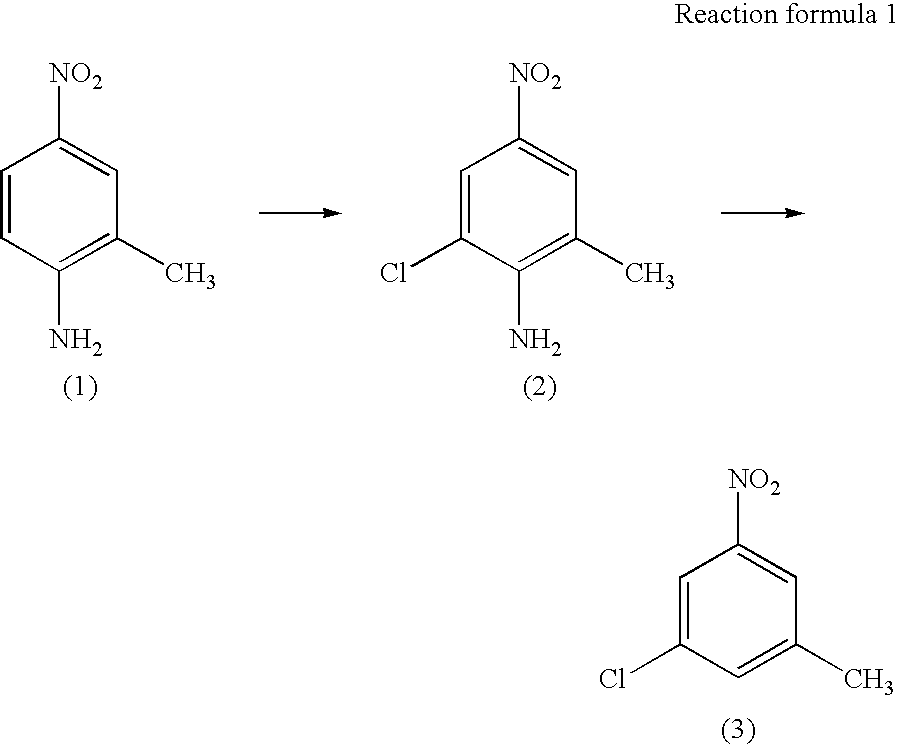
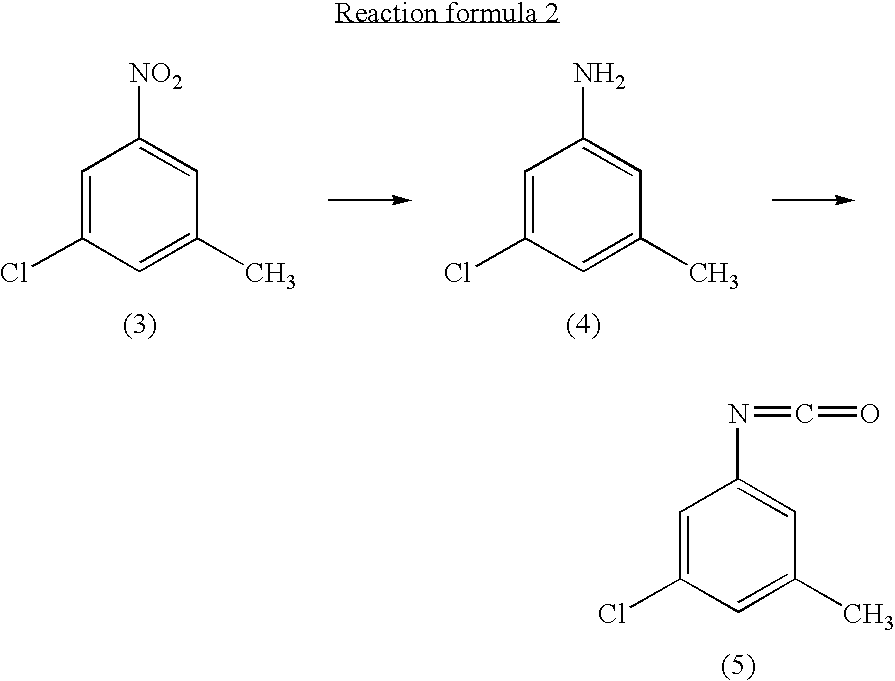
InactiveUS20040147776A1Preparation from carboxylic acid nitrogen analoguesOrganic compound preparationHypochloriteChemistry
A preparation process of 3-chloro-5-nitrotoluene is provided in mild conditions. It is a process for preparing 3-chloro-5-nitrotoluene, which comprises reacting 2-methyl-4-nitroaniline with a chlorinating agent such as 5-butyl hypochlorite in a neutral condition to obtain 2-chloro-4-nitro-6-methylaniline and deaminating the 2-chloro-4-nitro-6-methylaniline to obtain 3-chloro-5-nitrotoluene.
Owner:DAICEL CHEM IND LTD
Process for preparing 3-chloro-5-nitrotoluene
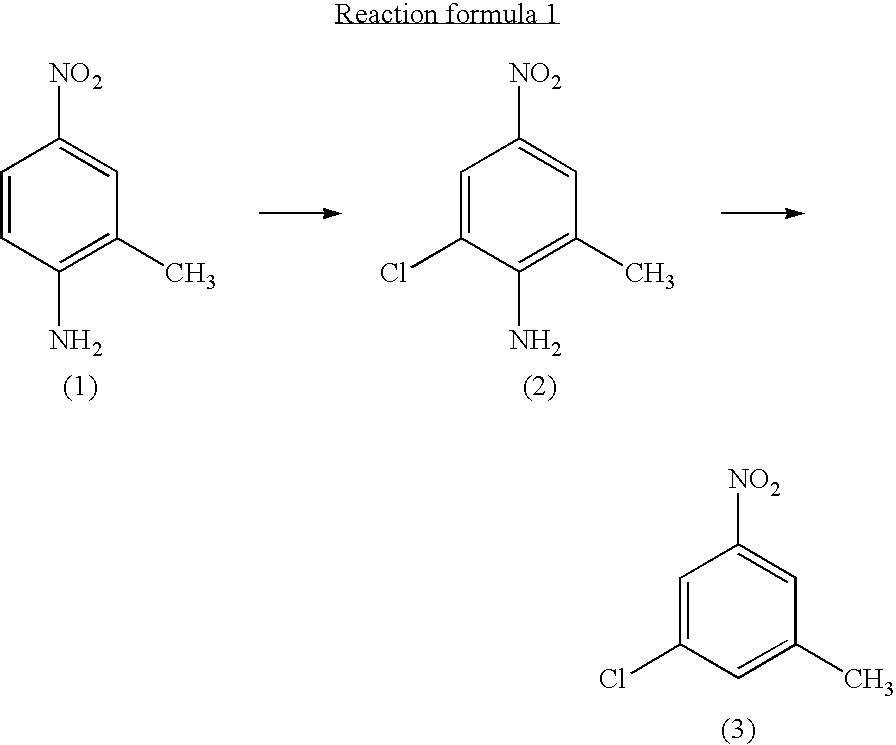
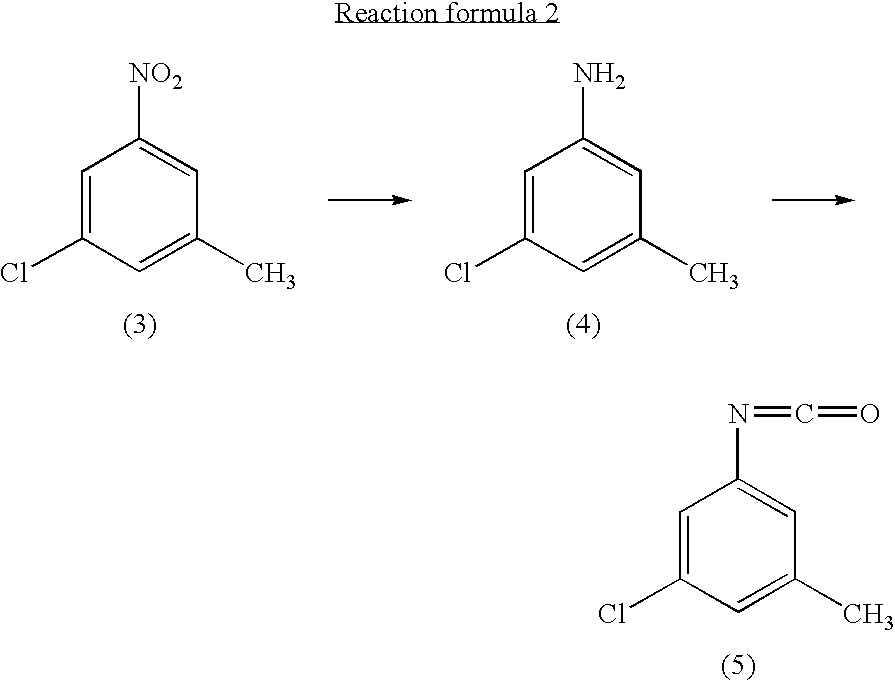
InactiveUS7081548B2Preparation from carboxylic acid nitrogen analoguesOrganic compound preparationMethylanilineHypochlorite
A preparation process of 3-chloro-5-nitrotoluene is provided in mild conditions. It is a process for preparing 3-chloro-5-nitrotoluene, which involves the steps of reacting 2-methyl-4-nitroaniline with a chlorinating agent such as 5-butyl hypochlorite in a neutral condition to obtain 2-chloro-4-nitro-6-methylaniline and deaminating the 2-chloro-4-nitro-6-methylaniline to obtain 3-chloro-5-nitrotoluene.
Owner:DAICEL CHEM IND LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com