Sulfonate type and sulfonate inner salt type fluorocarbon surfactant as well as preparation and applications thereof
A technology of fluorocarbon surface and sulfonic acid inner salt, which is applied in the direction of sulfonate preparation, sulfonic acid preparation, transportation and packaging, etc. It can solve the problems of complex synthesis, many steps, and great difficulty, and achieve simple preparation process and low cost , easily degradable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Synthesis of Fluorinated Intermediate Compound 2
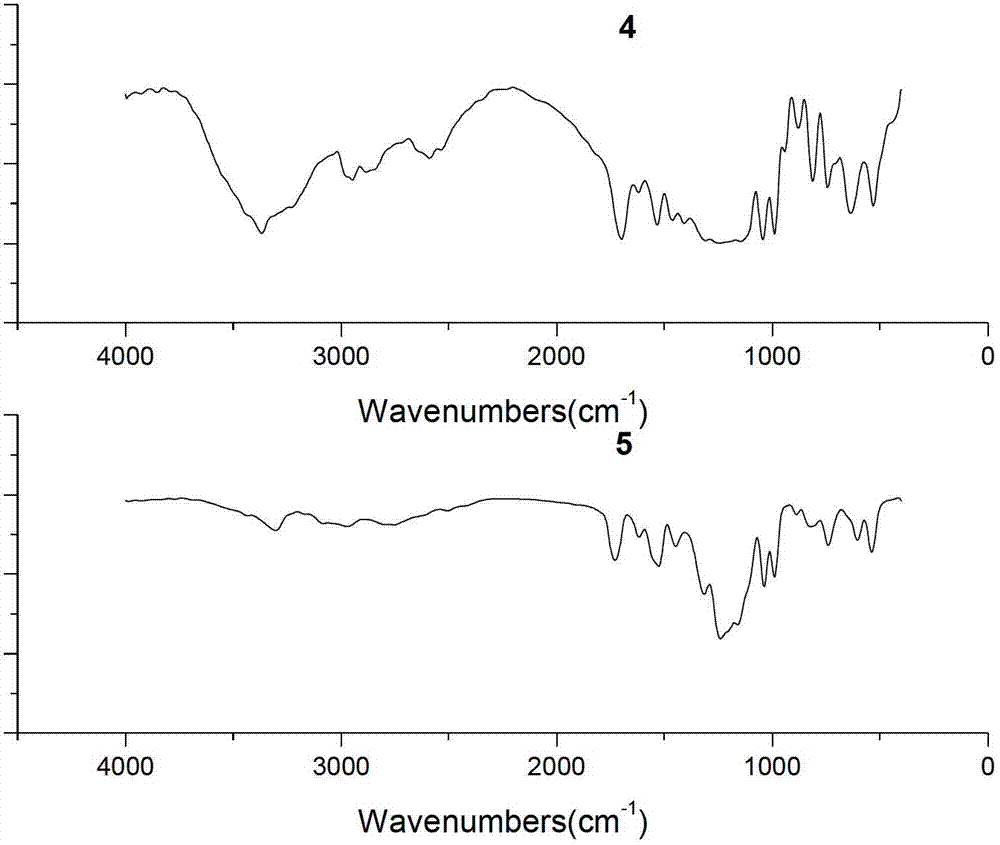
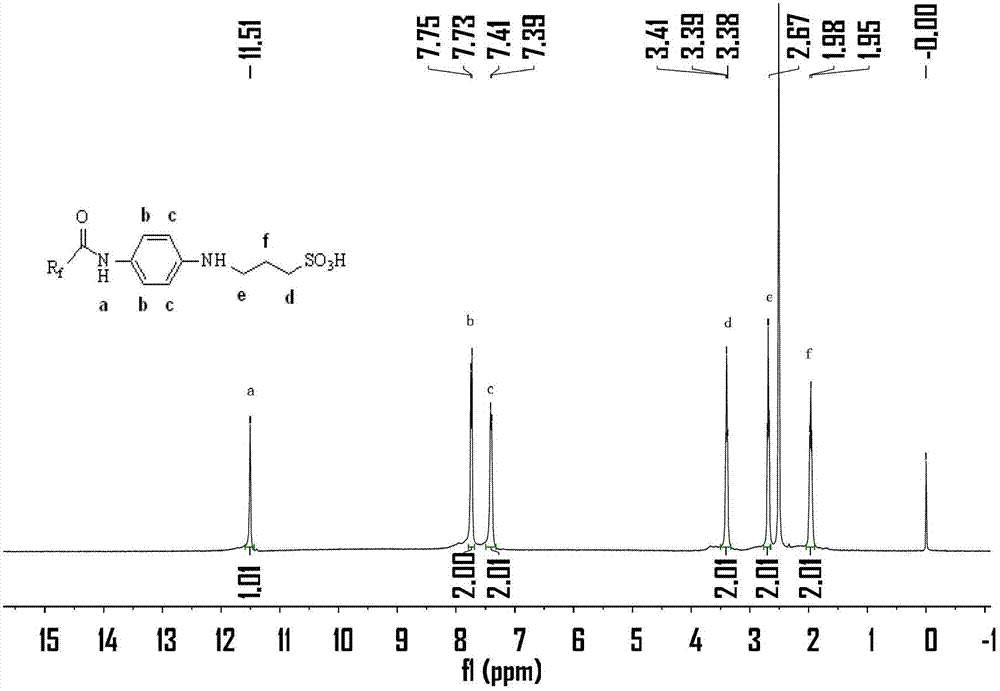
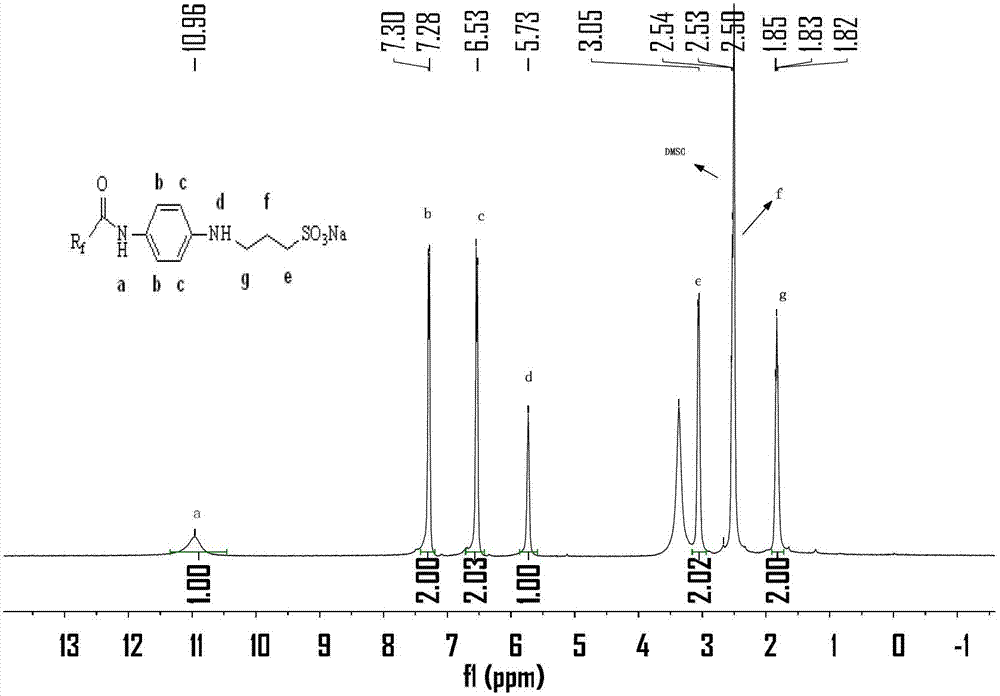
[0028] N 2 Protected, 250mL three-necked flask, add p-nitroaniline (6.62g, 48mmol) and chloroform (100mL) at room temperature, after fully dissolved, add Et 3 N (5.05g, 50mmol) was used as an acid-binding agent. Perfluoropolyetheryl fluoride 1 (19.92g, 40mmol) was slowly added dropwise at 0°C. Extract with chloroform (80mL×2), combine the extract with the organic phase, wash once with sodium bicarbonate (5%), then wash with water until neutral, dry the organic phase with anhydrous sodium sulfate, spin to dry the solvent, and perform column chromatography (silica gel, dichloromethane:methanol=20:1) separation and purification to obtain yellow oily solid product 2 (21.19g, yield 86%). m.p.31~31.9℃.IR(KBr,cm -1 )υ max 3325,3132,1720,1567,1511,1106,1033,989,538. 1 H NMR (400MHz, CDCl 3 ): δ8.33(d, J=9.2Hz, 3H, Note: Due to the strong electron-withdrawing property of the nitro group, the chemical shifts of the two hydr...
Embodiment 2
[0030] Synthesis of Fluorinated Intermediate Compound 3
[0031] In a 250mL three-neck flask, dissolve compound 2 (12.32g, 20mmol) in a mixture of 95% ethanol (64mL) and distilled water (24mL) at room temperature, and add reduced iron powder every 15min for a total of 3 times (4.48g, 80mmol), while slowly adding hydrochloric acid (4mL, 6mol / L) dropwise. Then heated to 90°C to continue the reaction for 25h, filtered while hot, and washed the filter residue with hot ethanol solution. After cooling, the filtrate was spin-dried to ethanol, and separated and purified by column chromatography (silica gel, petroleum ether: ethyl acetate = 6:1) to obtain product 3 as a light red solid (10.67 g, yield 91%). m.p.29.0~30.2℃.IR(KBr,cm -1 )υ max 3443,3350,3222,1716,1622,1547,1239,1038,522. 1 H NMR (400MHz, CDCl 3 ):δ7.88(s,1H),7.33(d,J=8.4Hz,2H),6.70(d,J=8.4Hz,2H),7.88(s,2H). 19 F NMR (376MHz, CDCl 3 ): δ-80.03, -81.30, -81.42, -82.13, -82.25, -129.79, -131.59, -145.31.
Embodiment 3
[0033] Synthesis of Fluorinated Intermediate Compound 3
[0034] N 2 Protected, 250mL three-neck flask, dissolved p-phenylenediamine (3.24g, 30mmol) in dry chloroform (120mL) at room temperature, after fully dissolved, added Et 3 N (3.03g, 30mmol) was used as an acid-binding agent, acid fluoride compound 1 (9.96g, 20mmol) was slowly added dropwise at 0°C, and after the dropwise addition was completed, it was raised to room temperature for 3 hours, and water was added to quench the reaction, and the reaction was washed with chloroform ( 100mL×2) extraction, the extract was combined with the organic phase, washed once with sodium bicarbonate (5%), then washed once with water, the organic phase was dried with anhydrous sodium sulfate, spin-dried the solvent, column chromatography (silica gel, petroleum ether: Ethyl acetate=6:1) was separated and purified to obtain product 3 as a light red solid (5.03 g, yield 43%). m.p.29~30.2℃.IR(KBr,cm -1 )υ max 3443,3350,3222,1716,1622,154...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com