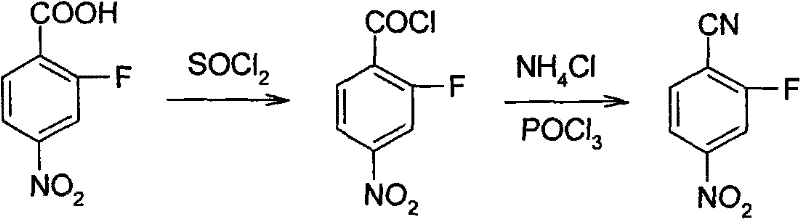
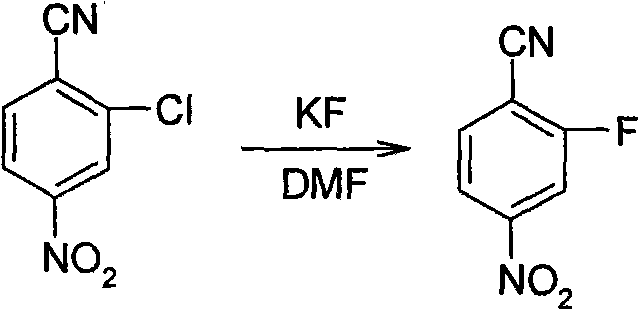
Synthesis method of 2-fluoro-4-nitrobenzonitrile
A technology of nitrobenzonitrile and synthetic method, which is applied in the field of synthesis of 2-fluoro-4-nitrobenzonitrile, and can solve the problem of lack of 2-fluoro-4-nitrobenzonitrile reaction conditions and yield, etc. problems, to achieve the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0032] The preparation of example 1,2-fluoro-4-nitroaniline
[0033] Put 1200mL of industrial alcohol, 750mL of 28% ammonia water, 27g of cuprous oxide and 480g of 3,4-difluoronitrobenzene into the high-pressure reactor, mix the above materials, raise the temperature to 120°C and control the pressure of the reactor at 1.7MPa , heat preservation reaction for 18 hours, GC tracking reaction is basically over; after cooling down, the material is pressed into water, and 451.5g of crude product 2-fluoro-4-nitroaniline is precipitated, the filter cake is washed with water and dried, and its content is 98.61%. (GC), the molar yield was 96.4% (based on 3,4-difluoronitrobenzene).
example 2
[0035] (1), pour the concentration of 2000g into a 5000mL four-necked flask and be 20% by weight sulfuric acid and 156g of 2-fluoro-4-nitroaniline prepared in Example 1, heat up to 90°C and keep it warm for 1 hour and then cool down to 0~5℃; Slowly add 235g of NaNO with a concentration of 30% by weight in this solution 2 Aqueous solution, keep warm at 0-5°C for 0.5 hours after adding, filter and remove a small amount of solid impurities to obtain about 2110mL of clear and transparent diazonium liquid, and store at 0-5°C;
[0036] (2), in another 5000mL four-neck flask, add 530g of hydrobromic acid solution with a concentration of 6.5% by weight of copper powder, stir and heat up, cool down to 65°C after being kept at 100°C for 1 hour, and add the above-mentioned prepared solution dropwise. Cold diazonium solution; keep warm for 0.5 hours after dripping, drop to room temperature, filter out orange solid crude product 2-fluoro-4-nitrobromobenzene, wash with water, the content of...
example 3
[0039] (1), in the four-neck flask of 5000mL, pour the concentration of 2000g into the 2-fluoro-4-nitroaniline that makes in the sulfuric acid of 24% by weight and 156g example 1, be heated up to 85 ℃ of insulation 1 hour and cool down to 0~5℃; Slowly add 210g of NaNO with a concentration of 33% by weight in this solution 2 Aqueous solution, keep warm at 0-5°C for 0.5 hours after adding, filter and remove a small amount of solid impurities to obtain about 2100mL clear and transparent diazonium solution, keep it at 0-5°C;
[0040] (2), in another 5000mL four-neck flask, add 530g of hydrobromic acid solution with a concentration of 6.0% by weight of copper powder, stir and heat up, cool down to 65°C after being kept at 100°C for 1 hour, and add the above-mentioned prepared solution dropwise. Cold diazonium solution; keep warm for 0.5 hours after dripping, lower to room temperature, filter out the orange solid crude 2-fluoro-4-nitrobromobenzene, wash with water, the crude 2-fluor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com