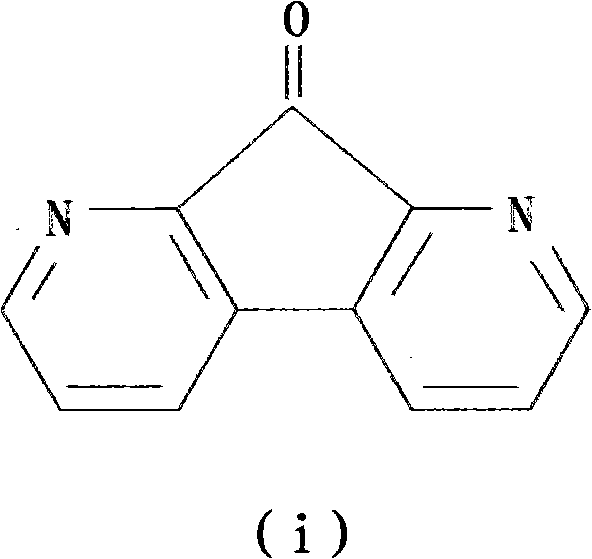
Preparation method of 1,8-dinitro-9-fluorenone
A technology of fluorenone and diketone, which is applied in the direction of organic chemistry and the like, achieves the effects of easy availability of preparation raw materials, simple and efficient preparation process, and cheap preparation of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0082] A kind of preparation method of 1,8-diaza-9-fluorenone, it comprises the following steps:
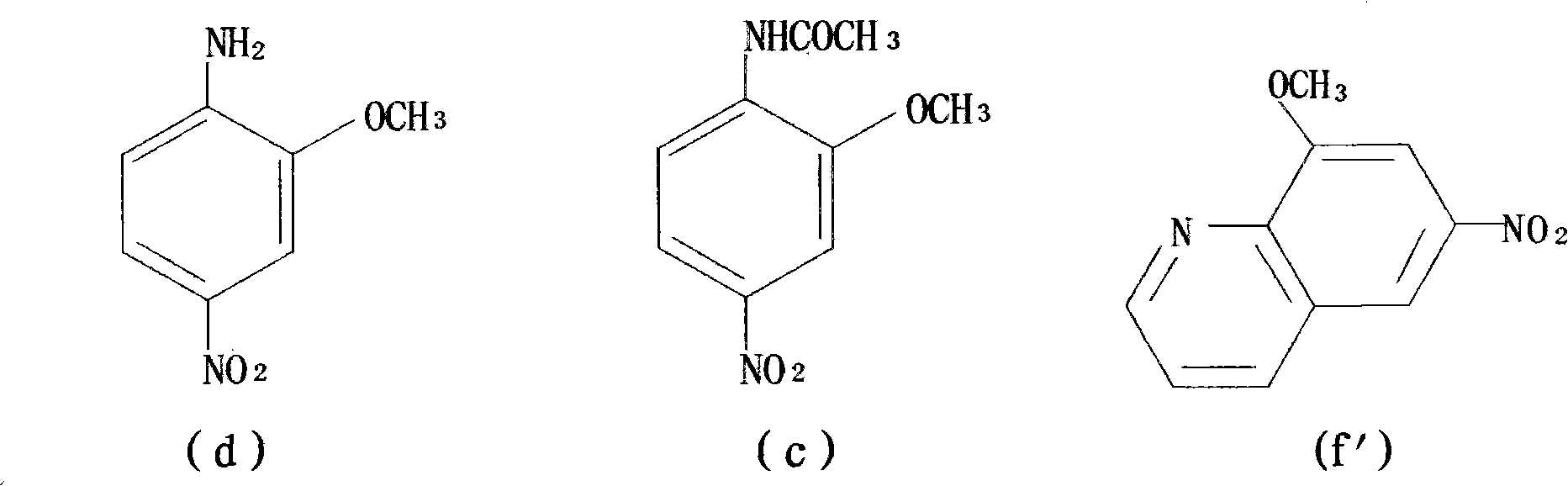
[0083] Step 1: Synthesis of 2-methoxy-4-nitroaniline from 2-methoxyaniline;
[0084] Step 2: the 2-methoxy-4-nitroaniline is reduced to generate 2-methoxy-p-phenylenediamine sulfate;
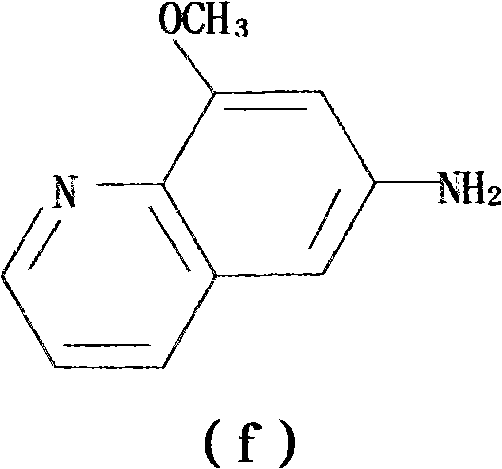
[0085] Step 3: The 2-methoxy-p-phenylenediamine sulfate is synthesized twice for the Skraup quinoline ring to generate 5(6)-methoxy-4,7-phenanthrene;
[0086] Step 4: The 5(6)-methoxy-4,7-phenanthroline is oxidized to generate 4,7-phenanthrene-5,6-dione;
[0087] Step 5: The 4,7-phenanthrene-5,6-dione is condensed in an alkaline aqueous solution to obtain the product DFO, and the total yield of DFO synthesized by five steps is 26.3%.
[0088] Hereinafter, the above steps will be described in detail in conjunction with specific experimental data.
[0089] Step 1: Synthesis of 2-methoxy-4-nitroaniline from 2-methoxyaniline;
[0090] 250ml four-neck flask, equipped with electromagnetic stirrer, re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com