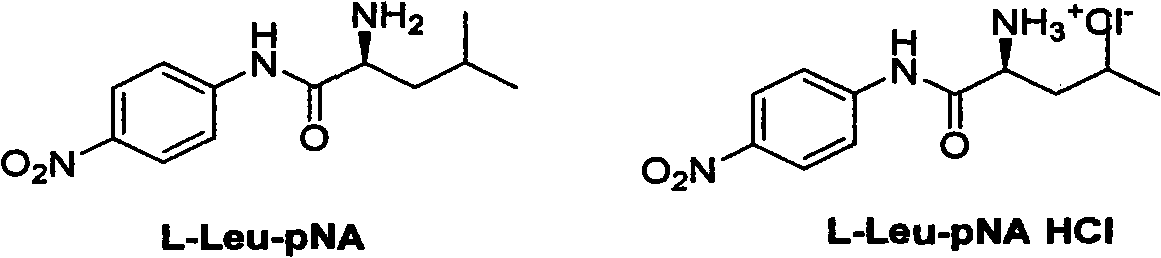
New synthesis method of important biochemical reagent L-leucine-4-nitroaniline hydrochloride
A technology of nitroaniline and biochemical reagents, applied in the field of synthesis of fluorescent peptidase substrates, can solve the problems of low direct condensation yield, non-reaction or low yield, high cost, etc. Easy-to-obtain, simple-to-operate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
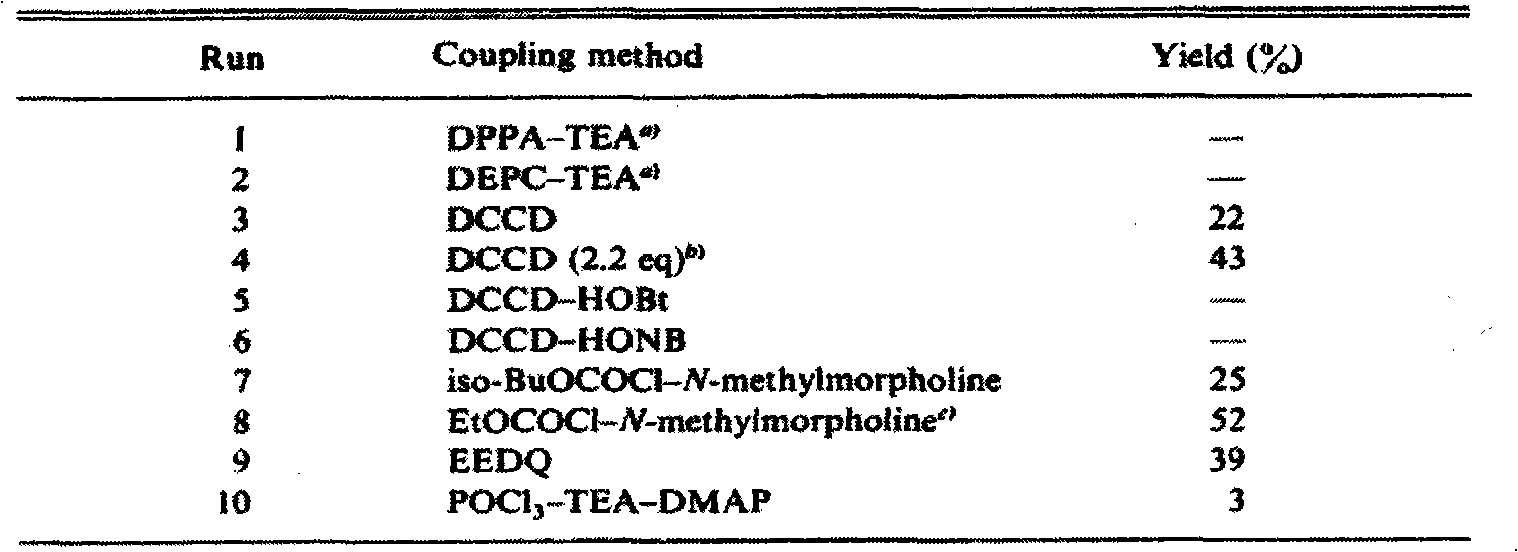
Method used
Image
Examples
Embodiment 1
[0028] The first step reaction: preparation of intermediate 3
[0029] Add compound 2 (12.2g, 93.1mmol, 1eq), dioxane / water (60mL / 60mL) into a 500mL four-necked flask under magnetic stirring, and configure 165mL aqueous solution of sodium hydroxide (7.5g, 210.4mmol, 2eq) , the solution was slowly added to the bottle, the temperature did not change significantly, after the solid was completely dissolved, under an ice-water bath, a mixed solution of BOC anhydride (42.6g, 210.4mmol, 2.26eq) and dioxane (42mL) was added dropwise. After dropping, the reaction was continued at room temperature. After TLC monitors the complete reaction of raw materials, it is adjusted to acidity with dilute hydrochloric acid. The reaction solution was extracted with ethyl acetate (200 mL*3). The organic phase was washed three times with saturated sodium chloride solution (100 mL*3), dried over sodium sulfate, and concentrated under reduced pressure to obtain 21.8 g of intermediate 3, a colorless vi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com