Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
57 results about "3-phenylpropionic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Analyte injection system
InactiveUS20050133370A1Increase in sizeShort amount of timeSludge treatmentVolume/mass flow measurementGlutaric acidAntibody conjugate
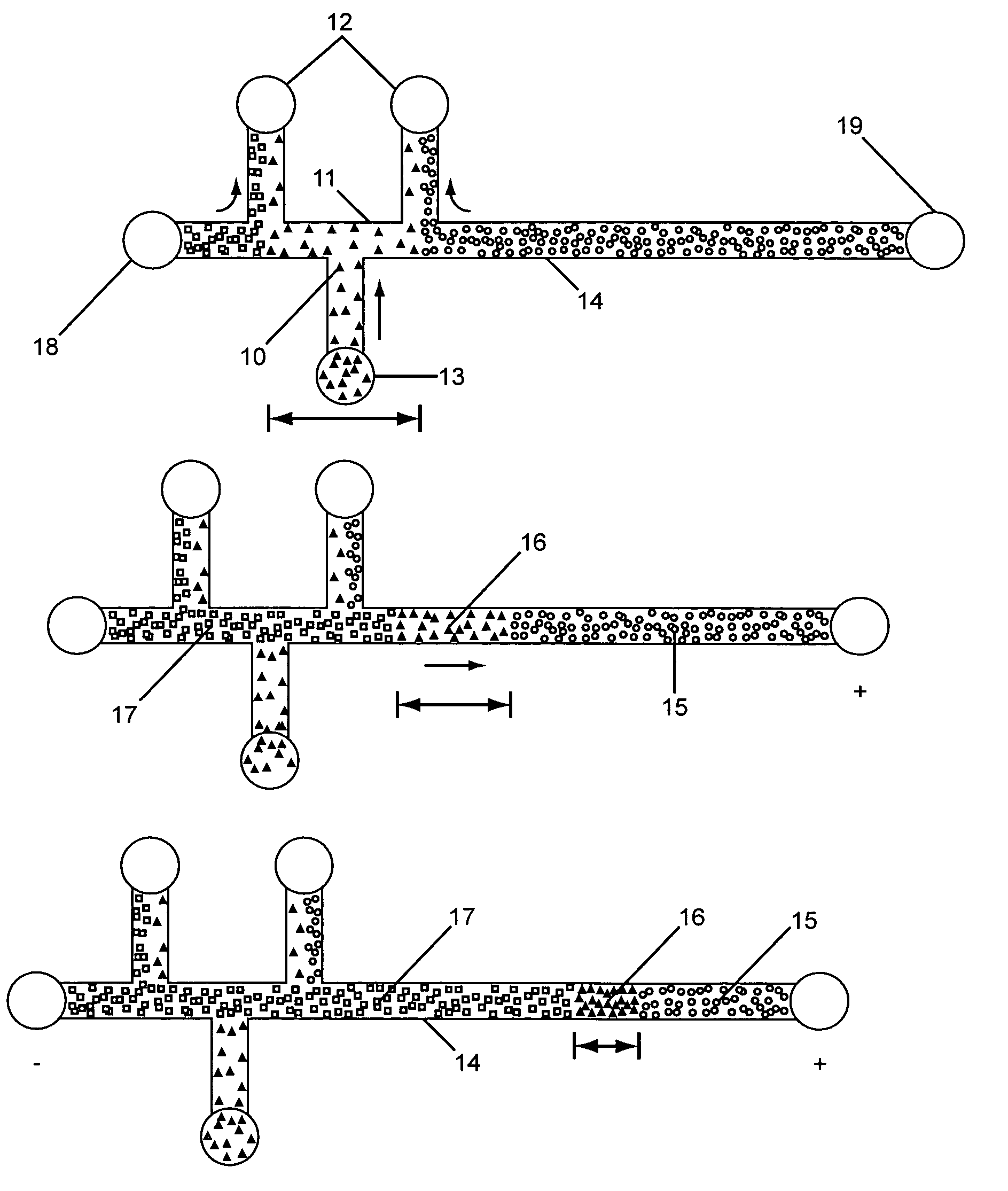
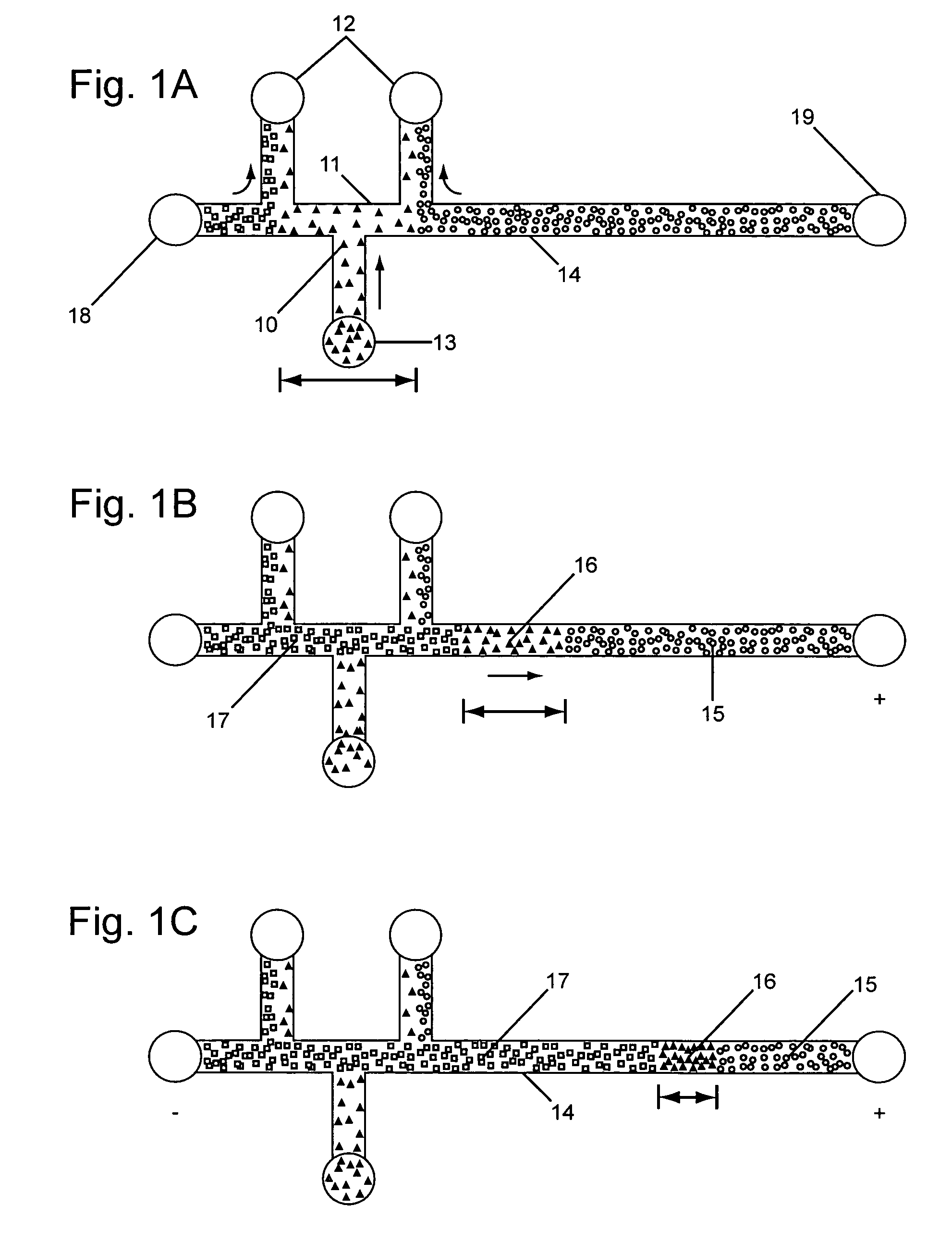
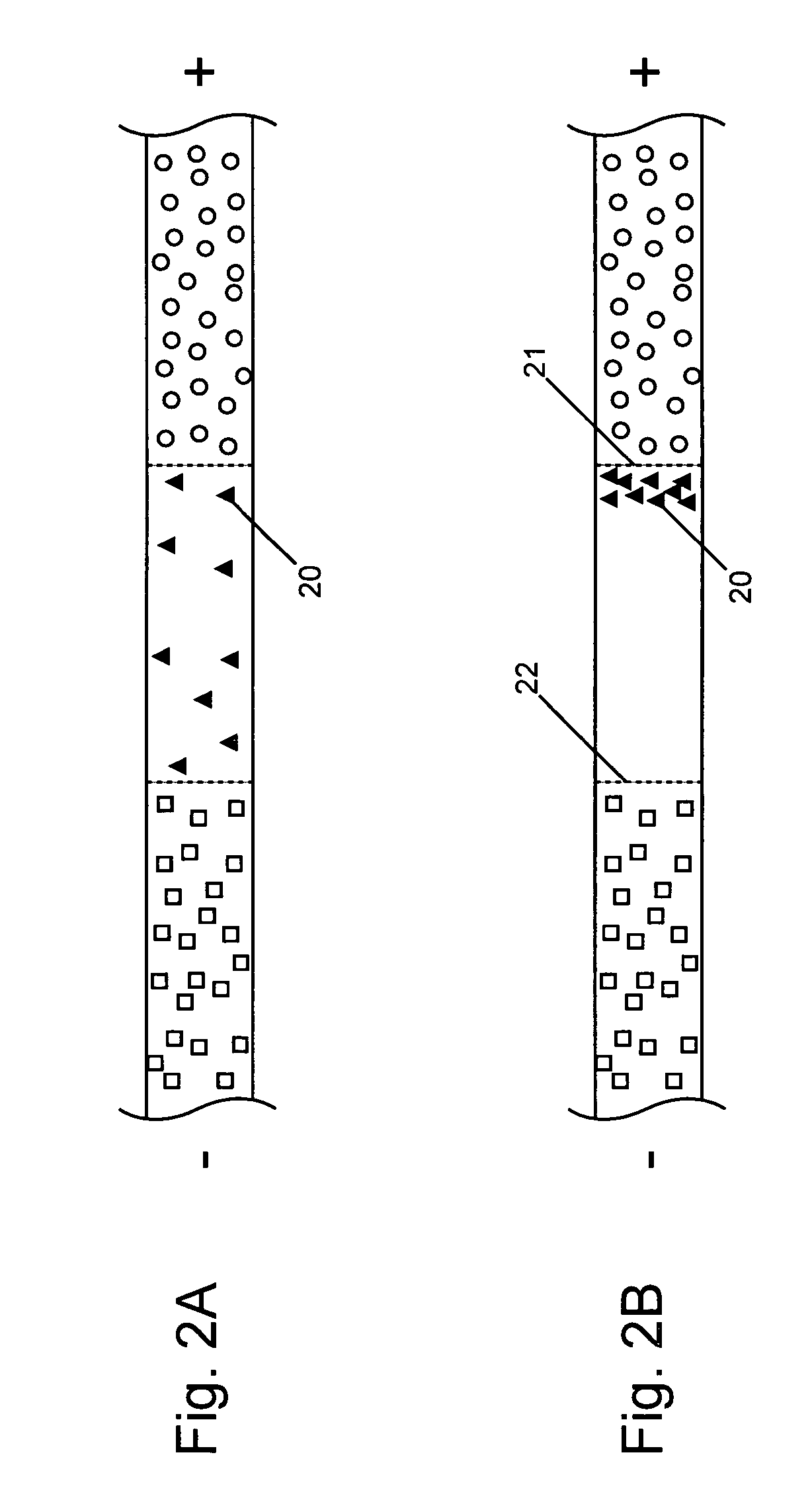
This invention provides methods and devices for spatially separating at least first and second components in a sample which in one exemplary embodiment comprises introducing the first and second components into a first microfluidic channel of a microfluidic device in a carrier fluid comprising a spacer electrolyte solution and stacking the first and second components by isotachophoresis between a leading electrolyte solution and a trailing electrolyte solution, wherein the spacer electrolyte solution comprises ions which have an intermediate mobility in an electric field between the mobility of the ions present in the leading and trailing electrolyte solutions and wherein the spacer electrolyte solution comprises at least one of the following spacer ions MOPS, MES, Nonanoic acid, D-Glucuronic acid, Acetylsalicyclic acid, 4-Ethoxybenzoic acid, Glutaric acid, 3-Phenylpropionic acid, Phenoxyacetic acid, Cysteine, hippuric acid, p-hydroxyphenylacetic acid, isopropylmalonic acid, itaconic acid, citraconic acid, 3,5-dimethylbenzoic acid, 2,3-dimethylbenzoic acid, p-hydroxycinnamic acid, and 5-br-2,4-dihydroxybenzoic acid, and wherein the first component comprises a DNA-antibody conjugate and the second component comprises a complex of the DNA-antibody conjugate and an analyte.
Owner:WAKO PURE CHEMICAL INDUSTRIES +1
Chiral MOF-graphene hybrid material, and preparation method and application thereof
InactiveCN107490610ASimple processEasy to industrializeMaterial electrochemical variablesEnantiomerHybrid material
The invention discloses a chiral MOF-graphene hybrid material, a preparation method thereof, and the application of the chiral MOF-graphene hybrid material to the detection of chiral drug enantiomers, and belongs to the technical fields of nanometer composite materials, macromolecule-based composite materials, graphene-based composite materials and chiral sensing detection. The method comprises the following main steps: blending an alkaline aqueous solution of L-aspartic acid and an aqueous solution of cupric nitrate-graphene oxide, adding an ethanol solution of 4, 4'-bipyridine, leaving the mixture to stand over a night, performing centrifugal separation, washing and drying, so as to obtain the chiral MOF-graphene hybrid material. A chiral MOF-graphene hybrid material sensor constructed through the hybrid material is applied to the sensitive detection of the contents of R-2-amino-3-phenylpropionic acid and S-2-amino-3-phenylpropionic acid enantiomers.
Owner:UNIV OF JINAN
3-phenylpropionic acid derivatives
Owner:ADAMED SP ZOO
2-carbonyl-3-phenylpropionic acid salicyloyl hydrazone di-n-butyltin complex as well as preparation method and application thereof
ActiveCN106220668AHas a thermally stable rangeFriendly and beneficialTin organic compoundsOrganic chemistry methodsHydrazoneStructural formula
The invention discloses a 2-carbonyl-3-phenylpropionic acid salicyloyl hydrazone di-n-butyltin complex of which the structural formula is as shown in the specification; in the formula, Ph is phenyl, and R is n-butyl. The invention further discloses a preparation method and application of the 2-carbonyl-3-phenylpropionic acid salicyloyl hydrazone di-n-butyltin complex in preparation of anti-cancer medicines.
Owner:HENGYANG NORMAL UNIV
2-carbonyl-3-phenylpropionic acid p-methyl benzoyl hydrazone bis-n-butyl tin complex and preparation method and application thereof
ActiveCN106279261AFriendly and beneficialHigh anticancer activityTin organic compoundsOrganic chemistry methodsHydrazoneStructural formula
The invention discloses a 2-carbonyl-3-phenylpropionic acid p-methyl benzoyl hydrazone bis-n-butyl tin complex. The complex has the structural formula (I) shown in the specification, wherein Ph is phenyl, and R is n-butyl. The invention further discloses a preparation method of the 2-carbonyl-3-phenylpropionic acid p-methyl benzoyl hydrazone bis-n-butyl tin complex and application in preparation of anti-cancer drugs.
Owner:HENGYANG NORMAL UNIV
3-Phenylpropionic acid derivatives
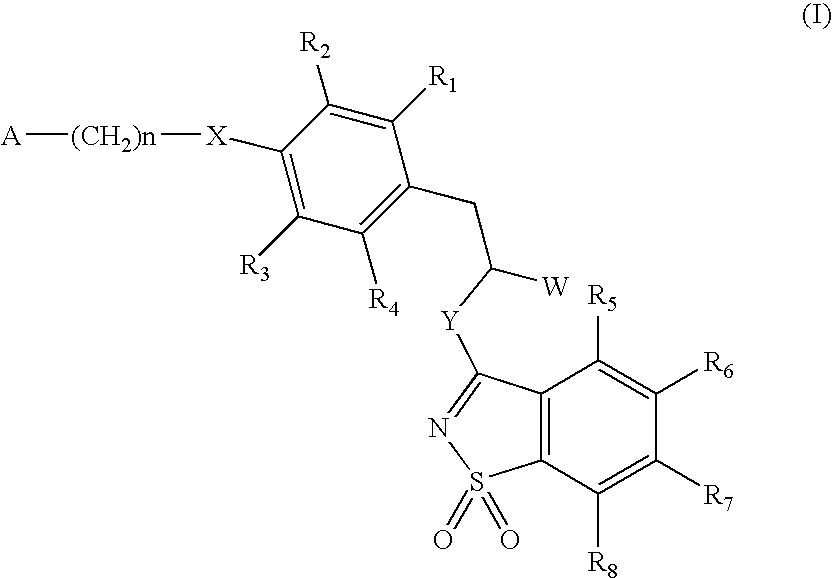
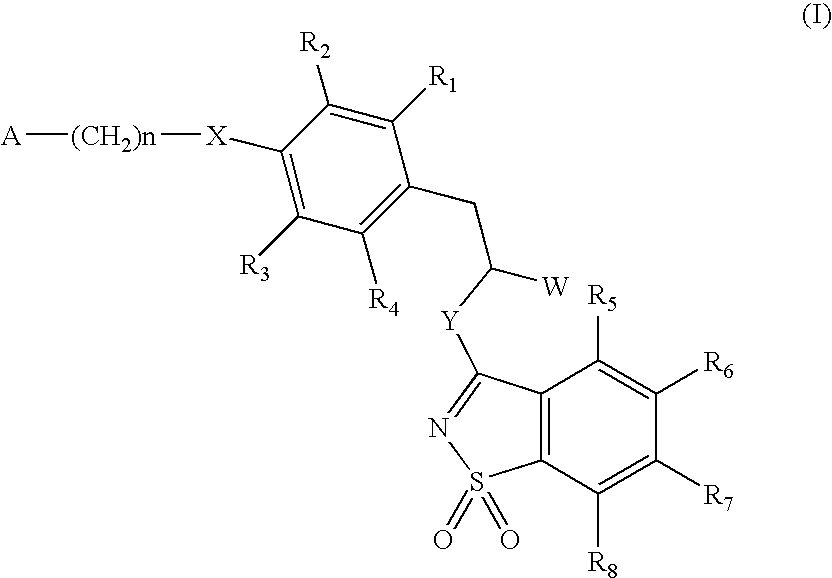
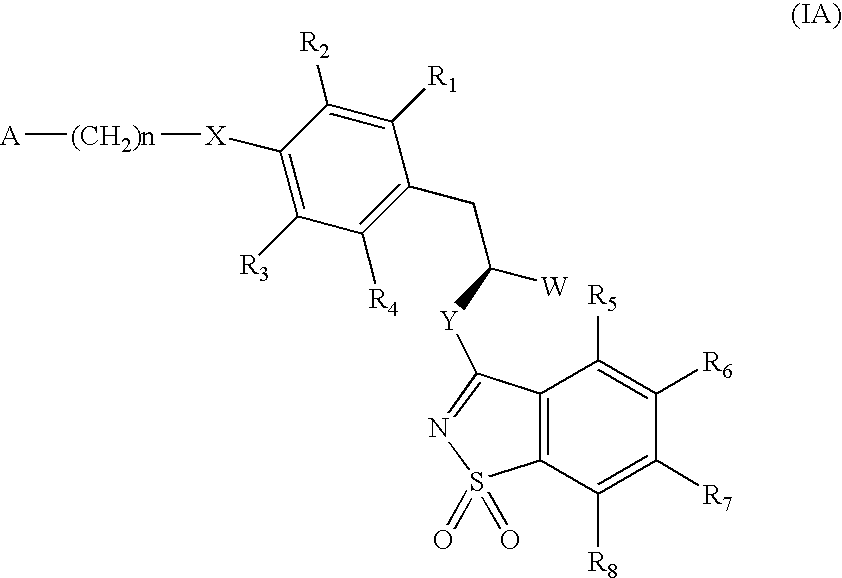
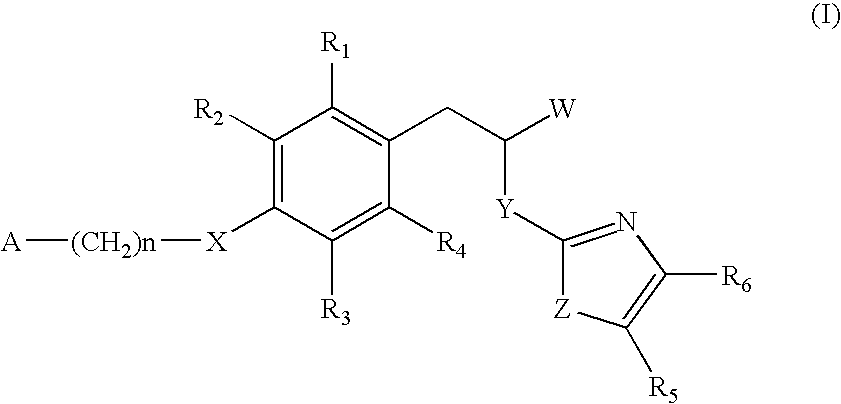
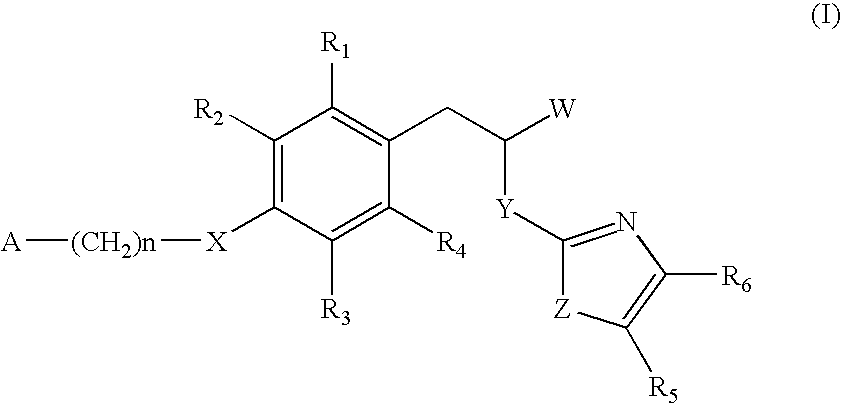
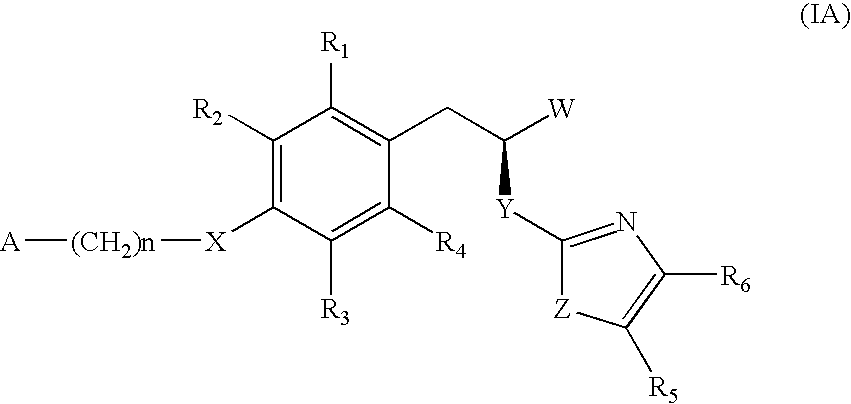
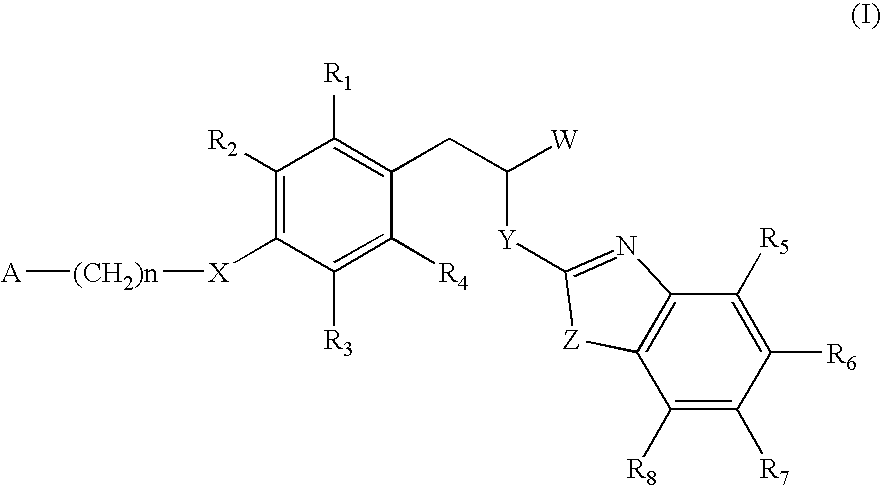
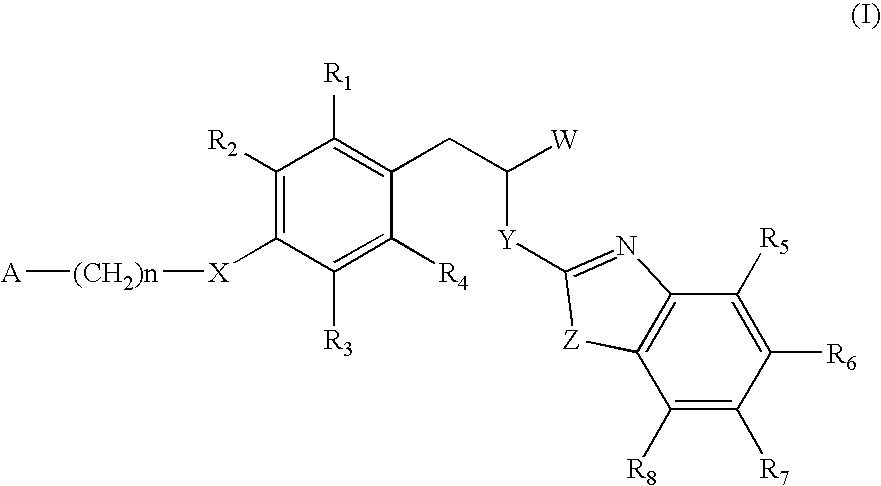
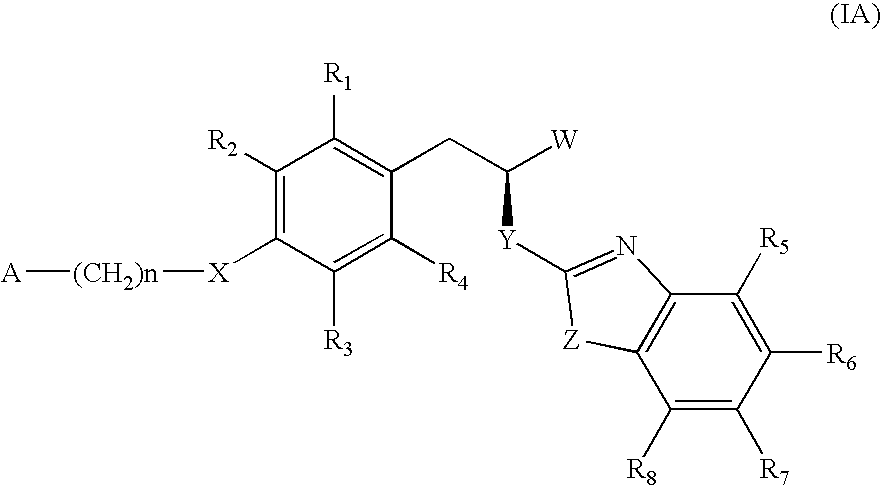
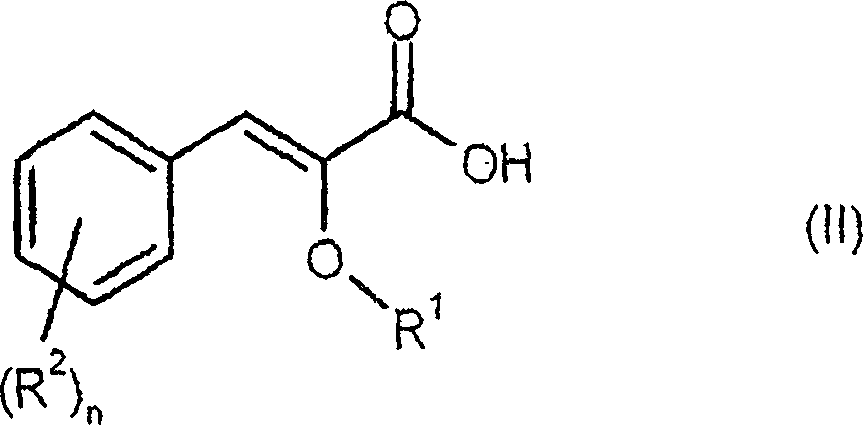
The invention relates to new compounds, being 3-phenylpropionic acid derivatives of formula I wherein W represents COOH group or its bioisosters, or —COO—C1-C4-alkyl group; Y represents NH, N—C1-C10-alkyl, O, or S; Z represents NH, N—C1-C10-alkyl, N-aryl, N-heteroaryl, S, or O; X represents O, S, NH, N—C1-C10-alkyl, N-aryl, NSO2—C1-C10-alkyl, N—SO2-aryl, or N—SO2-heteroaryl; R1, to R6 each independently represent hydrogen atom or a substituent defined in the description; A is as defined in the description; n represents an integer from 0 to 4, inclusive; and pharmaceutically acceptable salts thereof. The compounds are the ligands of PPAR-gamma receptor and are useful as medicaments.
Owner:ADAMED SP ZOO
2-carbonyl-3-phenylpropionic acid p-nitrobenzoyl hydrazone di-p-methyl benzyl tin complex as well as preparation method and application thereof
ActiveCN106220670AFriendly and beneficialHigh anticancer activityTin organic compoundsOrganic chemistry methodsHydrazoneStructural formula
The invention discloses a 2-carbonyl-3-phenylpropionic acid p-nitrobenzoyl hydrazone di-p-methyl benzyl tin complex of which the structural formula is shown in the description; in the formula, Ph is phenyl, and R is methyl benzyl. The invention further discloses a preparation method and application of the 2-carbonyl-3-phenylpropionic acid p-nitrobenzoyl hydrazone di-p-methyl benzyl tin complex in preparation of anti-cancer medicines.
Owner:HENGYANG NORMAL UNIV
2-carbonyl-3-phenylpropionic acid benzoyl hydrazone di-n-butyltin complex and preparation method and application thereof
ActiveCN106279252AHas a thermally stable rangeFriendly and beneficialTin organic compoundsOrganic chemistry methodsHydrazoneStructural formula
The invention discloses a 2-carbonyl-3-phenylpropionic acid benzoyl hydrazone di-n-butyltin complex. The 2-carbonyl-3-phenylpropionic acid benzoyl hydrazone di-n-butyltin complex is a complex with the structural formula (I) (please see the formula (I) in the description), wherein Ph is phenyl, and R is n-butyl. The invention further discloses a preparation method of the 2-carbonyl-3-phenylpropionic acid benzoyl hydrazone di-n-butyltin complex and application of the 2-carbonyl-3-phenylpropionic acid benzoyl hydrazone di-n-butyltin complex to anti-cancer drug preparation.
Owner:HENGYANG NORMAL UNIV
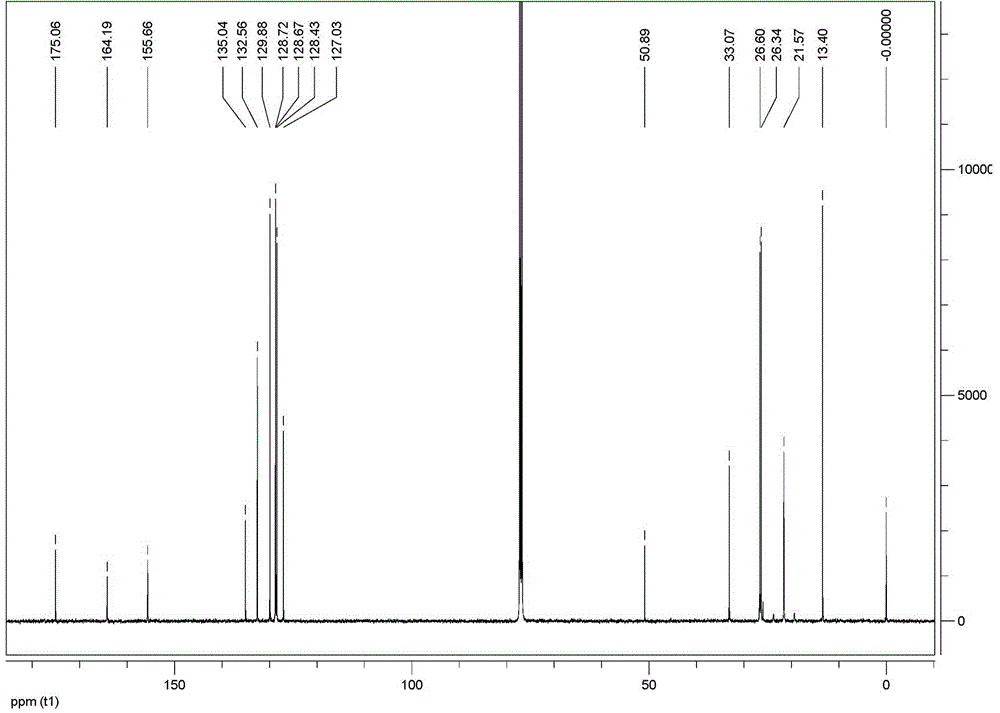
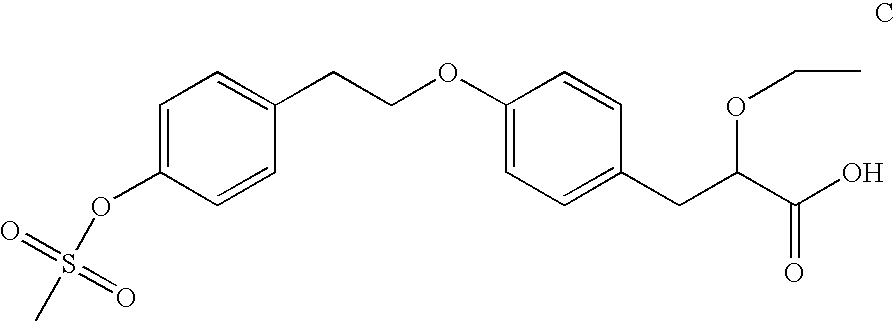
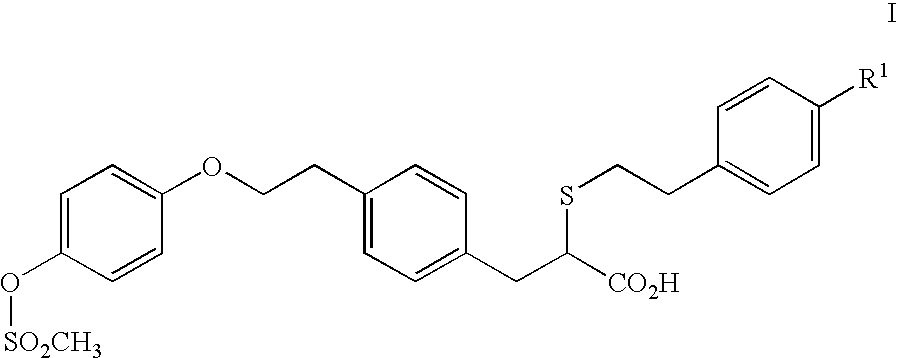
2-ethoxy-3-phenylpropionic acid derivatives for the treatment of lipid disorders
A compound of formula (I) wherein T represents O, S or NR and wherein R represents a H, a C1-6alkyl group or a phenyl C1-6alkyl group and pharmaceutically acceptable salts thereof, processes for preparing such compounds, their the utility in treating clinical conditions including lipid disorders (dyslipidemias) whether or not associated with insulin resistance, methods for their therapeutic use and and pharmaceutical compositions containing them
Owner:ASTRAZENECA AB
New 3-phenylpropionic acid derivatives
The invention relates to new compounds, being 3-phenylpropionic acid derivatives of formula I wherein W represents COOH group or its bioisosters, or —COO—C1-C4-alkyl group; Y represents NH, N—C1-C10-alkyl, O, or S; Z represents NH, N—C1-C10-alkyl, N-aryl, N-heteroaryl, S, or O; X represents O, S, NH, N—C1-C10-alkyl, N-aryl, NSO2—C1-C10-alkyl, N—SO2-aryl, or N—SO2-heteroaryl; R1 to R8 each independently represent hydrogen atom or a substituent defined in the description; A is as defined in the description; n represents an integer from 0 to 4, inclusive; and pharmaceutically acceptable salts thereof. The compounds are the ligands of PPAR-gamma receptor and are useful as medicaments.
Owner:ADAMED SP ZOO
Process for preparing enantiomerically enriched 2-alkoxy-3-phenylpropionic acids
InactiveCN1986516AOrganic compound preparationCarboxylic preparation by ozone oxidationAsymmetric hydrogenation3-phenylpropionic acid
Owner:SALTIGO GMBH
Preparation method and application of acid or active ester of polyethylene glycol with tail end connected with aminophenyl propionic acid
InactiveCN102964588APeptide preparation methodsEnzyme stabilisationPropanoic acidPolyethylene glycol
The invention discloses a preparation method and application of acid or active ester of polyethylene glycol with the tail end connected with aminophenyl propionic acid, belonging to the field of medicine. In water, the polymer active ester has proper activity of reacting with the amino of bioactive substances. The structural formula is R1-(CH2CH2O)n-CH2-CO-NH-R2-CO-OR3, wherein n is equal to 43-680; the R1 can be CH3O- or -O-CH2-CO-NH-R2-CO-OR3; the R2 is the residue of the aminophenyl propionic acid except the amino and the carboxyl; aminophenyl propionic acid can be 2-amino-3-phenylpropionic acid (namely, alpha-phenylalanine) or 3-amino-3-phenylpropionic acid (namely, beta-phenylalanine); and the R3 can be H- or -N-succinimide.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
Compound and preparation method thereof and midbody compound prepared with same and preparation method thereof
InactiveCN101857557AMild reaction conditionsGood choiceCarbamic acid derivatives preparationOrganic compound preparationTert-Butyloxycarbonyl protecting groupSide chain
The invention discloses a novel phenylisoserine derivative: (2R, 3S)-3-(tert-butoxycarbonylamino)-2(2-ethoxypropene-2-oxo)-3-phenylpropionic acid as a side chain for the synthesis of docetaxel, and a preparation method for the compound. The preparation method includes the following steps that: (2R, 3S)2-hydroxy-3-amino phenylalanine methylester is prepared with (3R, 4S)3-hydroxy-4-phenylazetidine-2-ketone ring-opening product, the amino group is protected by the tert-butoxycarbonyl group, the hydroxyl group is protected by the 2-ethoxypropene group, the methyl ester is unshackled, and thereby the novel phenylisoserine derivative is obtained. The invention also discloses a midbody compound which is prepared with the compound and used for synthesizing docetaxel, and a preparation method for the midbody compound. The novel synthesis route for synthesizing docetaxel has the advantages of mild reaction conditions and high condensation efficiency, can be used for industrialized production, and can be widely popularized.
Owner:CHONGQING TAIHAO PHARM CO LTD +2
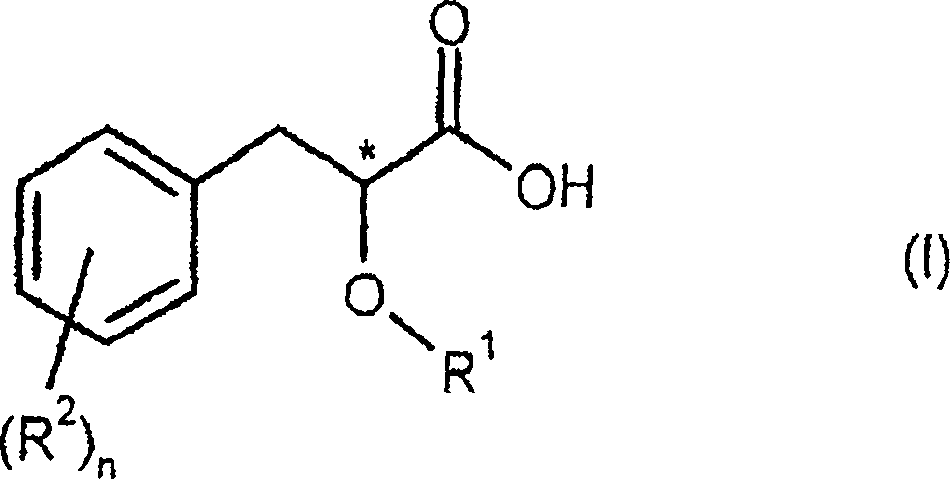
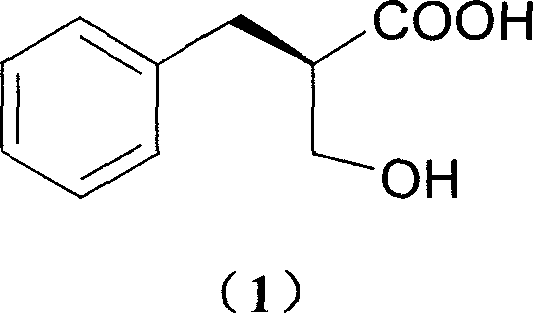
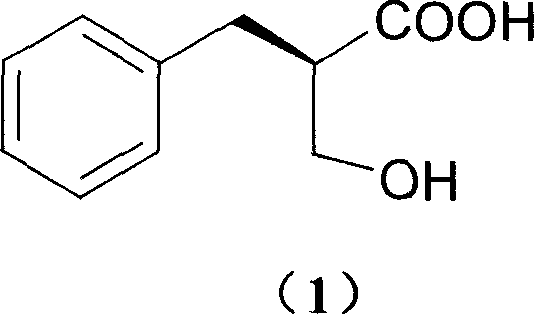
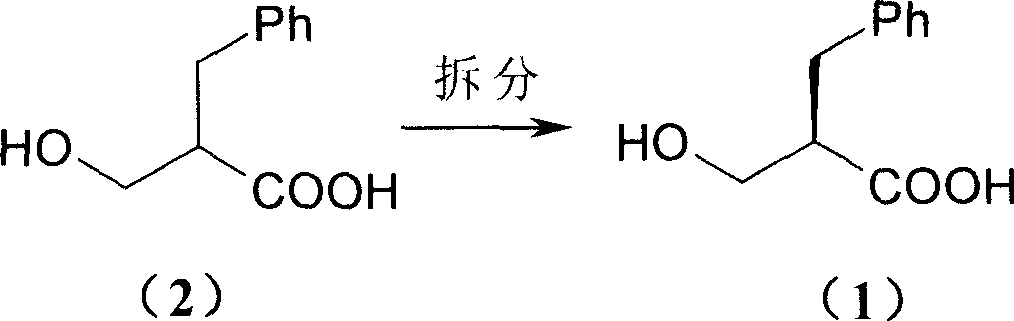
Process of resolving optical isomer of 2-hydroxmethyl-3-phenylpropionic acid
ActiveCN1931821ALow priceEasy to recycleCarboxylic compound separation/purificationPropanoic acidDiastereomer
The present invention provides one kind of process of resolving optical isomer of 2-hydroxymethyl-3-phenyl propionic acid. By using glucooctylamine as resolving agent, racemized 2-hydroxymethyl-3-phenyl propionic acid forms two diastereomers in certain solvent. Through separation, re-crystallization and acid or alkali liberation, one diastereomer with relatively low dissolubility is obtained. The resolving solvent is methanol, ethanol, isopropanol, acetone, etc or their mixed solvent with water. The process has high resolving efficiency, facile resolving agent and easy recovery of resolving agent, and is suitable for industrial production.
Owner:SHANGHAI SHYNDEC PHARMA CO LTD +1
Preparing method and application of corrosion inhibitor restraining carbon steel acid corrosion
InactiveCN107385449AEasy to produceEasy to synthesizeOrganic chemistryDiethylenetriamineAcid corrosion
The invention provides corrosion inhibitor for carbon steel in the oil field acid environment, and particularly relates to preparation and application of low-toxicity and efficient corrosion inhibitor for restraining corrosion of carbon steel and products of the carbon steel in oil field acid pickling liquid or a saturated CO2 medium. According to the preparing method of the corrosion inhibitor, 3-phenylpropionic acid and diethylenetriamine are subjected to the dehydration reaction, and the phenylpropionic acid imidazoline ramification is obtained. According to the product, comprehensive corrosion and local corrosion is prevented from being generated in the process that the carbon steel makes contact with the acid pickling liquid or the CO2 medium. The compound does not serve as metal corrosion inhibitor at current. By adoption of the corrosion inhibitor, production is simple and convenient, toxicity is small, the inhibition rate is high, corrosion damage to the carbon steel from the acid pickling liquid or CO2 can be effectively restrained, and wide application prospects are achieved. The steps are simple, operation is convenient, and practicability is high.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
Method for removing ethanol, isopropanol and octyl alcohol from amino resin workshop wastewater
InactiveCN105084495AStrong complexing abilityFast precipitationWater contaminantsNature of treatment waterOctanolAcetophenone
The invention relates to a method for removing ethanol, isopropanol and octyl alcohol from amino resin workshop wastewater. The components adopted by the method comprises 1,3,3-trimethyl-2-oxabicyclo[2.2.2]octane, 1,8-dihydroxy-3-methoxy-6-methylanthraquinone, S(-)-2-amino-6-n-propyle-4,5,6,7-tetrahydrobenzothiazole dihydrochloride, 4-octylphenol ethoxylate, 3',4'-dihydoxy-2-(methylamino)acetophenone hydrochloride, 4-acetoxy-3-methoxy-(2-propenyl) benzene, 4-methoxybenzyl acetate, 6,6,10-trimethyl bicyclo-3,1,1-hept-2-ene, and 2-[[1-(3-acetylthio-2-methylpropionyl)pyrrolidine-2-formyl]amino]-3-phenylpropionic acid. The components adopted by the method have strong complexing capability with target substances, high speed of forming complex precipitates, and high removal rate up to 99.9%.
Owner:李海兰
Method for synthesizing 1, 8-diazabicycle
The invention provides a synthesis method of 1, 8-diazabicycle. According to the technical scheme, a synthesis route of DBU is innovatively designed. Specifically, the method comprises the steps of firstly, reacting epsilon caprolactam, acrylonitrile and hydroquinone to generate N-(beta-cyanoethyl)-epsilon caprolactam, on the basis, reacting the N-(beta-cyanoethyl)-epsilon caprolactam with p-toluenesulfonic acid under a catalytic condition, then carrying out ice bath, reacting the N-(beta-cyanoethyl)-epsilon caprolactam with benzyl chloride under the protection of nitrogen, reacting the obtained product with sodium perborate by taking EDTA as a catalyst, and mixing a reaction product with 3-phenylpropionic acid and then reacting the mixture with 4-nitrochlorobenzyl to obtain the product; filtering the reaction product, extracting the filtrate with ethyl acetate after filtering, performing solid-liquid separation, merging solid phases, and drying the solid phases under reduced pressureto obtain the final product. According to the method, a brand-new synthetic route is adopted, the reaction condition is mild, the cost of raw materials is low, and the method has quite high popularization prospect.
Owner:淄博鸿润新材料有限公司
Aromatic composition reproducing fragrance of ficus carica l.
Provided is an aromatic composition containing, as active ingredients, a fragrance substance of Ficus carica L. analyzed by the solid-phase microextraction (SPME) method, and a 3-phenylpropionic acid which is warm, balsamic, and has a sweet fragrance, thereby reproducing the unique fragrance of Ficus carica L. and having excellent palatability.
Owner:AMOREPACIFIC CORP
(R)-beta-phenylacetylamino-beta-phenylpropionic acid compounds
The present invention, (R)-beta-phenylacetylamino-beta-phenylpropionic acid compound, is one compound for treating diabetes with obvious blood sugar lowering effect and less side effect. It is produced through condensation between phenylacetyl compound and 3-amino-beta-3-phenylpropionic acid compound.
Owner:SHENYANG PHARMA UNIVERSITY
Viscosity reducer for low-temperature eutectic thickened oil, preparation method and application thereof
InactiveCN109111367ALow viscosityImprove liquidityOrganic compound preparationPipeline systemsPotential marketViscosity
The invention relates to the field of viscosity reduction of thickened oil and specifically relates to a viscosity reducer for low-temperature eutectic thickened oils, a preparation method and application thereof. The viscosity reducer for low-temperature eutectic thickened oil in the invention is prepared according to the step of stirring and heating choline chloride and 3-phenylpropionic acid for 1-3h in oil bath at 80-100 DEG C till the reaction mixture is transparent liquid. Compared with common surfactant viscosity reducers, polymer viscosity reducers and oil-soluble viscosity reducers inother markets, the viscosity reducer for low-temperature eutectic thickened oils has the advantages of low raw material cost, simple synthesis operation, environmental protection, no toxicity, obvious effect in viscosity reduction of thickened oil and excellent viscosity reduction property under high temperature. The viscosity reducer for low-temperature eutectic thickened oil has a potential market value.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
Carbonyl reductase with self-assembled interface, and application of same in synthesis of (R)-3-hydroxy-3-phenylpropionic acid ethyl ester
ActiveCN108998430AHigh catalytic efficiencyRealize subsequent separation and extractionMicroorganism based processesOxidoreductasesPolystyreneCatalytic function
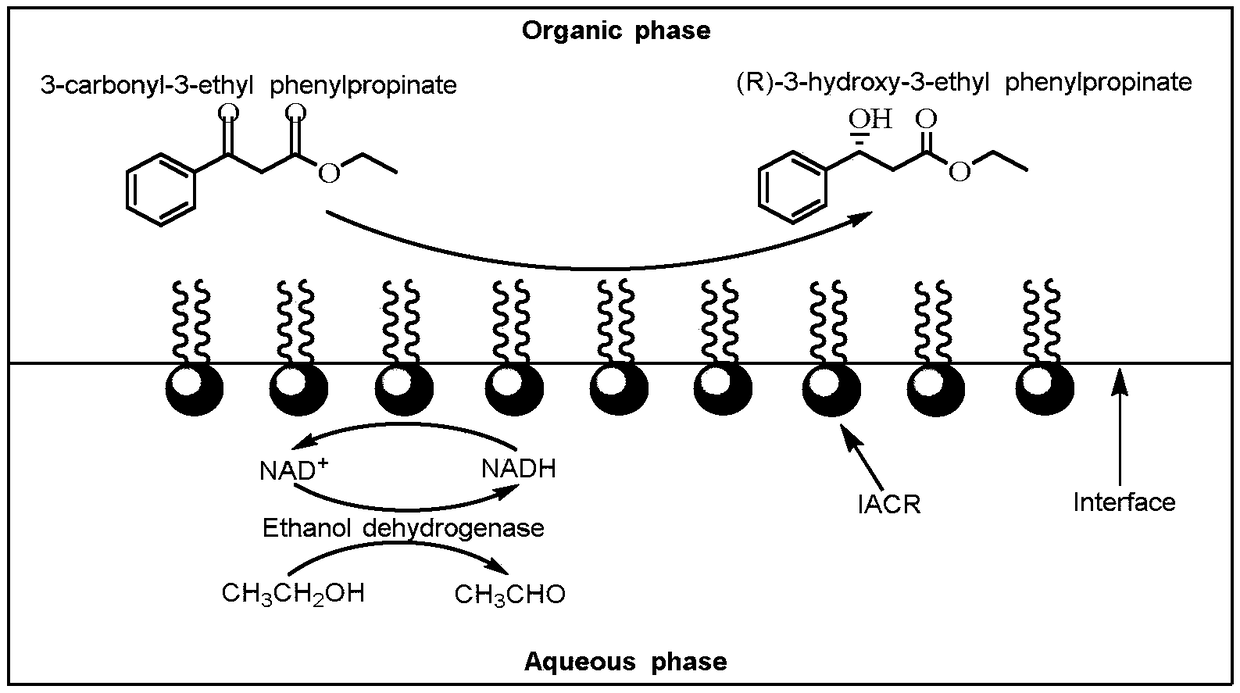
The invention discloses a carbonyl reductase with a self-assembled interface, and application of the same in synthesis of (R)-3-hydroxy-3-phenylpropionic acid ethyl ester. The carbonyl reductase withthe self-assembled interface is prepared by using the following steps: adding carbonyl reductase to a Tirs-HCl buffer, adding a toluene solution of polystyrene, shaking in a shaking bed for 1-4 hoursin the dark at 40-100 r / min and 20-40DEG C, and centrifuging to obtain a reaction system in which an organic phase is at the upper layer, the carbonyl reductase with the self-assembled interface is atthe middle layer, and an aqueous phase is at the lower layer; and washing the middle layer with toluene and Tirs-HCl buffer to obtain the carbonyl reductase with the self-assembled interface. The carbonyl reductase with the self-assembled interface, constructed in the invention, has the relatively good catalytic function at the interface between the organic phase and the aqueous phase, and can beused for converting 3-hydroxy-3-phenylpropionic acid ethyl ester to (R)-3-hydroxy-3-phenylpropionic acid ethyl ester, wherein the enantiomer excess value of (R)-3-hydroxy-3-phenylpropionicacidethylester is greater than 99%.
Owner:ZHEJIANG UNIV OF TECH
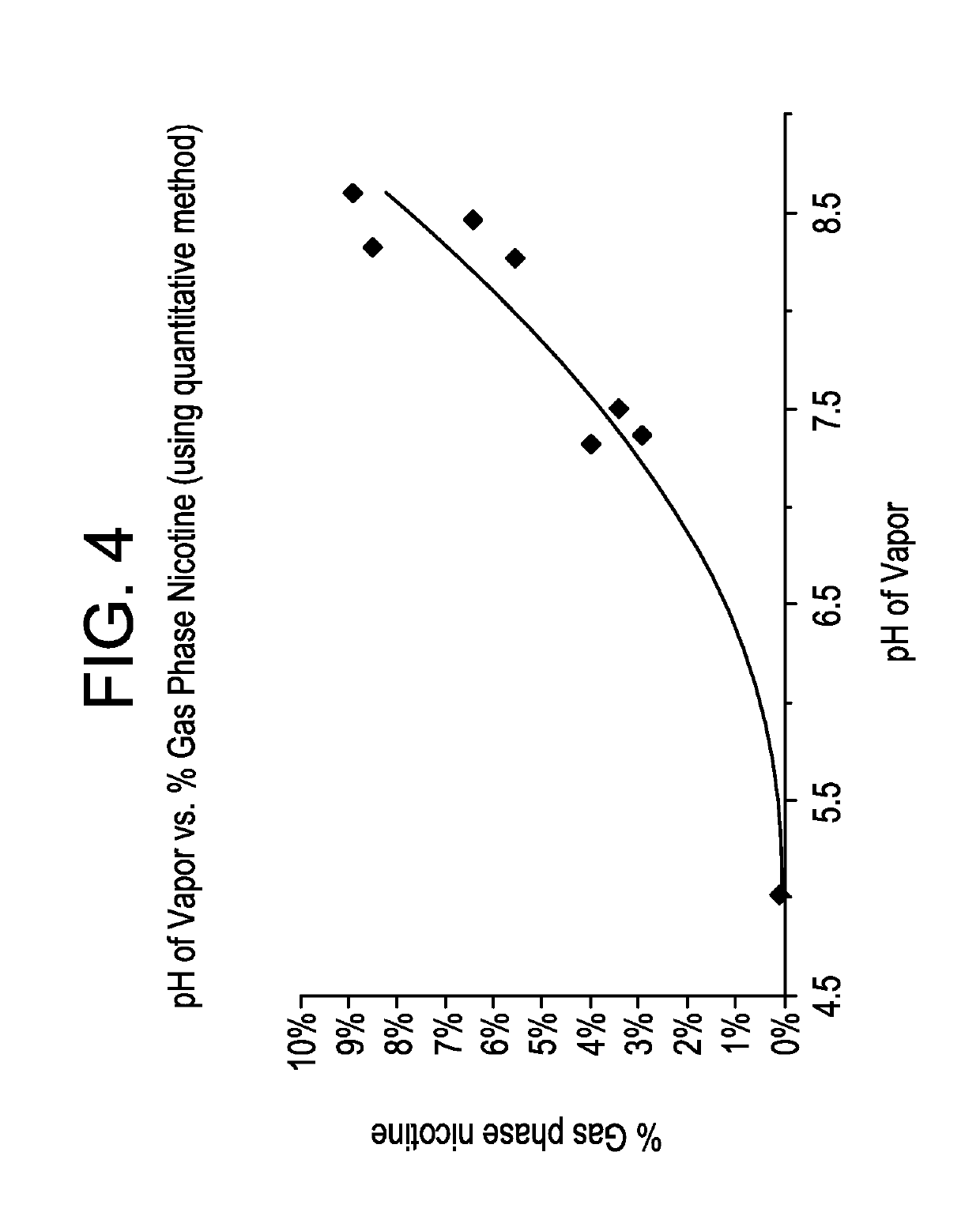
Pre-vaporization formulation for controlling acidity in an e-vaping device
ActiveUS10327472B2Increase acidityGreat proportionTobacco treatmentPharmaceutical delivery mechanismBenzoic acid4-pentenoic acid
Owner:AKRIA CLIENT SERVICES LLC
Optically active salts of 2-hydroxymethyl-3-phenylpropionic acid with cis-1-amino-2-indanol, alpha -methylbenzylamine, or 3-methyl-2-phenyl-1-butylamine
InactiveUS6028217AReadily availableEfficient use ofOrganic compound preparationOptically-active compound separationHydroxymethyl3-phenylpropionic acid
A compound selected from the group consisting of (a) optically active 2-hydroxymethyl-3-phenylpropionic acid cis-1-amino-2-indanol salt, (b) optically active 2-hydroxymethyl-3-phenylpropionic acid alpha -methylbenzylamine salt, (c) optically active 2-hydroxymethyl-3-phenylpropionic acid 3-methyl-2-phenyl-1-butylamine salt, (d) a salt of (S)-2-hydroxymethyl-3-phenylpropionic acid with (1R, 2S)-(+)-cis-1-amino-2-indanol, (e) a salt of (R)-2-hydroxymethyl-3-phenylpropionic acid with (1S, 2R)-(-)-cis-1-amino-2-indanol, (f) a salt of (R)-2-hydroxymethyl-3-phenylpropionic acid with (S)-(-)- alpha -methylbenzylamine, (g) a salt of (S)-2-hydroxymethyl-3-phenylpropionic acid with (R)-(+)- alpha -methylbenzylamine, (h) a salt of (S)-2-hydroxymethyl-3-phenylpropionic acid with (S)-(-)-3-methyl-2-phenyl-1-butylamine, and (i) a salt of (R)-2-hydroxymethyl-3-phenylpropionic acid with (R)-(+)-3-methyl-2-phenyl-1-butylamine.
Owner:AJINOMOTO CO INC
Treating agent for removing ethyl alcohol, isopropyl alcohol and octyl alcohol in waste water of amino resin workshop
InactiveCN105016450AStrong complexing abilityFast precipitationWater contaminantsNature of treatment waterOctanolAcetophenone
The invention relates to a treating agent for removing ethyl alcohol, isopropyl alcohol and octyl alcohol in waste water of an amino resin workshop. The treating agent is formed by compounding 1,3,3-trimethyl-2-oxabicyclo[2.2.2]octane, 1,8-dihydroxy-3-methoxy-6-methylanthraquinone, S(-)-2-amino-6-n-propyl-4,5,6,7-tetrahydrobenzothiazole hydrochloride, 4-octylphenol ethoxylate, 3',4'-dihydroxy-2-(methyl amino)acetophenone hydrochloride, 4-acetoxy-3-methoxy-(2-allyl) benzene, acetic acid-4-methoxy-benzyl ester, 6,6,10-trimethylbicyclo-3,1,1-hep-2-ene, 2-[[1-(3-acethythio-2-methypropionyl)pyrrolidine-2-formyl]amino]-3-phenylpropionic acid and the like. The capacity of the treating agent for complexing with a target substance is high, the complex precipitate forming speed is high, and the removing rate can reach 99.9%.
Owner:李海兰
Therapeutic agents
Substituted 3-phenylpropionic acid derivatives, processes for preparing such compounds, their utility in treating clinical conditions including lipid disorders (dyslipidemias) whether or not associated with insulin resistance, methods for their therapeutic use and pharmaceutical compositions containing them.
Owner:ASTRAZENECA AB
Process for preparing optically active 2-hydroxy-methyl-3-phenylpropionic acid
InactiveUS6093846AReadily availableEfficient use ofOrganic compound preparationOptically-active compound separationPhenyl groupMethyl group
A process for preparing optically active 2-hydroxymethyl-3-phenylpropionic acid by resolving (RS)-2-hydroxymethyl-3-phenylpropionic acid via crystallization of an optically active amine salt, where the amine is optically active cis-1-amino-2-indanol salt, optically active alpha -methylbenzylamine salt, or optically active 3-methyl-2-phenyl-1-butylamine salt.
Owner:AJINOMOTO CO INC
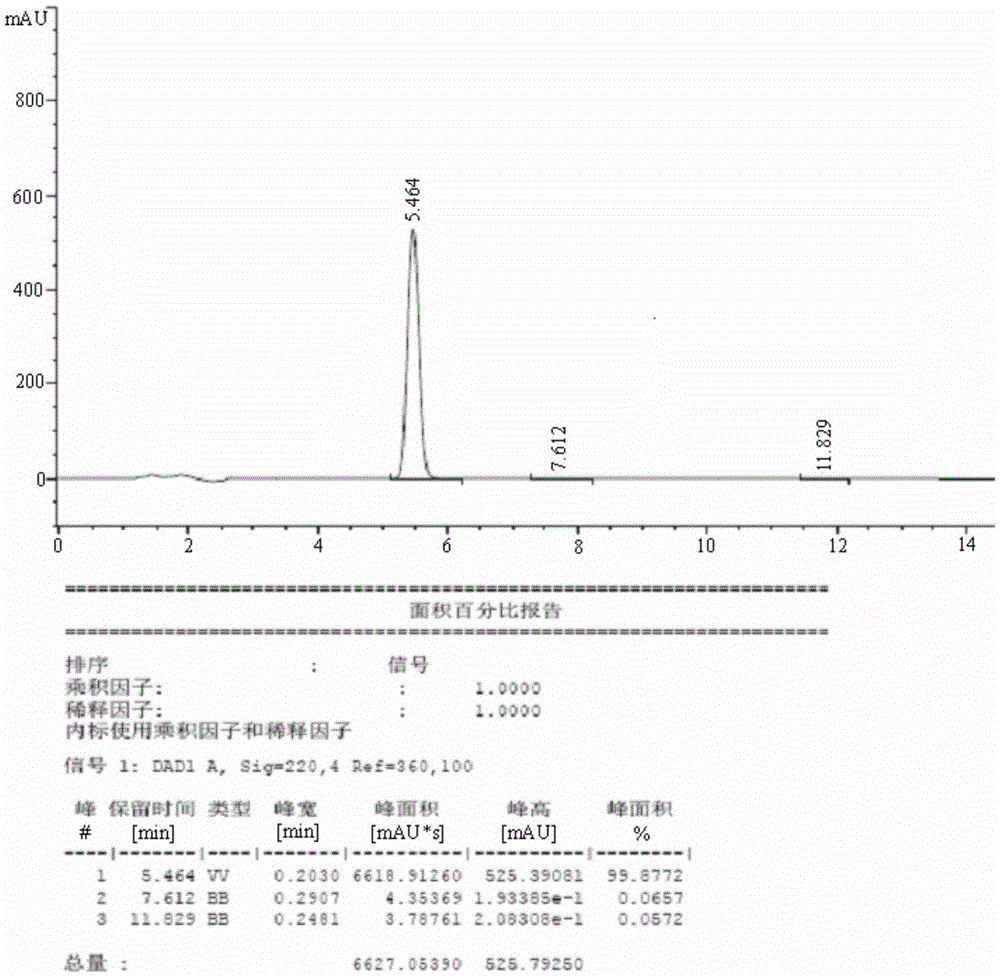
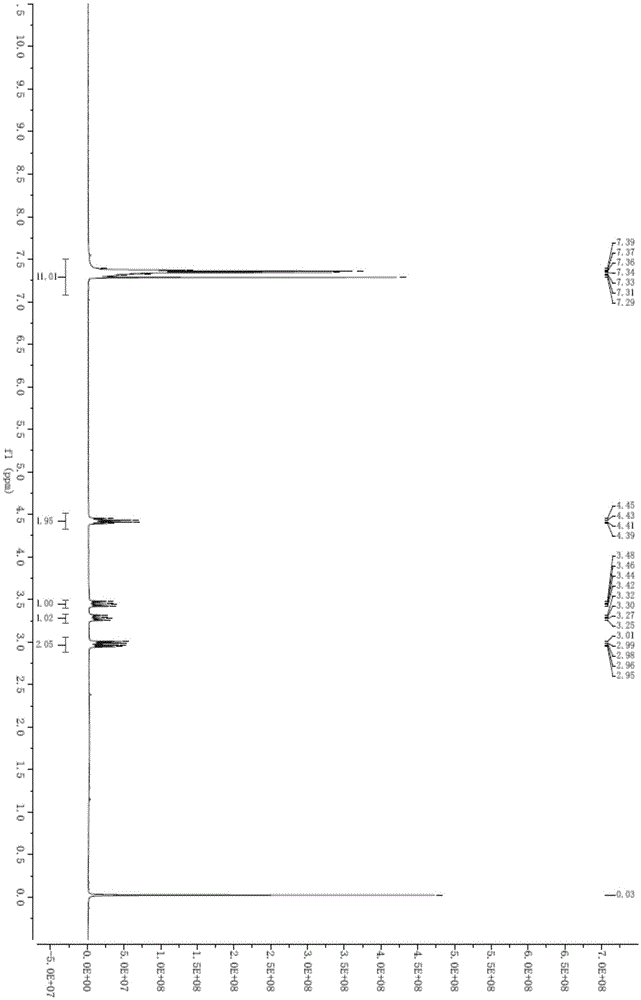
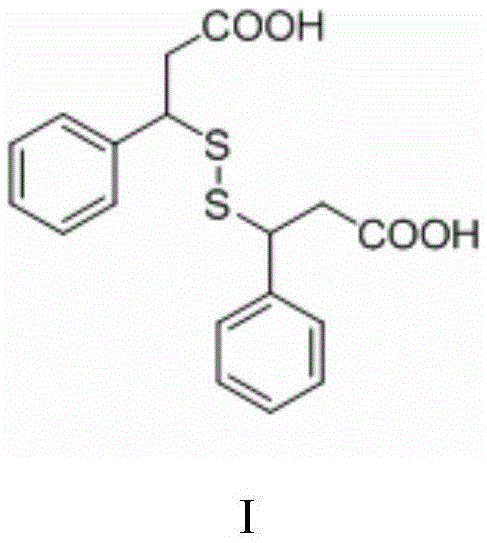
Preparing method of beta,beta'-dithiobis(dihydrocinnamicacid)
ActiveCN105669505ALess side effectsEasy to operateHydropoly/poly sulfide preparationOrganic synthesisSolvent
The invention relates to the field of organic synthesis, in particular to a preparing method of high-purity beta,beta'-dithiobis(dihydrocinnamicacid). The invention provides a preparing method of the beta,beta'-dithiobis(dihydrocinnamicacid), which comprises the following steps: taking 3-sulfydryl-3 phenylpropionic acid as a raw material, reacting in the presence of an oxidant and a catalyst in an environment of pH being 6-12 to generate the beta,beta'-dithiobis(dihydrocinnamicacid), wherein the reaction is performed in the presence of a reaction solvent being water. When the preparing method is used for synthesizing the beta,beta'-dithiobis(dihydrocinnamicacid), the water is used as the solvent, generation of side reactions in the reaction is reduced, waste liquid has no poison and harm, and operation is simple. The product is separated by adjusting to acidity with acids after the reaction, and the beta,beta'-dithiobis(dihydrocinnamicacid) with the purity up to 99.88% can be obtained by filtering.
Owner:SHANGHAI BETTERSYN BIOTECH
Method for preparing docetaxel anhydrous crystal
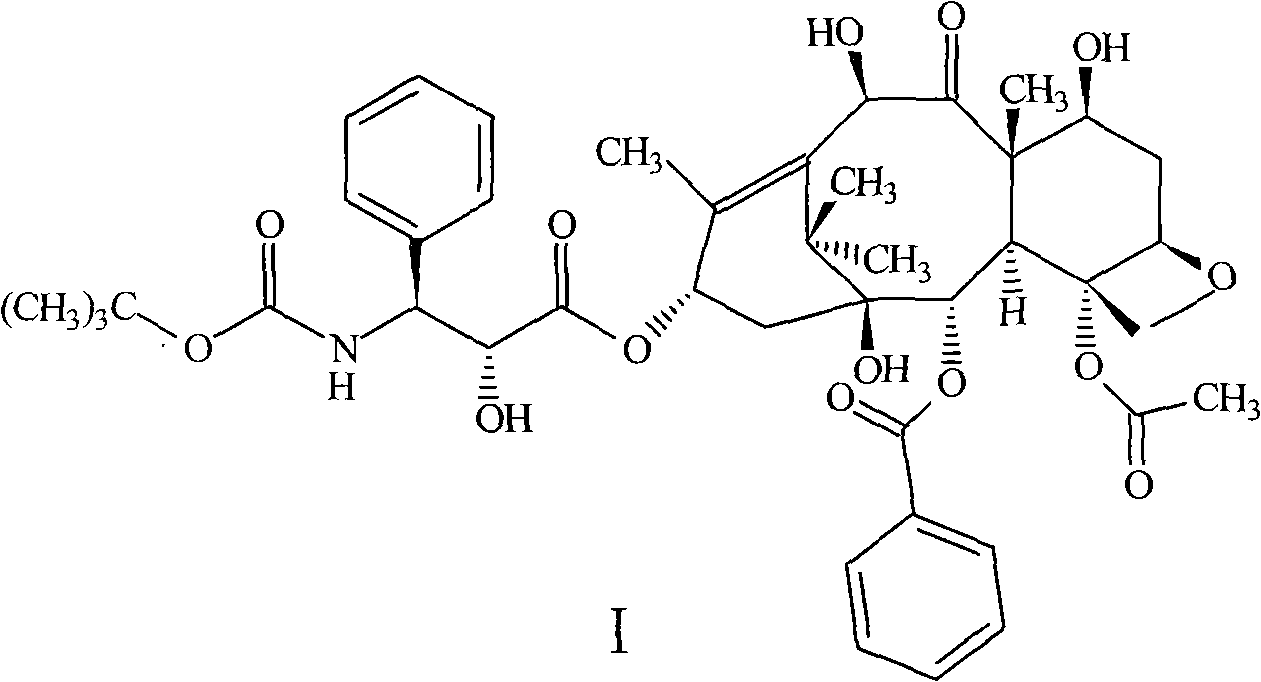
ActiveCN101337951BKeep dryEasy to controlOrganic chemistryOrganic solventTert-Butyloxycarbonyl protecting group
The invention provides a method for preparing (2R, 3S)-3-boc amino group-2-oxhydryl-3-phenylpropionic acid-4-carbethoxy-2Alpha-benzoyloxy-5Beta and 20-epoxide-1, 7Beta, 10Beta-trihydroxy-9-oxygen-taxus-11-alkene-13Alpha-ester (docetaxel) anhydrous crystallaria. The method uses mixed organic solvent composed of ethanol / aether or the mixed organic solvent composed of acetone / aether for recrystallization. The method has the characteristics of high yield, good product stability, easy drying and large scale production, etc.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
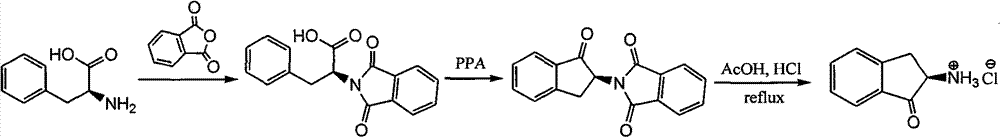
Synthesizing method of chiral amino indanone
The invention discloses a synthesizing method of chiral amino indanone. The chiral amino indanone as a chiral midbody is used for the synthesis of drugs, natural products and chiral ligands. The synthesizing method of the chiral amino indanone comprises the following steps of: reacting L-phenylalanine with phthalic anhydride to obtain (S)-2-phthalimide-3-phenylpropionic acid, and subjecting the (S)-2-phthalimide-3-phenylpropionic acid to an intramolecular friedel-crafts acylation reaction under the catalysis of polyphosphoric acid to obtain (S)-2-phthalimide-1-indanone; heating for hydrolyzing to strip a phthalyl protective group of the (S)-2-phthalimide-1-indanone contained in a concentrated hydrochloric acid and glacial acetic acid solution so as to obtain (S)-2-amino-1-indanone hydrochloride; substituting the L-phenylalanine by the D-phenylalanine so as to obtain (R)-2-amino-1-indanone hydrochloride, and using DL-phenylalanine so as to obtain a racemic product. The synthesizing method has the characteristics of environment friendliness, atom economy, low cost and easy obtaining of raw materials, and the like.
Owner:JIANGSU BAOZONG & BAODA PHARMACHEM
Carboxyl-containing difluoro monomer, preparation method of difluoro monomer and application of difluoro monomer to preparation of carboxyl-containing polyarylether
InactiveCN105037141AImprove conductivityImprove hydrophilicityOrganic compound preparationCarboxylic compound preparationPropanoic acidN dimethylformamide
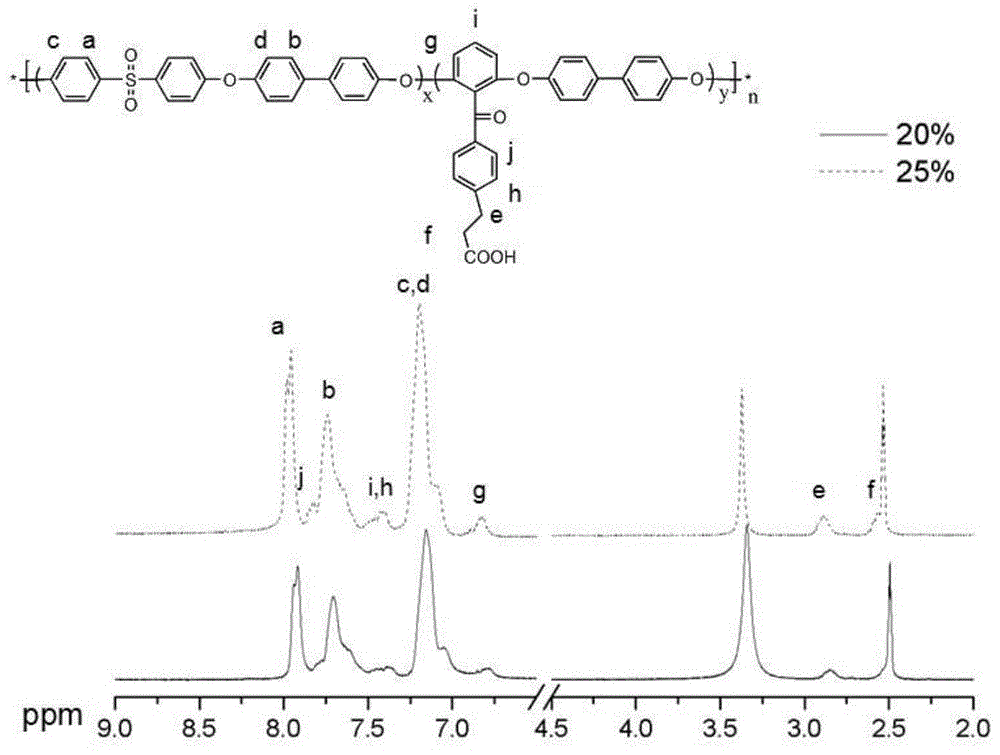
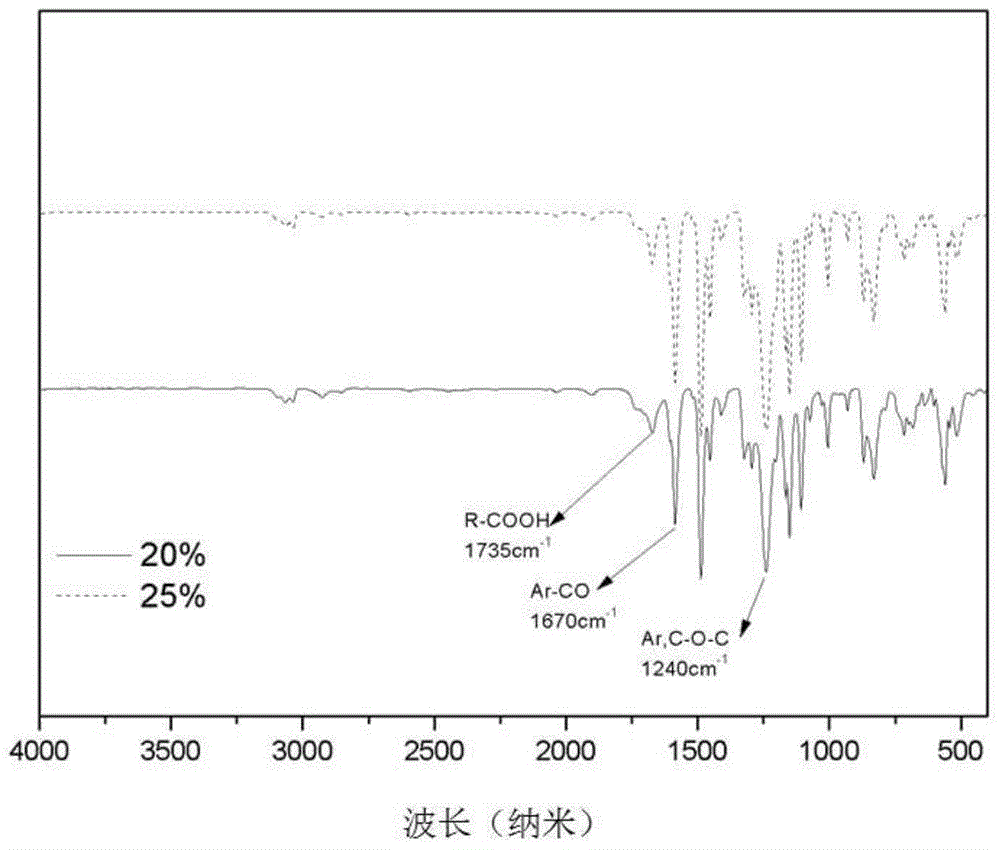
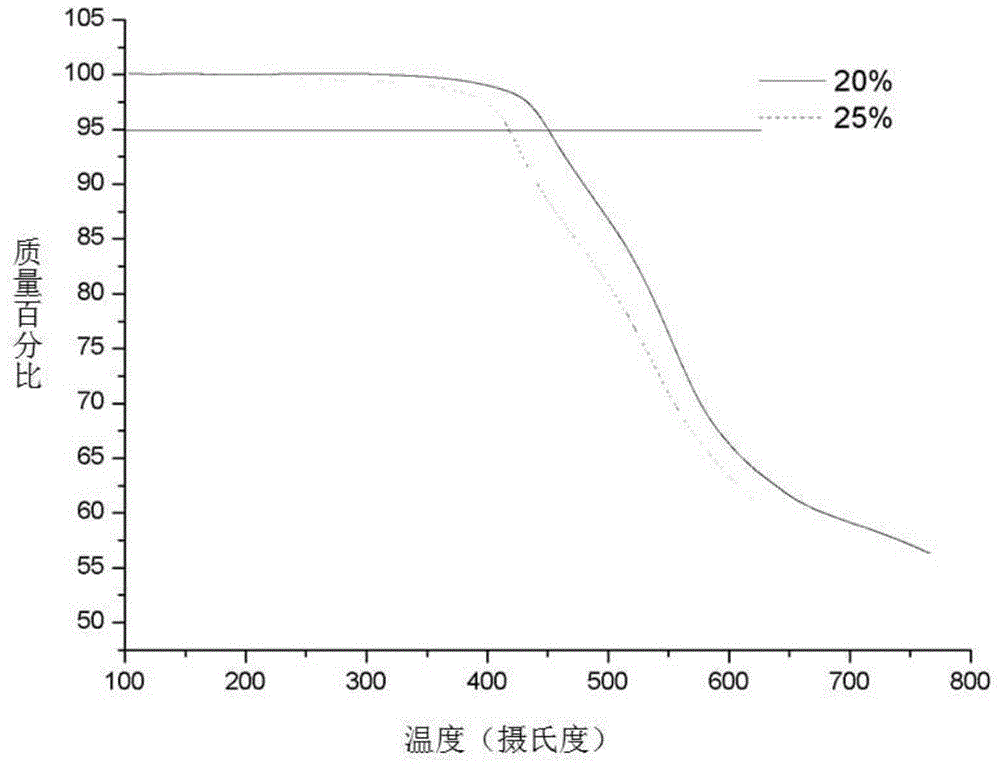
The invention provides a carboxyl-containing difluoro monomer, a preparation method of the difluoro monomer and application of the difluoro monomer to preparation of carboxyl-containing polyarylether, and belongs to the technical field of difluoro monomers and preparation of the difluoro monomers. The preparation method comprises the following steps: under argon atmosphere, using 2,6-difluorobenzoic acid as a raw material, thionyl chloride as a solvent and a reactant, and N,N-dimethylformamide as a catalyst to prepare 2,6-difluorobenzoyl chloride; under argon atmosphere and anhydrous condition, using anhydrous aluminium trichloride, 2,6-difluorobenzoyl chloride and 3-phenylpropionic acid as raw materials to prepare the white crystal difluoro monomer, namely, 3-[4-(2,6-difluorobenzoyl) phenyl] propionic acid. Compared with the common polyarylether polymer, the carboxyl-containing polyarylether prepared and synthesized in the invention introduces ionizable polar groups, can be applied to membrane separation technology, and can be used for promoting the hydrophilic performance and anti-fouling performance of a water separation membrane and improving the conductivity of a proton exchange membrane due to the electronegative carboxyl.
Owner:JILIN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com