Carbonyl reductase with self-assembled interface, and application of same in synthesis of (R)-3-hydroxy-3-phenylpropionic acid ethyl ester
A technology of ethyl phenylpropionate and carbonyl reductase, applied in the directions of oxidoreductase, microorganism-based methods, enzymes, etc., can solve the problems of containing precious metals, low substrate processing capacity, high price, etc., and reduce the enzyme activity. loss, improving catalytic efficiency, and improving the effect of conversion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
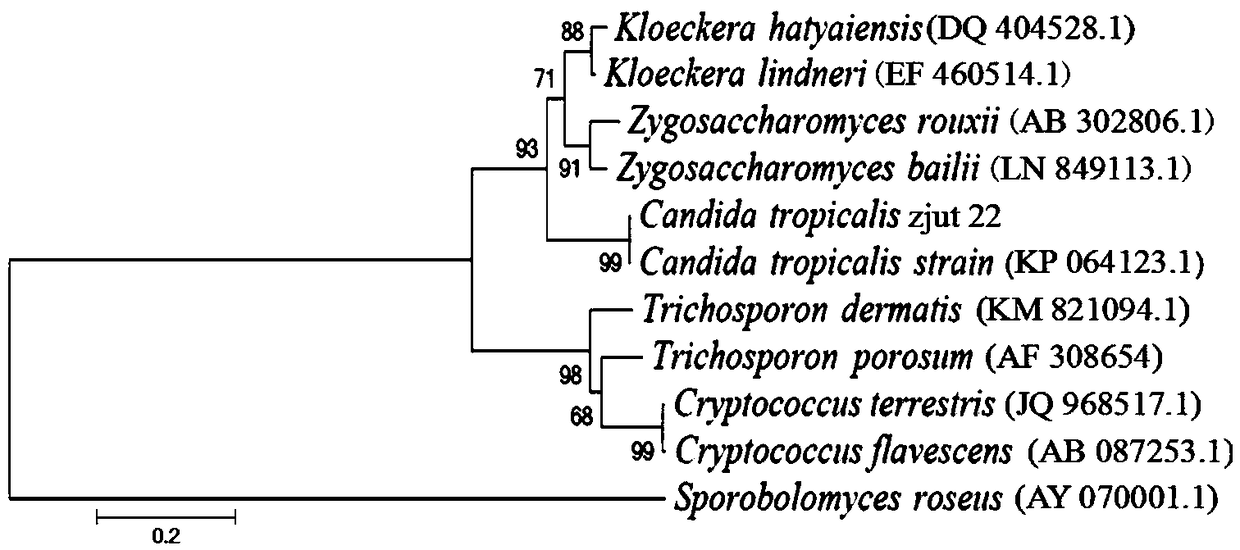
[0025] Example 1 Screening and identification of Candida tropicalis CGMCC No.15016
[0026] 1. Strain screening process:
[0027] (1) culture medium
[0028] Enriched plate medium (g / L): Glucose 20, (NH 4 ) 2 SO 4 1, KH 2 PO 4 0.5,K 2 HPO 4 1.5, NaCl1, MgSO 4 0.1, agar 20, the solvent is deionized water, and the pH value is natural.
[0029] Slant Medium (g / L): Glucose 20, (NH 4 ) 2 SO 4 1, KH 2 PO 4 0.5,K 2 HPO 4 1.5, NaCl 1, MgSO 4 0.1, agar 20, the solvent is deionized water, and the pH value is natural.
[0030] Seed medium (g / L): glucose 20, yeast powder 3, (NH 4 ) 2 SO 4 1, KH 2 PO 4 0.5,K 2 HPO 4 1.5, NaCl1, MgSO 4 0.1, the solvent is deionized water, and the pH value is natural. The composition of the fermentation medium is the same as that of the seed medium.
[0031] (2) Culture method
[0032] Incline culture: culture at 30°C for 3-5 days.
[0033] Seed cultivation: transfer the cultivated slant into a 100mL Erlenmeyer flask f...
Embodiment 2
[0057] Embodiment 2: the preparation of carbonyl reductase
[0058] 1. Wet bacteria
[0059] (1) Inoculate Candida tropicalis CGMCC No.15016 into the slant medium and culture at 30°C for 3-5 days to obtain slant bacteria; slant medium composition: glucose 20g / L, ammonium sulfate 1g / L, potassium dihydrogen phosphate 0.5g / L, dipotassium hydrogen phosphate 1.5g / L, sodium chloride 1g / L, magnesium sulfate 0.1g / L, agar 20g / L, the solvent is deionized water, and the pH value is natural.
[0060] (2) Inoculate the bacterium on the slope into a 100 mL Erlenmeyer flask filled with 25 mL of seed medium, and culture at 30° C. and 120 r / min for 24 hours to obtain seed liquid. Seed medium composition: glucose 46g / L, yeast juice 25g / L, potassium dihydrogen phosphate 4g / L, dipotassium hydrogen phosphate 4g / L, sodium chloride 0.1g / L, solvent is deionized water, pH value is natural.
[0061] (3) Put the seed solution cultivated for 24 hours into a 1000 mL Erlenmeyer flask filled with 250 mL ...
Embodiment 3
[0070] Example 3 Preparation and Application of Interfacial Self-Assembly Carbonyl Reductase
[0071] (1) Preparation of interfacial self-assembled carbonyl reductase
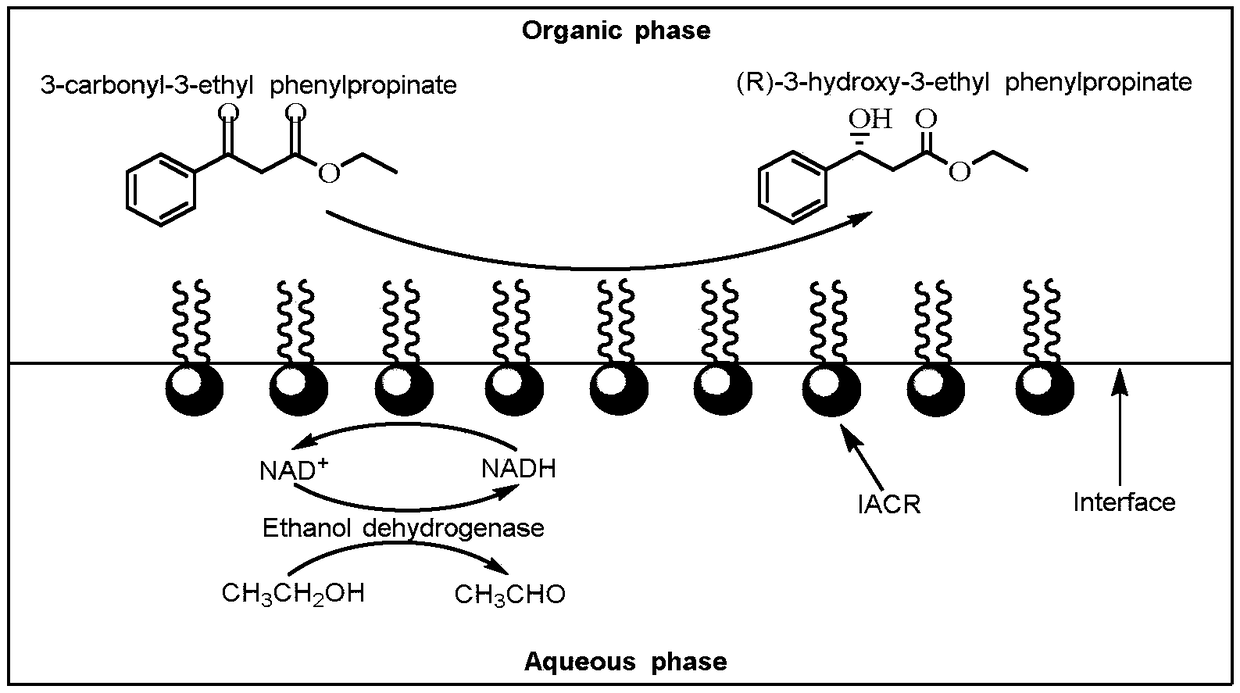
[0072] Add 5mg of carbonyl reductase enzyme solution (180U / mg) prepared in Example 2 into 6.25ml, pH 7.9, 0.05M Tris-HCl buffer solution to prepare 0.8g / L enzyme solution, then add 3.75g / L poly Styrene (Mw 10000Da) toluene solution 10ml was reacted for 1 hour in the dark at a shaker speed of 80r / min at 30°C. After the reaction is completed, centrifuge at 1200r / min for 10min, and the interfacial self-assembling enzyme is distributed on the two-phase interface of water and organic phase (that is, the upper layer is toluene, the middle is the interfacial self-assembling enzyme, and the lower layer is Tris-HCl buffer solution). The buffer and organic solvent were removed, and the middle layer was washed 3 times with toluene and pH 7.9, 0.05M Tris-HCl buffer solution successively to remove free carbonyl reductase a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific vitality | aaaaa | aaaaa |
| Specific vitality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com