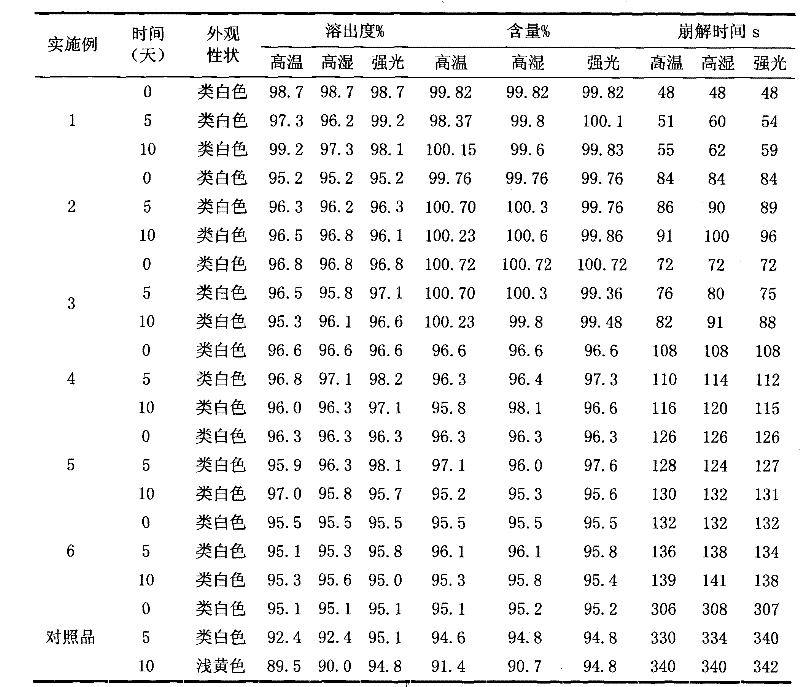
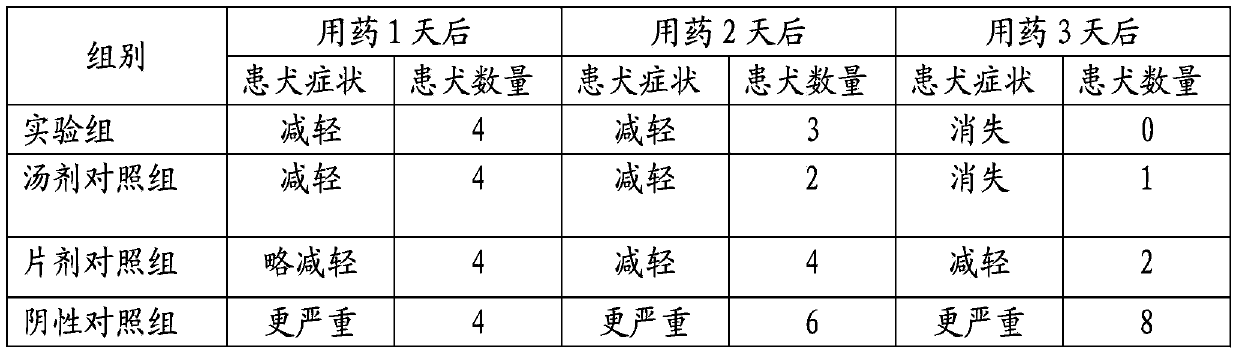
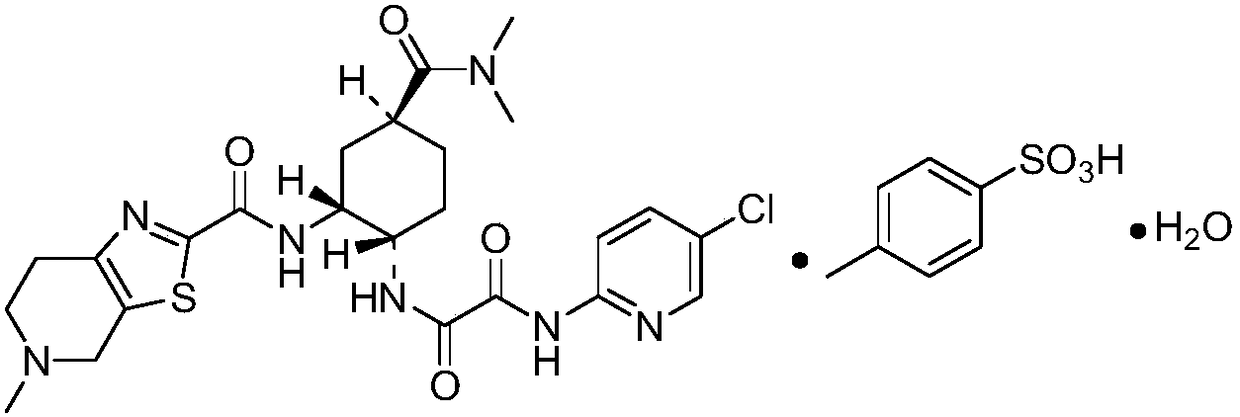
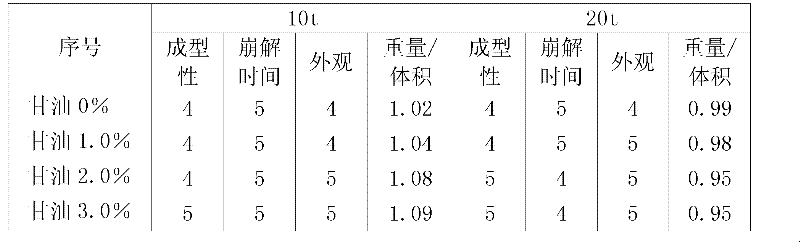
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30results about How to "Prolonged disintegration time" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
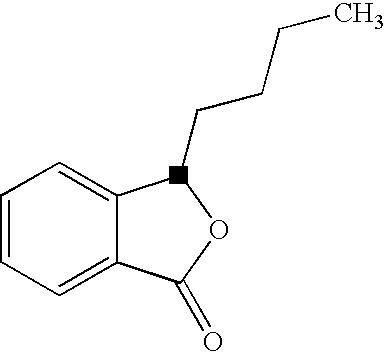
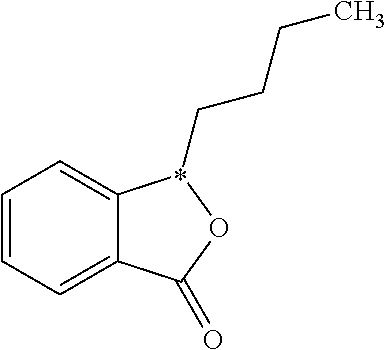
Butylphthalide Self-Emulsifying Drug Delivery System, Its Preparation Method and Application
ActiveUS20080319056A1Improve absorption rateReduce power consumptionBiocideNervous disorderAdditive ingredientButylphthalide
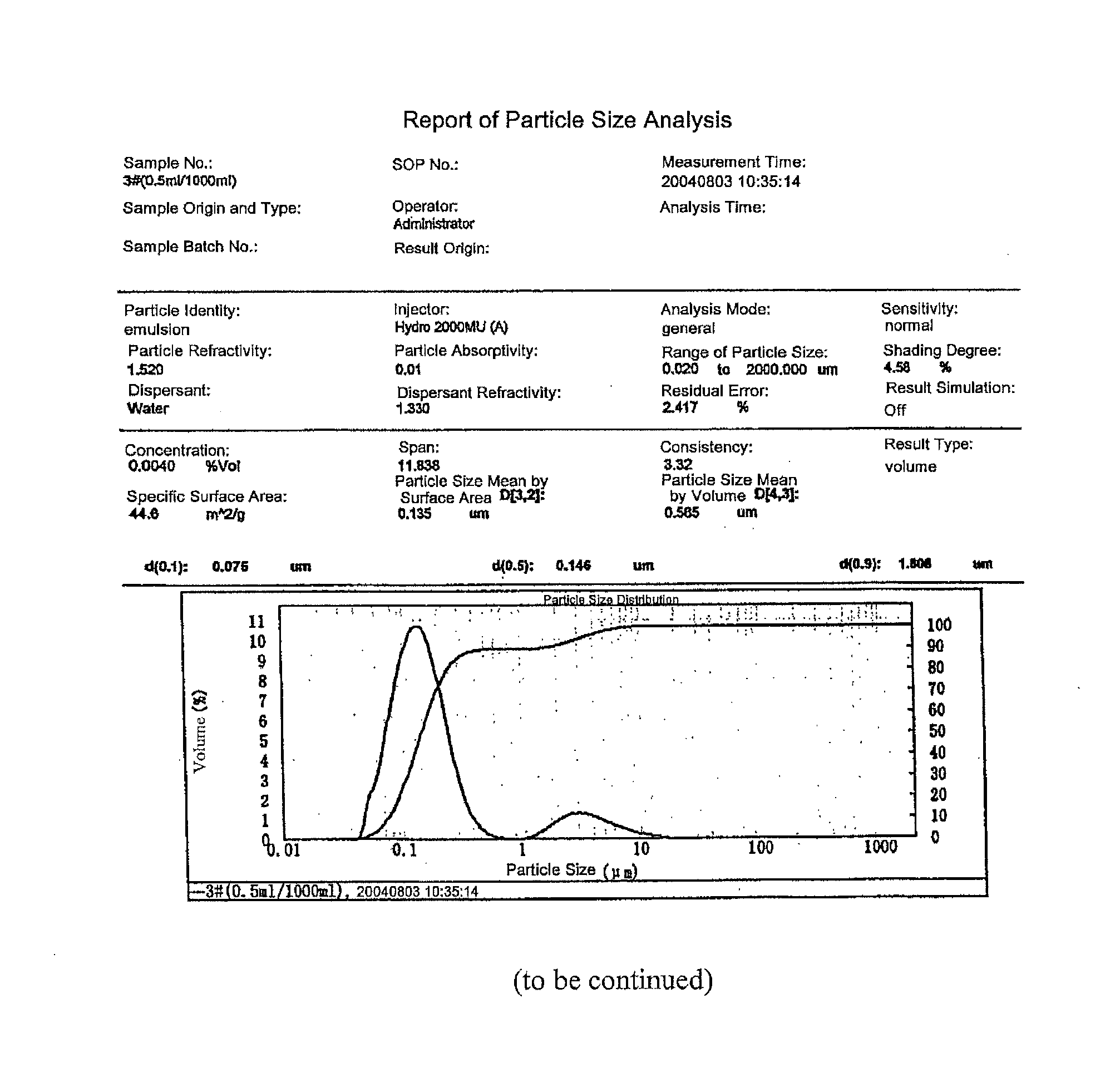
The present invention relates to a novel drug delivery and release system, i.e. Self-emulsifying Drug Delivery System (SEDDS), of butylphthalide, to a preparation process thereof, and to a use thereof in a pharmaceutical formulation. The drug delivery system comprises as essential ingredients 1% to 65% of butylphthalide and 10% to 65% of a emulsifying agent, together with various excipients as required depending on the desired dosage forms. The present invention significantly increases the contact area between butylphthalide and the mucous membrane of the gastrointestinal tract, and therefore improves the absorptivity of the drug.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
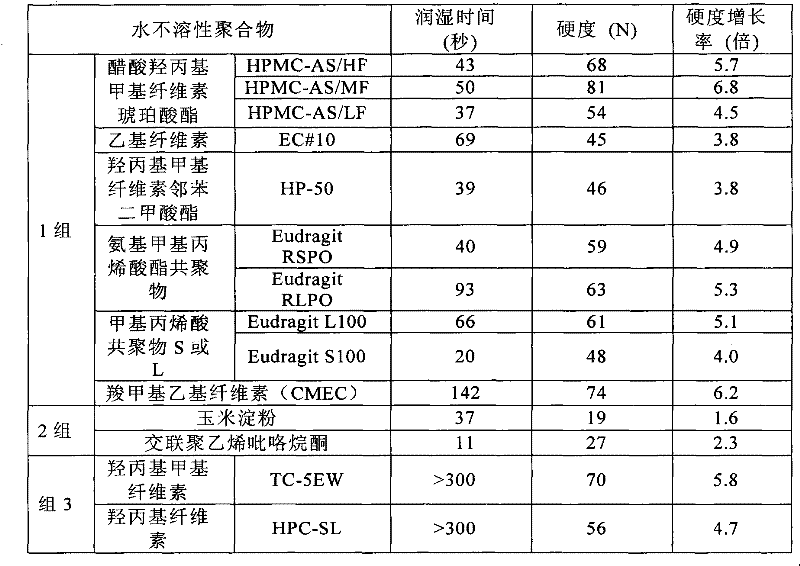
Orally rapidly disintegrating tablet, and process for producing same
ActiveCN102223880ASolve the lack of hardnessProlonged disintegration timeOrganic active ingredientsNervous disorderCompression moldingWater insoluble
Disclosed is an orally rapidly disintegrating tablet characterized in that the tablet can be produced in a conventional tablet production facility and has a satisfactory level of hardness for practical applications, and the change in properties of the tablet (i.e., decreased in hardness of the tablet, and delay of the time required for disintegration of the tablet in the oral cavity) are rarely caused by factors such as humidity. The orally rapidly disintegrating tablet has hardness of 40 N or more, can be disintegrated in the oral cavity within 60 seconds, and is produced by compression molding of a mixture of (a) an active ingredient, (b) an excipient having good water wettability, (c) a water-insoluble polymer that has good moldability and does not substantially cause the decrease in the water wettability of the excipient and (d) a disintegrating agent.
Owner:MITSUBISHI TANABE PHARMA CORP
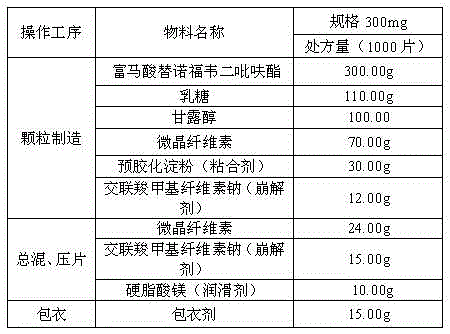
Tenofovir disoproxil fumarate tablets
InactiveCN104644598AGuaranteed stabilityGood compressibilityOrganic active ingredientsPharmaceutical delivery mechanismAdhesiveCombinatorial chemistry
The invention belongs to the technical field of medicinal preparations, and specifically relates to tenofovir disoproxil fumarate tablets. The tablets comprise tenofovir disoproxil fumarate, a filler, an adhesive, a disintegrating agent, a lubricant and a coating agent; each tablet is 600-800mg and contains 300mg of tenofovir disoproxil fumarate, 25-65% of the filler, 2-5% of the adhesive, 3-15% of the disintegrating agent, 1-3% of the lubricant and 2-3% of the coating agent. The tenofovir disoproxil fumarate tablets have the advantages of low cost and stable product quality.
Owner:LEPU PHARMACEUTICAL CO LTD
Tablet containing faropenem sodium
ActiveCN101744782ASignificant progressDissolution constantAntibacterial agentsDigestive systemHigh humidityOlder people
The invention relates to a tablet containing faropenem sodium, belonging to the field of pharmaceutical preparation. The faropenem sodium tablet of the invention comprises 20-50 parts of faropenem sodium, 20-25 parts of disintegrant, 2-10 parts of 95% ethanol solution containing 5% of polyvinylpyrrolidone, 20-30 parts of filler and 0.5-2 parts of lubricant. The faropenem sodium tablet of the invention has the advantages of fast disintegration speed and stable properties in high-temperature and high-humidity environment, can effectively improve the effect-taking concentration and bioavailability of faropenem, and is very suitable for old people and children with dysphagia to take.
Owner:LUNAN BETTER PHARMA
Pet's scourge-clearing toxin-vanquishing chewable tablet and preparation process thereof
InactiveCN103417865AImprove efficacyReduce dosageAntibacterial agentsDigestive systemSucroseMoisture absorption
The invention discloses a pet's scourge-clearing toxin-vanquishing chewable tablet and a preparation process thereof. The pet's scourge-clearing toxin-vanquishing chewable tablet is composed of gypsum, rhizoma rehmanniae, cornu bubali, coptis chinensis, gardenia, moutan bark, scutellaria baicalensis, red peony, radix scrophulariae, rhizoma anemarrhenae, fructus forsythiae, lophatherum gracile, magnesium stearate, starch, extractum glycyrrhizae, ballonflower extractum and a flavoring agent. The preparation process is simple and includes three steps of preparing traditional Chinese medicine fine powder, preparing a mixed solution and mixing, pelletizing, tableting and forming. Compared with scourge-clearing toxin-vanquishing powder, the pet's scourge-clearing toxin-vanquishing chewable tablet has the advantages that a forming form of chewable tablet is adopted, traditional Chinese medicinal materials are subjected to smashing, tableting and forming, and extracted extractum glycyrrhizae and ballonflower extractum are adopted, so that dissolution, absorption and quick working of active ingredients are facilitated; medicine binding is realized through PVP (poly vinyl pyrrolidone), so that less proneness to moisture absorption is realized, efficacy can last longer, and full utilization of medicine is facilitated; mannitol used for flavoring can prevent the pet's scourge-clearing toxin-vanquishing chewable tablet from absorbing moisture, thereby having no hygroscopicity compared with common cane sugar, sorbitol and the like.
Owner:SICHUAN JIANYANG AIDI FEED PHARMA
Pharmaceutical composition for edoxaban tablets
InactiveCN108175753ASignificant progressShort disintegration timeOrganic active ingredientsPharmaceutical non-active ingredientsMedicineBioavailability
The invention provides pharmaceutical composition for edoxaban tablets. The composition is prepared from 10-50 parts of p-toluenesulfonic acid edoxaban monohydrate, 15-35 parts of a disintegrant, 2-10parts of a 5% polyvinylpyrrolidone 95% ethanol solution, 20-45 parts of filler and 0.5-2 parts of a lubricant, and has the advantages of high disintegration speed, high bioavailability, good stability and the like.
Owner:SICHUAN HAISCO PHARMA CO LTD
Spirulina tablet added with glycerol and processing method thereof
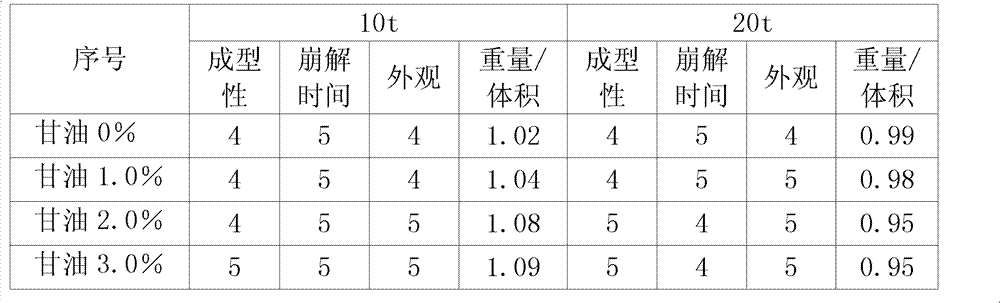
ActiveCN102406178AGood molding effectProlonged disintegration timeFood preparationMicroorganismWater activity
The invention discloses a spirulina tablet added with glycerol and a processing method thereof. The method comprises the following steps: uniformly mixing 60-120 meshes of the spirulina powder with the glycerol according to the mass percent rate of 100 : 1 to 3; and pressing in a tablet press of which the pressure is 10-20 tons to form the tablets. The glycerol has the function of reducing water activation, so the water activity of the product can be reduced after the glycerol is added in the spirulina powder, the storage time of the spirulina powder is effectively prolonged, and the number of microorganism is reduced.
Owner:LIJIANG CHENGHAI BAOER BIOLOGICAL DEV
Caraway-containing compound capsule composition and preparation method thereof
ActiveCN106344536AExcellent long-term storage stabilityProlonged disintegration timeAntipyreticAnalgesicsMedicinePeppermints
The invention provides a caraway-containing compound capsule composition and a preparation method thereof. The content of the caraway-containing compound capsule composition consists of caraway oil, peppermint oil, sesame oil and olive oil. The caraway-containing compound capsule composition has the advantages of a short disintegrating time, high storage stability, high medicinal safety and the like.
Owner:HEBEI IDEAL & HIGHTECH PHARMA
Skeleton tablet core for digestive tract evacuation-detecting slow-release tablet and its preparing process
InactiveCN1814307ALow disintegration rateDelayed disintegration rateEmulsion deliveryCross-linked polyethyleneLow speed
This invention is framework core used to alimentary tract emptying function detection slow release pill and its preparation technique. The core includes following components and quality percentage: hard dissolve medicine using materials =30-90%, X ray developer =5-50%, disintegration using auxiliary materials =5-65%. The hard dissolve medicine using materials are ethyl cellulose, or cerotic acid cellulose, or high viscosity hydroxypropyl cellulose, or hydroxide third concha cellulose phthalidyl, or hydroxymethyl ethyl cellulose, or acrylic resin. The X ray developer is barium sulfate developer, or iodine developer. The disintegration using auxiliary materials are hydroxymethyl starch natrium, or crosslinked polyethylene pyrrolidone, or low substitution hydroxypropyl cellulose. its preparation technique main including getting framework core materials and suppress framework core. The core can be observed by X ray perspective equipment in alimentary canal, is has low speed slaking characteristic.
Owner:CHONGQING UNIV
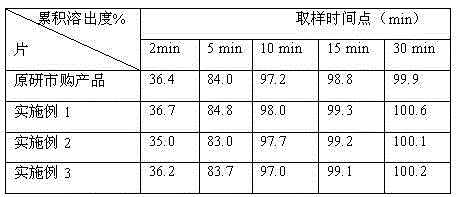
Mesalazine tablet having improved dissolution
InactiveCN103108629ALow variabilityDissolution with low variabilitySalicyclic acid active ingredientsDigestive systemHardnessDissolution
The invention provides a method for preparing a mesalazine enteric coated tablet comprising: (i) granulating a composition comprising mesalazine, a pharmaceutically acceptable salt, or ester thereof, into mesalazine granulates; (ii) tabletting a core composition comprising the mesalazine granulates obtained in (i) to obtain a tablet core; (iii) coating the tablet core obtained in (ii) with at least an enteric coating; where the tablet core hardness is controlled to be comprised between 80 N and 105 N.
Owner:DISPHAR INTERNATIONAL BV
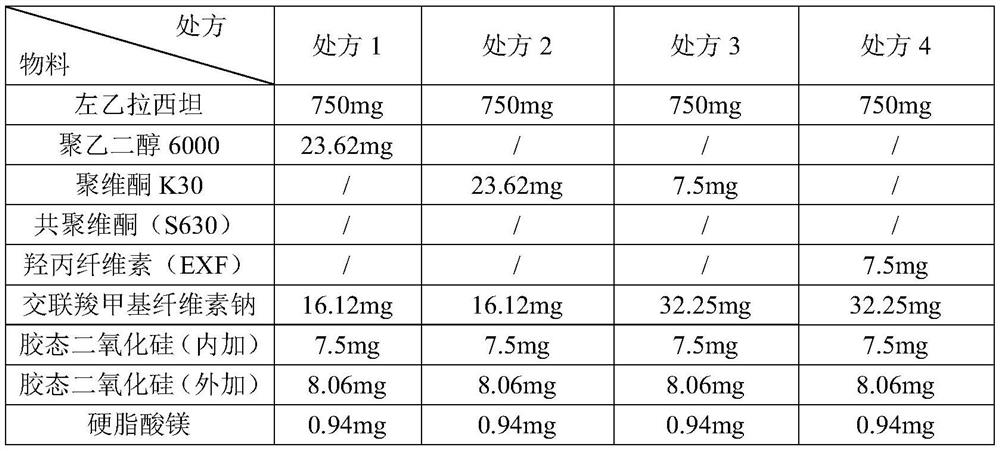
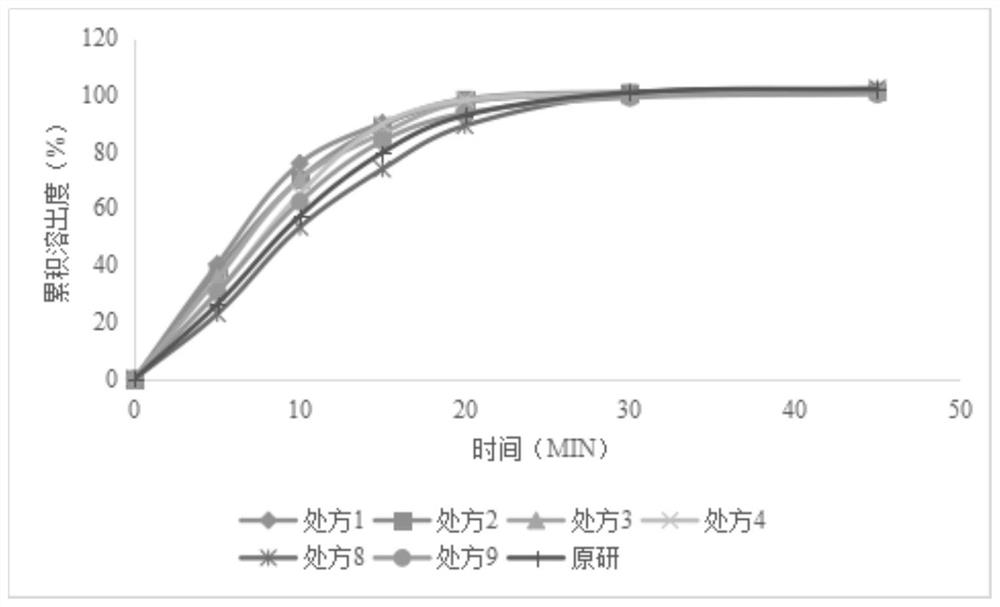
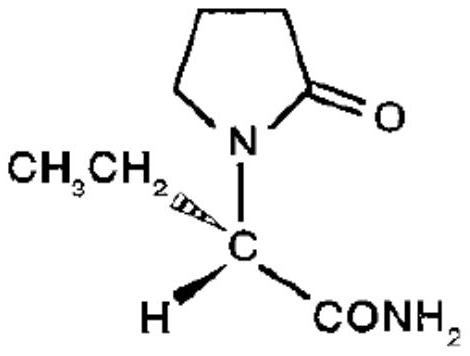
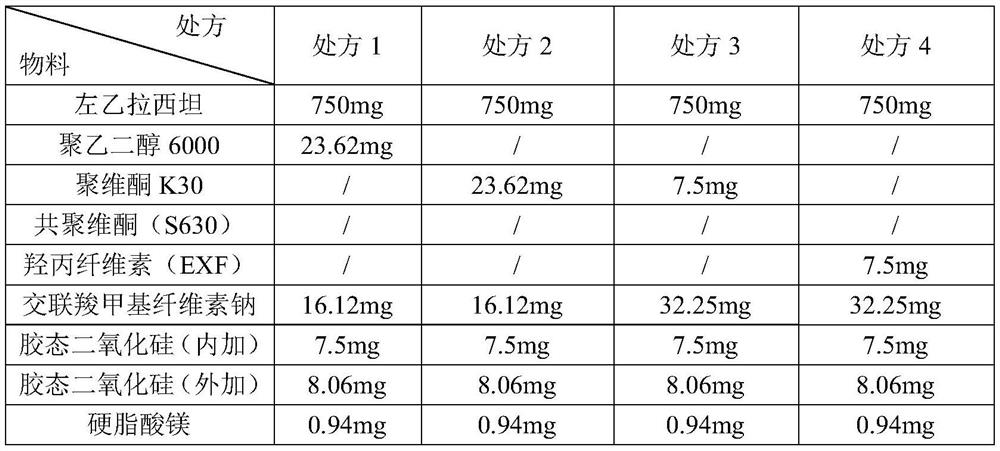
A kind of levetiracetam tablet and preparation method thereof
ActiveCN112870176BSimple processEasy to operateOrganic active ingredientsNervous disorderCellulosePowder mixture
The invention belongs to the technical field of pharmaceutical preparations, in particular to a levetiracetam tablet and a preparation method thereof. The preparation method comprises the following steps: step 1: sieve levetiracetam, hydroxypropyl cellulose, croscarmellose sodium and part of colloidal silicon dioxide together for later use; step 2: sift the After the granulation materials are mixed, magnesium stearate is added and mixed evenly to obtain a premixed powder; step 3: the premixed powder in step 2 is placed in dry granulation and granulated to obtain a mixture of granules and powder; step 4: The mixture of granules and powder after the granulation in Step 3 is mixed with the remaining colloidal silicon dioxide and then compressed into tablets; Step 5: Coating. The preparation method provided by the invention has the advantages of simple technological process, strong operability, low production cost and stability.
Owner:HINYE PHARM CO LTD
Irbesartan tablet and preparation method thereof
ActiveCN105078913AImprove stabilitySimple processOrganic active ingredientsPill deliverySucrosePrill
The invention relates to an irbesartan tablet and a preparation method thereof. The irbesartan tablet is prepared from the following raw materials in parts by mass: 150-450 parts of irbesartan, 15-50 parts of montmorillonite, 85-270 parts of sucrose, 0.5-1.5 parts of magnesium stearate and 0.5-1.5 parts of silicon dioxide. The invention also provides a preparation method of the irbesartan tablet. The irbesartan tablet disclosed by the invention, by matching special accessories and main drugs and through special proportions, can be used for solving the problems of existing drug particles which are poor in liquidity, non-ideal in drug disintegration, low in drug dissolution, poor in stability, not high in bioavailability in human body and the like.
Owner:SHANDONG SBOND PHARMA
Coating agent containing hydroxyalkyl cellulose
InactiveCN105283204AHigh hardnessNo lossPharmaceutical non-active ingredientsCoatingsCelluloseFoaming agent
Obtained is a coating agent that contains a hydroxyalkyl cellulose, which contains more than 50% by mass but 60% by mass or less of a hydroxy alkyl group relative to the total mass of the hydroxyalkyl cellulose, in an amount of from 1% by mass to 7% by mass (inclusive) relative to the total mass of the coating agent. A solid preparation is obtained by spraying this coating agent onto a base tablet and drying this coating agent thereon.
Owner:NIPPON SODA CO LTD
Butylphthalide self-emulsifying drug delivery system, its preparation and method and application
ActiveUS8728518B2Improve absorption rateEasy to operatePowder deliveryBiocideButylphthalideSelf emulsifying
The present invention relates to a novel drug delivery and release system, i.e. Self-emulsifying Drug Delivery System (SEDDS), of butylphthalide, to a preparation process thereof, and to a use thereof in a pharmaceutical formulation. The drug delivery system comprises as essential ingredients 1% to 65% of butylphthalide and 10% to 65% of a emulsifying agent, together with various excipients as required depending on the desired dosage forms. The present invention significantly increases the contact area between butylphthalide and the mucous membrane of the gastrointestinal tract, and therefore improves the absorptivity of the drug.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Compound indigo pill
InactiveCN101040993AProlonged disintegration timeLong onset timePill deliveryDermatological disorderAlkanna tinctoriaSmoked Plum
The invention relates to a concentrated compound natural indigo pill which is prepared from the following raw materials: natural indigo 60g, purslane 200g, dahurian angelica root 100g, poria cocos 200g, alkanna tinctoria 80g, basket fern 60g, dandelion 80g, root of red rooted saliva 100g, rhizoma dioscoreae hypoglaueae 100g, Chinese dittany bark 100g, smoked plum 200g, schisandra fruit 100g, haw 60g and medicated leaven 60g.
Owner:焦林海
Spirulina tablet added with glycerol and processing method thereof
ActiveCN102406178BGood molding effectProlonged disintegration timeFood preparationMicroorganismWater activity
The invention discloses a spirulina tablet added with glycerol and a processing method thereof. The method comprises the following steps: uniformly mixing 60-120 meshes of the spirulina powder with the glycerol according to the mass percent rate of 100 : 1 to 3; and pressing in a tablet press of which the pressure is 10-20 tons to form the tablets. The glycerol has the function of reducing water activation, so the water activity of the product can be reduced after the glycerol is added in the spirulina powder, the storage time of the spirulina powder is effectively prolonged, and the number of microorganism is reduced.
Owner:LIJIANG CHENGHAI BAOER BIOLOGICAL DEV
A kind of levoxiracetam oral fast-dissolving film and preparation method thereof
ActiveCN106821959BMedication conveniencePhysical stabilityOrganic active ingredientsNervous disorderSide effectFiller Excipient
The invention relates to an L-oxiracetam oral instant membrane, which is prepared from 3 to 20 parts of L-oxiracetam, 40 to 70 parts of membrane-forming material, 10 to 30 parts of filter, 5 to 20 parts of plasticizer and 2 to 4 parts of corrigent, wherein the membrane-forming material is maltodextrin. The L-oxiracetam oral instant membrane can be dissolved by a small amount of saliva in the oral cavity, and can be taken without water for delivery, so administration is convenient. After being stuck to the tongue, the L-oxiracetam oral instant membrane cannot be easily spit out, and therefore is suitable for children with dysphagia. Moreover, because the L-oxiracetam oral instant membrane can be absorbed through the mucous membrane, the first pass elimination effect is prevented, the bioavailability is increased, the dosage of medication is reduced, and thereby the side effect of the medicine is reduced. A preparation method of the invention is simple, the process cost is low, and therefore the preparation method is suitable for mass production.
Owner:CHONGQING RUNZE PHARM CO LTD
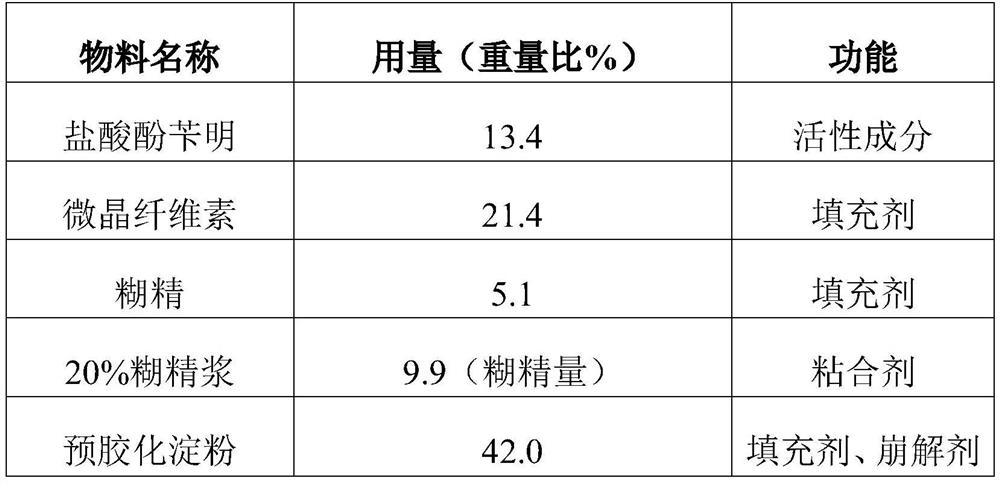
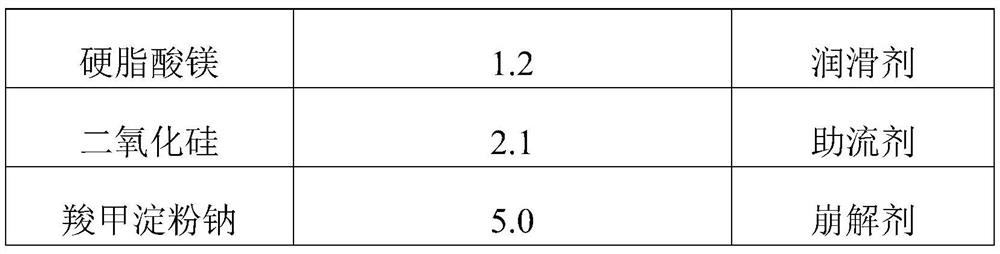
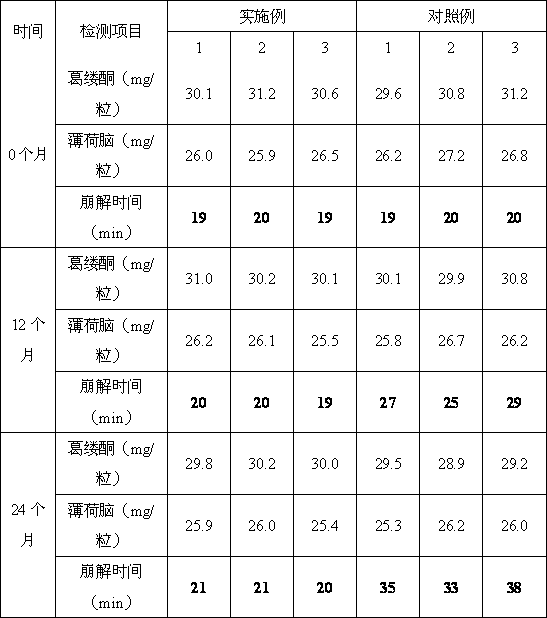
Phenoxybenzamine hydrochloride tablet and preparation method thereof
ActiveCN111743871BImprove bindingGood compressibilityOrganic active ingredientsUrinary disorderPhenoxybenzamine HydrochlorideSodium Starch Glycolate
The invention discloses a phenoxybenzamine hydrochloride tablet, which comprises: phenoxybenzamine hydrochloride, dextrin, pregelatinized starch, disintegrant, filler, binder, lubricant and flow aid. The viscosity of the dextrin is 4.5-8.5mpa.s; the specific surface area of the pregelatinized starch is ≤350m 2 / kg; The disintegrating agent is sodium starch glycolate. Add disintegrants such as carboxymethyl starch sodium and dextrin with a viscosity of 4.5-8.5mpa.s and a specific surface area of ≤350m in the components 2 The pregelatinized starch per kg can make the obtained phenoxybenzamine hydrochloride tablet have improved dissolution rate and uniformity of dissolution rate, and the difference within the group is small and the stability is good.
Owner:TOPFOND PHARMA CO LTD
L-oxiracetam oral instant membrane and preparation method thereof
ActiveCN106821959AMedication conveniencePhysical stabilityOrganic active ingredientsNervous disorderSide effectFiller Excipient
The invention relates to an L-oxiracetam oral instant membrane, which is prepared from 3 to 20 parts of L-oxiracetam, 40 to 70 parts of membrane-forming material, 10 to 30 parts of filter, 5 to 20 parts of plasticizer and 2 to 4 parts of corrigent, wherein the membrane-forming material is maltodextrin. The L-oxiracetam oral instant membrane can be dissolved by a small amount of saliva in the oral cavity, and can be taken without water for delivery, so administration is convenient. After being stuck to the tongue, the L-oxiracetam oral instant membrane cannot be easily spit out, and therefore is suitable for children with dysphagia. Moreover, because the L-oxiracetam oral instant membrane can be absorbed through the mucous membrane, the first pass elimination effect is prevented, the bioavailability is increased, the dosage of medication is reduced, and thereby the side effect of the medicine is reduced. A preparation method of the invention is simple, the process cost is low, and therefore the preparation method is suitable for mass production.
Owner:CHONGQING RUNZE PHARM CO LTD
Oxiracetam oral membrane preparation and preparation method thereof
InactiveCN106822055AMedication convenienceUniform appearanceOrganic active ingredientsNervous disorderSide effectOlder people
The invention relates to an oxiracetam oral membrane preparation and a preparation method thereof. The oxiracetam oral dispersion membrane preparation is prepared from Oxiracetam, a membrane forming material, a plasticizing agent and a filling agent. The oxiracetam oral membrane preparation has the advantages that the preparation can be dissolved by less saliva in an oral cavity, and can be taken without using water, so that the taking is convenient; the easiness in vomiting is avoided after sticking onto a tongue, and the oxiracetam oral preparation is suitable for old people with difficulty in swallowing; by utilizing mucosae to absorb, the first pass elimination effect is avoided, the bioavailability is improved, the medicine dosage is reduced, and the side effect is decreased. The preparation method has the advantages that the preparation is simple, the technology cost is low, and the preparation method is suitable for industrialized production.
Owner:CHONGQING RUNZE PHARM CO LTD
A kind of compound Tibetan fennel capsule composition and preparation method thereof
ActiveCN106344536BExcellent long-term storage stabilityProlonged disintegration timeAntipyreticAnalgesicsMedicinePeppermints
The invention provides a caraway-containing compound capsule composition and a preparation method thereof. The content of the caraway-containing compound capsule composition consists of caraway oil, peppermint oil, sesame oil and olive oil. The caraway-containing compound capsule composition has the advantages of a short disintegrating time, high storage stability, high medicinal safety and the like.
Owner:HEBEI IDEAL & HIGHTECH PHARMA
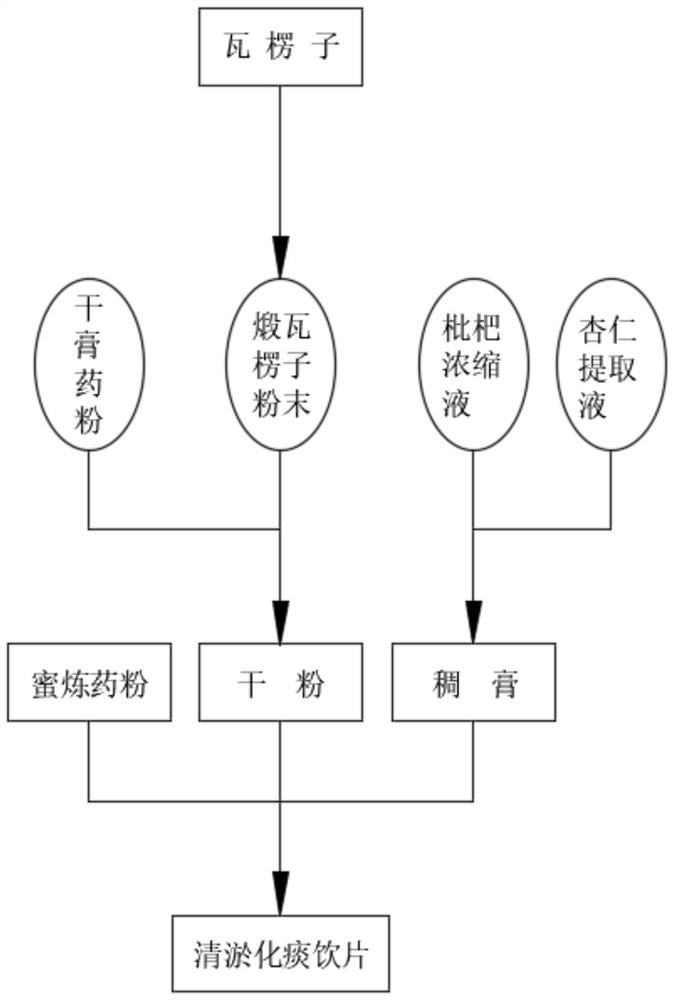
Production process of decoction pieces for dredging and reducing phlegm based on calcined concha arcae
ActiveCN114177250APrevent diseaseIncrease viscosityPharmaceutical non-active ingredientsPill deliveryAnticarcinogenic EffectAceric acid
The invention discloses a production process of decoction pieces for dredging and reducing phlegm based on calcined concha arcae, and belongs to the technical field of traditional Chinese medicine decoction pieces, the calcined concha arcae is a monarch drug and is easily ground into powder after being calcined and quenched by adopting an acetic acid solution, and the calcined concha arcae powder is finer and more convenient to disperse after being ball-milled; the lucid ganoderma extract in the dry plaster powder contains ganoderan, so that the dry plaster powder has an anti-cancer effect and can prevent inflammatory cytopathy; the rhizoma gastrodiae has the effects of clearing and activating the channels and collaterals, and the pericarpium citri reticulatae has the effects of regulating qi-flowing for strengthening the spleen and eliminating dampness and phlegm and has fragrance, so that the decoction piece is more delicious; the loquat concentrated solution has the effects of moistening the lung to arrest cough, and the almonds also have the effects of relieving cough and asthma; the liquorice has the effects of expelling phlegm to arrest coughing and harmonizing all the medicines, the fried fructus gardeniae has the effects of cooling blood, detoxifying, diminishing swelling and the like, after the liquorice and the fried fructus gardeniae are further fried through the refined honey, the viscosity of the prepared refined honey medicine powder is increased, and after the liquorice and the fried fructus gardeniae are pressed into tablets, the disintegration time can be prolonged, and the medicine action time can be prolonged.
Owner:安徽省双辉生物科技有限公司
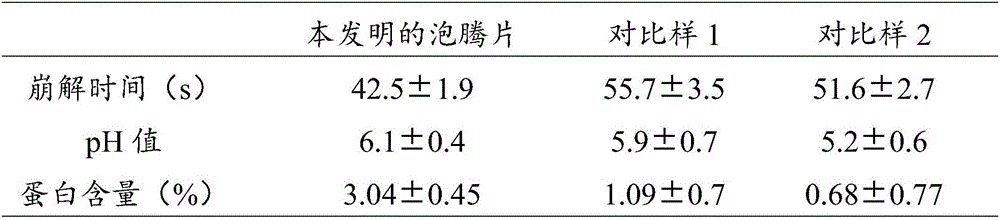
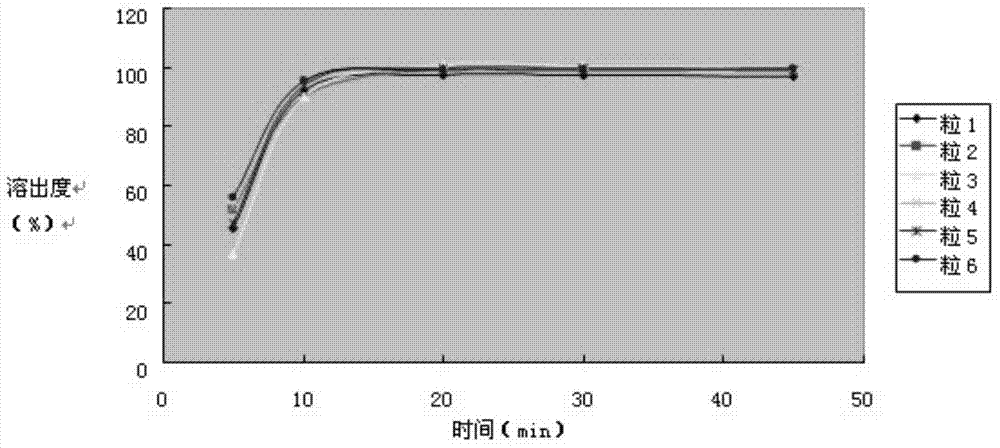
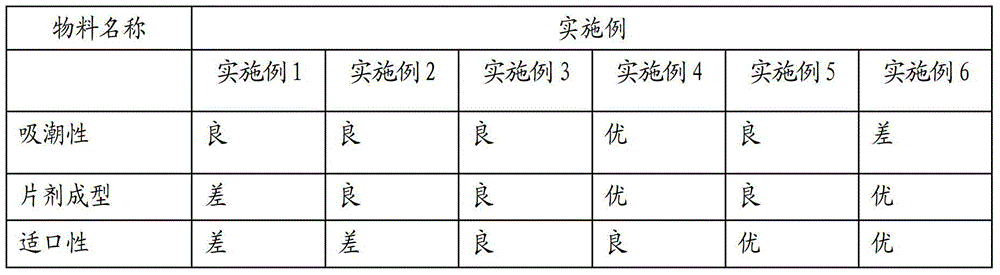
Milk powder high-protein effervescent tablet and preparation method thereof
ActiveCN106490164AFast disintegrationShort disintegration timeMilk preparationCompression moldingEffervescent tablet
The invention provides a milk powder high-protein effervescent tablet and a preparation method thereof. The preparation method comprises the following steps: pelleting by using an acid-base separated type disintegrant, uniformly mixing base source granules and acid source granules, mixing with a lubricant, and performing compression molding. By adopting the preparation method provided by the invention, on the premise that the content of protein in the effervescent tablet is increased, the defects that a milk product has unstable properties, for example, the sticking and disintegration time is long, foams can be easily generated, and the like in a disintegration period can be remarkably overcome; the stability of a disintegrated product can be improved by innovatively changing the shape of the effervescent tablet and increasing the specific surface area, so that the disintegration time of the milk powder effervescent tablet can be effectively shortened, the stability of a protein system can be improved, and bubble and sticking phenomena in the disintegration process can be avoided; the milk powder high-protein effervescent tablet prepared by using the preparation method provided by the invention is high in disintegration speed, stable in product shape, applicable to various fast-moving consumption people, and capable of completely meeting industrial production demands.
Owner:BRIGHT DAIRY & FOOD CO LTD
Compound paracetamol and amantadine hydrochloride capsule and preparation method thereof
ActiveCN103933069AQuality is easy to controlEasy to useAntiviralsUnknown materialsSucroseCalculus bovis
The invention relates to a compound paracetamol and amantadine hydrochloride capsule and a preparation method thereof. The compound paracetamol and amantadine hydrochloride capsule disclosed by the invention is prepared from the following raw materials in parts by mass: 250-400 parts of paracetamol, 100-160 parts of amantadine hydrochloride, 10-16 parts of calculus bovis factitius, 15-24 parts of caffeine, 2-3.2 parts of chlorpheniramine maleate, 5-30 parts of montmorillonite, and 6-36 parts of cane sugar. By means of innovative accessory selection and optimized accessory proportion, the capsule dosage form is prepared; the problems of being poor in liquidity, non-ideal in drug disintegration, low in drug dissolution rate, poor in stability, not high in human bioavailability and the like in existing drug particles are solved.
Owner:SHANDONG SBOND PHARMA +1
Pet's scourge-clearing toxin-vanquishing chewable tablet and preparation process thereof
InactiveCN103417865BImprove efficacyReduce dosageAntibacterial agentsDigestive systemSucroseSaccharum
The invention discloses a pet's scourge-clearing toxin-vanquishing chewable tablet and a preparation process thereof. The pet's scourge-clearing toxin-vanquishing chewable tablet is composed of gypsum, rhizoma rehmanniae, cornu bubali, coptis chinensis, gardenia, moutan bark, scutellaria baicalensis, red peony, radix scrophulariae, rhizoma anemarrhenae, fructus forsythiae, lophatherum gracile, magnesium stearate, starch, extractum glycyrrhizae, ballonflower extractum and a flavoring agent. The preparation process is simple and includes three steps of preparing traditional Chinese medicine fine powder, preparing a mixed solution and mixing, pelletizing, tableting and forming. Compared with scourge-clearing toxin-vanquishing powder, the pet's scourge-clearing toxin-vanquishing chewable tablet has the advantages that a forming form of chewable tablet is adopted, traditional Chinese medicinal materials are subjected to smashing, tableting and forming, and extracted extractum glycyrrhizae and ballonflower extractum are adopted, so that dissolution, absorption and quick working of active ingredients are facilitated; medicine binding is realized through PVP (poly vinyl pyrrolidone), so that less proneness to moisture absorption is realized, efficacy can last longer, and full utilization of medicine is facilitated; mannitol used for flavoring can prevent the pet's scourge-clearing toxin-vanquishing chewable tablet from absorbing moisture, thereby having no hygroscopicity compared with common cane sugar, sorbitol and the like.
Owner:SICHUAN JIANYANG AIDI FEED PHARMA
Sustained-release type orally disintegrating film with excellant oral mucoadhesion and the manufacturing method thereof
InactiveCN112402397ASmooth inflowMaintain fitCosmetic preparationsToilet preparationsFilm baseDentistry
The present invention relates to a sustained-release oral disintegrating film having excellent oral mucosa adhesion, which contains a component with improved oral mucosa adhesion to allow efficient introduction of effective ingredients contained in an oral mucosa film through a capillary inside a mucosal layer since long-term attachment can be performed when being attached to the oral mucosa for use, and a disintegration time can be delayed by oral saliva by increasing adhesion for oral mucosa through a combination of chitosan or a compound thereof and glutathione with respect to components ofan oral mucosa attachable sustained-release oral disintegrating film, and being formed by coating ethyl cellulose exhibiting hydrophobic characteristics on one side of the film, and to a manufacturing method thereof. In addition, the sustained-release oral disintegrating film is purposed for a health functional food or medicine and medical supplies by appropriately mixing and combining effectiveingredients having various functional effects on the basis of film base ingredients secured in the present invention.
Owner:株式会社艾福瑞特
Phenoxybenzylamine hydrochloride tablets and preparation method thereof
ActiveCN111743871AImprove bindingGood compressibilityOrganic active ingredientsPill deliveryCarboxymethyl starchPhenoxybenzamine Hydrochloride
The invention discloses a phenoxybenzylamine hydrochloride tablet which comprises the following components: phenoxybenzylamine hydrochloride, dextrin, pregelatinized starch, a disintegrating agent, afilling agent, an adhesive, a lubricating agent and a flow aid. The viscosity of the dextrin ranges from 4.5 mpa.s to 8.5 mpa.s; the specific surface area of the pregelatinized starch is less than orequal to 350m < 2 > / kg; the disintegrating agent is carboxymethyl starch sodium. A disintegrating agent such as carboxymethyl starch sodium, dextrin with viscosity of 4.5-8.5 mpa.s and pregelatinizedstarch with specific surface area of less than or equal to 350m < 2 > / kg are added into the components, so that the obtained phenoxybenzamine hydrochloride tablet has improved dissolution rate and dissolution rate uniformity, and is small in intra-group difference and good in stability.
Owner:TOPFOND PHARMA CO LTD
Skeleton tablet core for digestive tract evacuation-detecting slow-release tablet and its preparing process
InactiveCN100354007CDelayed disintegration rateSlow disintegration rateEmulsion deliveryLow speedAcrylic resin
This invention is framework core used to alimentary tract emptying function detection slow release pill and its preparation technique. The core includes following components and quality percentage: hard dissolve medicine using materials =30-90%, X ray developer =5-50%, disintegration using auxiliary materials =5-65%. The hard dissolve medicine using materials are ethyl cellulose, or cerotic acid cellulose, or high viscosity hydroxypropyl cellulose, or hydroxide third concha cellulose phthalidyl, or hydroxymethyl ethyl cellulose, or acrylic resin. The X ray developer is barium sulfate developer, or iodine developer. The disintegration using auxiliary materials are hydroxymethyl starch natrium, or crosslinked polyethylene pyrrolidone, or low substitution hydroxypropyl cellulose. its preparation technique main including getting framework core materials and suppress framework core. The core can be observed by X ray perspective equipment in alimentary canal, is has low speed slaking characteristic.
Owner:CHONGQING UNIV
Milk powder high-protein effervescent tablet and preparation method thereof
ActiveCN106490164BFast disintegrationShort disintegration timeMilk preparationPharmaceutical delivery mechanismEffervescent tabletCompression molding
The invention provides a milk powder high-protein effervescent tablet and a preparation method thereof. The preparation method comprises the following steps: pelleting by using an acid-base separated type disintegrant, uniformly mixing base source granules and acid source granules, mixing with a lubricant, and performing compression molding. By adopting the preparation method provided by the invention, on the premise that the content of protein in the effervescent tablet is increased, the defects that a milk product has unstable properties, for example, the sticking and disintegration time is long, foams can be easily generated, and the like in a disintegration period can be remarkably overcome; the stability of a disintegrated product can be improved by innovatively changing the shape of the effervescent tablet and increasing the specific surface area, so that the disintegration time of the milk powder effervescent tablet can be effectively shortened, the stability of a protein system can be improved, and bubble and sticking phenomena in the disintegration process can be avoided; the milk powder high-protein effervescent tablet prepared by using the preparation method provided by the invention is high in disintegration speed, stable in product shape, applicable to various fast-moving consumption people, and capable of completely meeting industrial production demands.
Owner:BRIGHT DAIRY & FOOD CO LTD
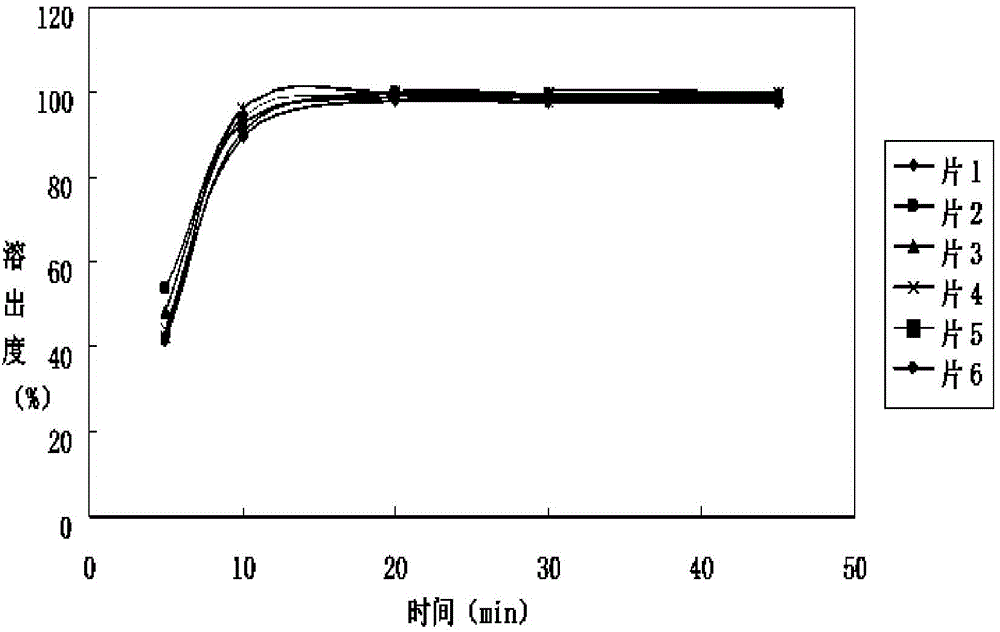
Levetiracetam tablet and preparation method thereof
ActiveCN112870176ASimple processEasy to operateOrganic active ingredientsNervous disorderCellulosePowder mixture
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to a levetiracetam tablet and a preparation method thereof. The preparation method comprises the following steps: 1, sieving levetiracetam, hydroxypropyl cellulose, croscarmellose sodium and part of colloidal silicon dioxide together for later use; 2, mixing the granulated materials in the step 1, adding magnesium stearate, and uniformly mixing to obtain premixed powder; 3, the premixed powder in the step 2 being subjected to dry granulation and size stabilization, and obtaining a particle and powder mixture; 4, mixing the granules and powder mixture granulated in the step 3 with the residual colloidal silicon dioxide, and tabletting; and 5, coating. The preparation method provided by the invention has the characteristics of simple process flow, strong operability, low production cost and stability.
Owner:HINYE PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com